Abstract
Tsetse flies (Diptera: Glossinidae) are the cyclical vectors of the trypanosomes, which cause human African trypanosomosis (HAT) or sleeping sickness in humans and African animal trypanosomosis (AAT) or nagana in animals. Due to the lack of effective vaccines and inexpensive drugs for HAT, and the development of resistance of the trypanosomes against the available trypanocidal drugs, vector control remains the most efficient strategy for sustainable management of these diseases. Among the control methods used for tsetse flies, Sterile Insect Technique (SIT), in the frame of area-wide integrated pest management (AW-IPM), represents an effective tactic to suppress and/or eradicate tsetse flies. One constraint in implementing SIT is the mass production of target species. Tsetse flies harbor obligate bacterial symbionts and salivary gland hypertrophy virus which modulate the fecundity of the infected flies. In support of the future expansion of the SIT for tsetse fly control, the Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture implemented a six year Coordinated Research Project (CRP) entitled “Improving SIT for Tsetse Flies through Research on their Symbionts and Pathogens”. The consortium focused on the prevalence and the interaction between the bacterial symbionts and the virus, the development of strategies to manage virus infections in tsetse colonies, the use of entomopathogenic fungi to control tsetse flies in combination with SIT, and the development of symbiont-based strategies to control tsetse flies and trypanosomosis. The results of the CRP and the solutions envisaged to alleviate the constraints of the mass rearing of tsetse flies for SIT are presented in this special issue.
Keywords: Tsetse, Glossinidae, Salivary gland hypertrophy virus, SIT, Symbionts, Pathogens
1. Introduction
Tsetse flies(Diptera:Glossinidae) are the cyclical vectors of the trypanosomes, which cause human African trypanosomosis (HAT) or sleeping sickness in humans and African animal trypanosomosis (AAT) or nagana in animals (Simarro et al., 2003) (Fig. 1). Human sleeping sickness is a zoonosis caused by the protozoan Trypanosoma brucei rhodesiense in East Africa and Trypanosoma brucei gambiense in West and Central Africa. The nagana-causing trypanosomatids, Trypanosoma vivax and Trypanosoma congolense, are major pathogens of cattle and other ruminants, whereas Trypanosoma simiae causes high mortality in domestic pigs and Trypanosoma brucei infects all livestock. Vaccines are not available and are unlikely to be developed due to the antigenic variation exhibited by the trypanosome in the mammalian host. The drug treatment of both HAT and ATT is in a perilous state and relies on old, often dangerous drugs and resistance is becoming an increasing problem (for more information see Holmes, 2013).
Fig. 1.
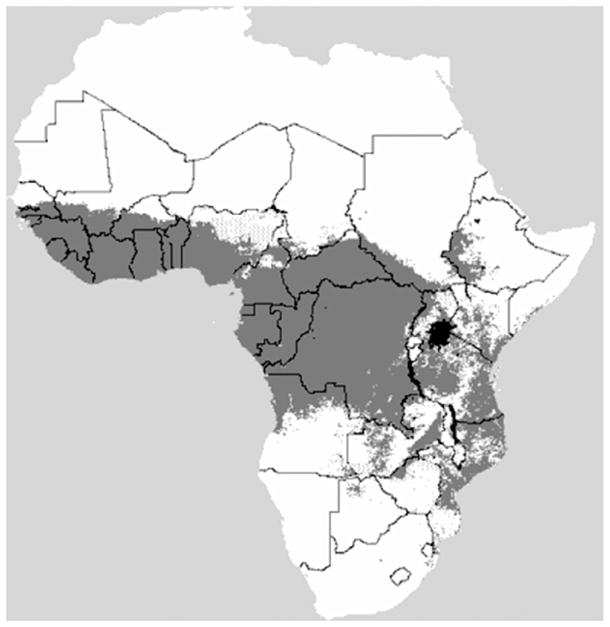
Tsetse fly distribution in African continent (modified from PAAT information system http://www.fao.org/ag/againfo/programmes/en/paat/infosys.html.
It is conservatively estimated by the World Health Organization (WHO) that there are currently 10,000–45,000 cases of human African trypanosomosis (HAT) with 60 million people at risk in 36 countries covering ~40% of Africa (almost 10 million km2). After a devastating epidemic in the early 20th century when a million people died of HAT the disease almost disappeared from Africa by the 1960s. In the 1990s we have witnessed yet another epidemic that killed tens of thousands of people. Through an ambitious campaign led by WHO and many Non-Governmental Organizations (NGOs) and thanks to a public–private partnership with Sanofi-Aventis and Bayer to donate the necessary drugs to WHO for distribution to affected countries, the latest epidemic has come under control although several foci in Congo, Uganda and Angola remain of major concern. Sustainable management of such diseases poses a formidable challenge to endemic countries and health ministries that are faced with limited infrastructure and financing. If the decline in the reported HAT cases trigger African governments to abandon their local control efforts and for funding agencies to relax their disease research priorities, it is certain that epidemics will continue to flare up in the near future as has happened in the recent past (Aksoy, 2011).
The impacts of African trypanosomosis are multidimensional and affect human health, livestock production, agricultural production (lack of draught animals and manure), rural socio-economic development, national economies (import and export of animal products), and the environment (insecticide applications). About fifty million cattle and tens of millions of small ruminants are at risk from AAT. Tsetse and trypanosomosis (T&T) are the major factors preventing the establishment of sustainable agricultural systems in many parts of sub-Saharan Africa. The Programme Against African Trypanosomosis (PAAT) estimates that AAT causes approximately 3 million cattle deaths per year. Farmers are required to administer approximately 35 million doses of costly trypanocidal drugs. Unfortunately, many of these chemicals fail because of development of resistance by the parasites. Direct losses in meat production and milk yield and the costs of programs that attempt to control trypanosomosis are estimated to amount to between US$600 million and $1.2 billion each year. In the absence of the AAT problem, a family that is currently dependent on manual labour alone could use draught animals and thus increase its income from agricultural work by 45% per unit of land and by 143% per unit of labor. If one includes this loss in livestock and crop production, trypanosomosis is estimated to cost sub-Saharan Africa US$4 billion or more each year (Budd, 2000), equivalent to the value of one-quarter of the area’s total livestock production.
The lack of effective vaccines and high costs of disease treatment, disease control, via control of the tsetse vector, has been found to be the most effective strategy. Present vector control efforts, which depend on trapping or killing the tsetse flies with insecticides, have been difficult to sustain at the local community level for human disease control. However these methods are widely used for control of animal diseases and this CRP has focused on one complement to current vector control methods, the Sterile Insect Technique (SIT). The eradication of the tsetse fly Glossina austeni from Unguja Island of Zanzibar by means of an area-wide integrated pest management program concluding with the release of sterile flies stimulated interest to expand this strategy to large areas on mainland Africa (Vreysen et al., 2000). In July 2000, at a summit in Lomé, Togo, the African Heads of State and Government passed a resolution (AHG/Dec.156 XXXVI), giving birth to the Pan African Tsetse and Trypanosomosis Eradication Campaign (PATTEC). Several UN Organizations, including WHO, the Food and Agricultural Organization (FAO) and the International Atomic Energy Agency (IAEA) subsequently passed resolutions supporting the PAT-TEC initiative. In May 2002, PATTEC and PAAT identified priority areas for T&T intervention and for related sustainable agriculture and rural development. Specific national/sub-regional strategies were developed so that the necessary national and international funding could be generated, including grants and soft loans from the African Development Bank.
2. FAO/IAEA sponsored Coordinated Research Project
The Joint Division of Nuclear Techniques in Food in Agriculture of the Food and Agriculture Organization (FAO) and the International Atomic Energy Agency (IAEA) sponsors Coordinated Research Projects (CRPs), research networks that focus participating scientists from both developing and developed countries on applying nuclear techniques to specific problems relevant to agriculture. A CRP entitled “Improving SIT for Tsetse Flies through Research on their Symbionts and Pathogens” was initiated in 2006. This CRP involved four Research Coordination Meetings (RCMs) held in Vienna, Austria (October, 2007), Bobo-Dioulasso, Burkina Faso, (February 2009), Nairobi, Kenya (July, 2010) and Vienna, Austria (March, 2012) to review results and plan future research. Twenty-three research teams from eighteen countries (Austria, Belgium, Burkina Faso, Cameroon, Canada, China, France, Germany, Ghana, Greece, Italy, Kenya, Slovakia, South Africa, Tanzania, The Netherlands, Uganda and the United States) participated and conducted research on different aspects of tsetse symbionts and pathogens.
The long-term goal of the CRP is to improve the quality of decision-making related to field implementation of SIT projects. Achieving the specific objectives outlined in the CRP (see below) is directed at improving the mass production of healthy colonies for production of sterile males, developing parasite-resistant SIT lines that can be applied in disease endemic areas, and incorporating natural incompatibilities for production of robust sterile males. The beneficiaries include: (i) people and livestock in the endemic areas through reduced incidence of trypanosomosis; (ii) livestock producers through increased profitability; (iii) the environment through reduced insecticide use; and (iv) tsetse-free countries by reducing, or possibly eliminating, the risk of introduction of these devastating diseases.
3. Objectives of the CRP
The overall objective of the CRP was to exploit interactions between tsetse flies and their microbes to enhance the efficacy of tsetse SIT programs. The specific objectives and the expected output of this CRP are listed in Table 1.
Table 1.
Specific objectives and the expected output of the CRP.
| Objectives of the CRP | Expected output of the CRP | Papers published during the CRP |
|---|---|---|
|
|
Alam et al. (2012), Bonomi et al. (2011), Bourtzis (2008), Muturi et al. (2011), Pais et al. (2008) Abd-Alla et al. (2010b), Adams et al. (2008, 2010), Doudoumis et al. (2012), Enyaru et al. (2010), Farikou et al. (2010a, 2010b, 2011a, 2011b), Geden et al. (2008, 2011b), Geiger et al. (2007, 2009, 2010, 2011), Hamilton et al. (2008), Lietze et al. (2011b), Malele et al. (2007, 2011a, 2011b) and Prompiboon et al. (2010) |
|
|
Abd-Alla et al. (2007, 2008, 2009a, 2009b, 2010a, 2011b), Garcia-Maruniak et al. (2008), Garcia-Maruniak et al. (2009), Geden et al. (2011a), Kariithi et al. (2010, 2011), Lietze et al. (2010, 2011c, 2011a), Wang et al. (2007) and Wang and Jehle (2009) Abd-Alla et al. (2010a, 2011b) and Sasanya et al. (2010) |
|
|
De Vooght et al. (2011, 2012) Akoda et al. (2009a, 2009b and 2009c) Scolari et al. (2011) |
|
|
Alam et al. (2012), Doudoumis et al. (2012), Ioannidis et al. (2007), Saridaki and Bourtzis (2009) and Saridaki and Bourtzis (2010) Ioannidis et al. (2007), Saridaki and Bourtzis (2009, 2010) |
|
|
http://entnemdept.ufl.edu/boucias/hytrosaviridae/Salivary_Gland_Hypertrophy_Viruses/Home6.html, http://www.sanger.ac.uk/cgi-bin/blast/submitblast/g_morsitans, www.cost-fa0701.com |
4. Current status
4.1. Tsetse symbionts
Tsetse flies feed exclusively on vertebrate blood and exhibit an unusual adenotrophic viviparity mode of reproduction during which all embryonic and larval stages develop within the female’s uterus (modified common oviduct). The mother develops a single oocyte at a time, then carries and nourishes the resulting embryo and larva in an intrauterine environment for the duration of its immature development. To supplement their single diet, which is restricted in many vitamins and coenzymes, tsetse have entered into symbiotic association with bacterial microbes. Throughout larvagenesis maternal milk gland secretions provide developing offspring with nourishment as well as 3 distinct endosymbiotic bacteria (Aksoy, 2000; Attardo et al., 2008). To date, members from the genera Wigglesworthia, Sodalis, and Wolbachia have been identified in both natural field populations as well as in colonies of different tsetse fly species. The paper presented by Balmand et al. (2013) in this volume describes the spatial distribution of tsetse’s three symbionts in different host tissues by applying fluorescence in situ hybridization (FISH) with symbiont specific 16S rDNA probes. These studies showed that Sodalis is absent from the germ cells (oocyte and embryo) while Wolbachia are present in these cells. Examination of the milk glands showed that Sodalis and Wigglesworthia were localized in the duct while Sodalis cells were also infecting the milk gland cells. In addition to the extracellular cells in the lumen of the milk gland, Wigglesworthia are also localized within bacteriocytes in the midgut bacteriome organ. Thus, it appears that while Wolbachia are transmitted transovarially, Sodalis and Wigglesworthia are acquired by the developing intrauterine larva from the mother’s milk glands.
4.1.1. Genus Wigglesworthia
The association between Wigglesworthia and its tsetse host is apparently ancient (50–80 million years old) and phylogenetic studies show that Wigglesworthia species display concordance with their insect host species, a phenomenon indicative of strict vertical transmission of the symbiont from generation to generation (Chen et al., 1999). The elimination of Wigglesworthia via antibiotic supplementation of tsetse’s diet results in loss of host fecundity (Nogge, 1976; Pais et al., 2008). The genome sequences of two Wigglesworthia species (from tsetse hosts Glossina brevipalpis and Glossina morsitans) indicates a drastic reduction to about 700 kb in size and a high A + T bias of over 80% (Akman et al., 2002; Rio et al., 2012). The related free living enterics typically have a genome size of 4000–10,000 kb (Wernegreen, 2002). Despite this reduction, and hence functional capacity, the small genome has retained many vitamin biosynthetic pathways, confirming the nutritional role of Wigglesworthia symbiosis in tsetse. The small Wigglesworthia genome has retained the ability to synthesize a functional flagellum. Gene expression studies indicate that the flagellum is expressed in the milk gland tissue possibly suggesting a role for flagella functions in the transmission process (Rio et al., 2012). Knowledge of Wigglesworthia products that support host fecundity and transmission biology to progeny is central for successful maintenance of tsetse colonies given its indispensable nature.
4.1.2. Genus Sodalis
The second organism, the commensal Sodalis, is harboured in all tsetse flies in the insectary colonies but is heterogeneous in natural populations. Symbionts closely related to Sodalis have been identified in insects from different taxa such as hippoboscid flies (Diptera), weevils (Coleoptera) and chewing lice (Phthiraptera) (Kaiwa et al., 2010; Novakova and Hypsa, 2007; Snyder et al., 2011). The taxonomic origin of Sodalis needs to be further clarified. The genome sequence of Sodalis is also available, further mediating functional studies into Sodalis’ biology in the tsetse host. Unlike Wigglesworthia the Sodalis genome, which is about 4.5 Mb in size, has not undergone a size reduction and is more in line with free-living enteric microbes. It does however contain many pseudogenes, especially in pathways that are unlikely to be active in the restricted nutritional ecology of its host biology. It represents an organism that is apparently in transition from a free-living state to a symbiotic microbe (McCutcheon and Moran, 2012).
Sodalis’s functional role in tsetse is unknown. An interesting hypothesis has focused on the influential role of Sodalis for trypanosome transmission. The presence of Sodalis has been implicated in enhancing the vectorial capacity of tsetse (Baker et al., 1990; Maudlin et al., 1990). Flies that harbour greater Sodalis densities have been suggested to be more susceptible to trypanosomes. In addition to density effects, it is also possible that different Sodalis genotypes may confer greater transmission ability to the host. These inferences can be evaluated further in populations both for density effects and for potentially different genotypes that may exist in natural populations that confer enhanced parasite transmission ability to host insects. In a previous work, a large tsetse fly sampling campaign had been conducted in Bipindi and Campo where that are two historical sleeping sickness foci located in the south of Cameroon. Each fly species was identified along with the presence/absence of Sodalis glossinidius, and the trypanosome species infections they may carry. Given the wide differences observed between the two fly populations, the genetic diversity in symbiont populations was investigated. Preliminary results using about 200 flies have confirmed the existence of Sodalis diversity in field flies and allowed for the identification of about twenty different genotypes (Tchouomene-Labou et al., personal communication).
It has been possible to establish in vitro culture conditions for Sodalis, to introduce and express foreign genes and to reintroduce the modified symbionts into tsetse (Beard et al., 1993a). Other desirable traits can now be introduced and expressed in Sodalis. One application of the paratransgenic process for SIT is the ability to engineer release lines that are unable to transmit trypanosomes in the field since both male and female tsetse blood feed and transmit trypanosomes. The establishment of such lines will further enable the on-going sterile male release programs and those planned in human disease endemic areas (Aksoy et al., 2008a, 2008b; Rio et al., 2004).
To enable paratransgenic applications two candidate trypanosome-interfering proteins were selected for expression in Sodalis: the human trypanolytic serum protein, Apolipoprotein L-1, and a trypanosome targeting Nanobody® Nb33. For trypanocidal impact, it is essential that the expressed proteins are released from the symbiont into the midgut lumen (Caljon et al., 2013). This requires improved knowledge of the functional secretion pathways to the periplasmatic and/or extracellular environment in Sodalis. Expression of a marker gene (Green Fluorescent Protein, GFP) fused to the twin-arginine signal peptide of TMAO reductase (TorA), which is known to target proteins across the cytoplasmic membrane via the twin-arginine translocation (Tat) system was successful. Thus, it appears that heterologous proteins can be exported to the periplasm of S. glossinidius in an active form by the Tat pathway. This periplasmic expression is an important first step in the development of a Sodalis expression system that will allow the release of the trypanosome-targeting nanobodies into the extracellular environment where they can tackle the trypanosome parasite.
4.1.3. Genus Wolbachia
The third symbiont Wolbachia is an obligatory intracellular and maternally transmitted alphaproteobacterium that infects many arthropod and filarial nematode species (Saridaki and Bourtzis, 2010; Werren et al., 2008). Wolbachia is responsible for the induction of a number of reproductive alterations and cytoplasmic incompatibility (CI) (Saridaki and Bourtzis, 2010; Serbus et al., 2008; Werren et al., 2008). In its simplest form, CI is expressed as embryonic lethality when an infected male is crossed with an uninfected female (Werren et al., 2008; Saridaki and Bourtzis, 2010). Wolbachia-induced CI has been proposed as a tool for the control of vector populations and diseases (Aksoy et al., 2001; Beard et al., 1993b, 1998; Bourtzis, 2008; Bourtzis and O’Neill, 1998; O’Neill et al., 1993). A prerequisite for the development of symbiont-based control strategies is the detection and characterization of the symbiotic communities of both laboratory and natural population of the target species. Although the presence of Wolbachia has been reported in a limited number of tsetse flies samples (Cheng et al., 2000; O’Neill et al., 1993), a thorough investigation for the presence of this symbiont, particularly in natural populations, as well the genotyping of these infections was lacking. One of the specific objectives of this CRP was to study the prevalence, genotyping and phylogenetic analysis of Wolbachia infections in both laboratory and natural populations of Glossina species including the investigation of the potential reproductive effects this infection may have in tsetse flies (see Doudoumis et al. 2013; Wang et al. 2013, Schneider et al. 2013; (Alam et al., 2012; Doudoumis et al., 2012).
4.1.4. Intercommunity dynamics
It has been possible to develop tsetse lines that are either devoid of Wigglesworthia or that are symbiont free by supplementation of the host diet with ampicillin and tetracycline along with yeast extract, respectively (Alam et al., 2012; Pais et al., 2008). These lines have allowed for investigations into the functional roles of tsetse’s different symbionts. In the absence of Wigglesworthia (while still harbouring Sodalis and Wolbachia) adult flies were found to be unusually susceptible to infections with trypanosomes. Molecular investigations into tsetse’s immune responses under these conditions have shown that larvae developed in the absence of Wigglesworthia give rise to adults that have compromised cellular immune responses (Weiss et al., 2011, 2012). Many studies are now indicating that interactions between the beneficial microbes harboured by insect hosts and pathogens represent highly dynamic processes. The paper presented by Wang et al., describes the intercommunity influences on symbiont density regulation by following symbiotic densities in these lines through multiple generations. These studies show evidence for cooperation between Wigglesworthia and Sodalis such that fly lines that lack Wigglesworthia while retaining Sodalis and Wolbachia eventually loose Sodalis infections by the third generation, while Wolbachia and GpSGHV densities initially decline but eventually reach normal levels. Interestingly, in the absence of tsetse’s symbionts, GpSGHV densities display relaxed density levels but reach homeostatic levels by the third generation. Thus, tsetse host responses as well as community interdependencies are apparently influential in shaping symbiotic densities.
4.1.5. Symbiotic influences on female mating behavior
Data have been obtained on spatial/temporal variation in the reproductive behavior of G. fuscipes fuscipes. In particular, the occurrence of female mating and remating has been assessed in two natural populations in Uganda (Bonomi et al., 2011). Different mating and remating estimates provide evidence that remating is a frequent behavior in the wild and what is more, females store sperm from multiple males, which are potentially available for insemination. In these two populations, Wolbachia infection frequency is high. Work is in progress to assess if the presence of Wolbachia infections may result in a selective advantage for polyandry and on the consequent sperm use.
4.2. Tsetse fly pathogens
The majority of research on the SGHV has been conducted under objective 2 to understand and manage the tsetse SGHV interactions in lab populations. The first of the five verifiable indicators for this objective was the analysis of the GpSGHV genome isolated from the Uganda tsetse strain maintained since 1979 at Seibersdorf. The collaborative research effort of the IAEA laboratories at Seibersdorf, and others have produced and published a full-length sequence of the GpSGHV (Abd-Alla et al., 2008). In addition, the complete genome sequence of the SGHV isolate from Ethiopian G. pallidipes colony has been sequenced. The two viruses share 98.7% nucleotide sequence identity, their overall organization is almost identical, and 80% of their ORFs have the same best blastp value, strongly supporting the conclusion that they are two isolates of the same virus (unpublished data). The putative 160 ORFs of both genome isolates correspond to expressed sequences, their transcriptome maps showed that over 90% of the putative ORFs are transcribed (unpublished data).
Phylogenetic analysis conducted on several genes demonstrated that the GpSGHV differs significantly from all known large dsDNA viruses of invertebrates (baculoviruses, nudiviruses, iridoviruses, ascoviruses, entomopoxviruses and whispoviruses) (Fig. 2). Based on these data and the structure of the virus particle, a new virus family, named Hytrosaviridae, has been proposed to and accepted by the International Committee on Taxonomy of Viruses (ICTV) by the newly established Hytrosaviridae Study Group (Abd-Alla et al., 2009b). The name Hytrosaviridae is derived from Hytrosa, a sigla from the Greek ‘Hypertrophia’ for ‘hypertrophy’, ‘sialoadenitis’ for ‘salivary gland inflammation and consist of two genera: (1) Glossinavirus with the type species Glossina hytrosavirus, and (2) Muscavirus with the type species Musca hytrosavirus. GpHV belongs to the species Glossina hytrosavirus. The genus Muscavirus includes the SGHV from Musca domestica. Its genome, although smaller (124,241 bp) than that of the GpSGHV, produces similar SGH symptoms (Fig. 2), and contains a number of putative ORFs which are homologues to those of GpSGHV and which are distinct from other insect viruses (Garcia-Maruniak et al., 2008, 2009). Phylogenetic analyses have been performed to elucidate the relationship of SGHVs to the baculoviruses and nudiviruses, as these viruses share a number of core genes involved in DNA replication, RNA transcription and virion host cell interaction (Wang et al., 2011; Wang and Jehle, 2009). There is strong evidence that hytrosaviruses, nudiviruses and baculoviruses comprise a supergroup of invertebrate-specific nuclear-replicating circular dsDNA viruses, warranting the establishment of a new virus Order (Jehle et al., 2013).
Fig. 2.
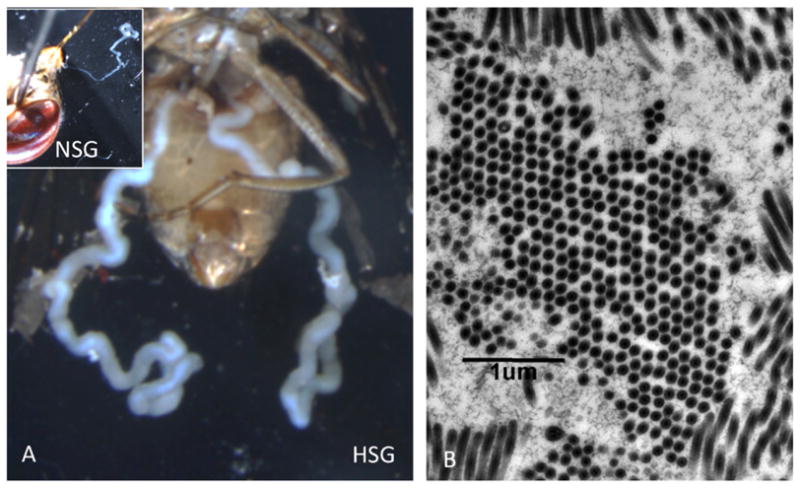
(A) Normal (NSG) and hypertrophied salivary gland (HSG) of Glossina pallidipes fly. (B) Thin section of a heavily infected salivary gland cell showing bundles of GpHV virions in both longitudinal and cross sections.
The availability of sequence information from multiple closely related and disparate SGHVs has provided a framework to identify and design molecular probes that target common domains. These data have been used to develop universal primer pairs designed to detect SGHVs (Abd-Alla et al., 2011b). Experiments have demonstrated that these primers generated a virus-specific PCR product from both MdHV and GpHV whereas no non-specific PCR product was found when using genomic DNA of healthy flies as templates. Therefore, these primers sets could be used to detect novel hytrosaviruses in other insects. A similar approach can be taken in designing dsRNA to target homologous regions to reduce viral replication. Significantly, this approach will allow validation of the suppressive activities of RNAi in the easily manipulated house fly host.
LC-MS/MS analysis revealed that GpSGHV contains 61 virus-encoded proteins (Kariithi et al., 2010). The most abundant proteins (GP10 and GP96) are products of the ORF10 and ORF96 genes. The proteome contained homologues to four per os infectivity factors (PIFs), that are essential for oral infectivity in baculoviruses (Slack and Arif, 2007) and a giant protein (511 kDa) encoded by ORF62. Antiserum generated against virions strongly reacted with GP10 and GP96 and a few other virion proteins, such as the PIF proteins. Among the GpSGHV structural proteins, 32 had homolog genes in the MdHV genome, although only 12 of these were detected in the MdHV proteome. This supports the recent assembly of GpSGHV and MdHV into two separate genera within the accepted family Hytrosaviridae. Information on the GpHV proteome is pivotal for both neutralization of virus infection and interruption of virus transmission in tsetse fly colonies. Boucias et al. (2013) described the major envelope protein of MdHV and showed that the neutralization of the virus particle of MdHV with the specific envelope protein antibodies reduced levels of the virus infection. The analysis of the saliva of hypertrophied flies indicated, in addition of the virion proteins, the presence of a suite of host specific proteins providing new leads for virus control studies (Kariithi et al., 2011).
The second verifiable indicator was deciphering the pathology of the SGHVs. Utilizing quantitative PCR-based diagnostic methods (Abd-Alla et al., 2009a, 2010b), non-infected and infected flies have been identified and were used to demonstrate that this virus can be both vertically and horizontally transmitted in colonized tsetse flies. In addition to salivary glands that display overt pathologies, a wide range of tissues was found to support replication (Abd-Alla et al., 2009b). A parallel study, conducted by Lietze et al. (2011c), demonstrated that the MdHVDNA and resulting transcripts can be detected at high levels in a wide range of tissues without inducing hypertrophy. EM studies suggested that the tracheole system extending throughout all tissues is an alternate site of replication for MdHV. In heterologous fly species, evidence is accumulating that the SGHVs can replicate without causing detectable hypertrophy symptoms. For example, challenge of the stable fly (Stomoxys calcitrans) and the black dump fly (Hydrotaea aenescens) with MdHV sterilized females without inducing hypertrophy (Geden et al., 2011a).
Replication of GpHV (Seibersdorf) and of MdHV (Florida) was attempted in 21 cell lines derived from Lepidoptera, Hymenoptera, Hemiptera and Coleoptera. Inoculated cells were observed daily for evidence of virus-induced cytopathic effects and alteration in cell growth. To date, no evidence of replication of either virus was observed in the tested cell lines, which underscores the need to generate novel cell lines from the tsetse fly and house fly (see Arif, 2013).
The third verifiable indicator was the establishment and maintenance of asymptomatic colonies. To date, not all colonized flies have been shown to harbour GpHV. In Tanzania, molecular analysis of laboratory Glossina pallidipes did not detect SGHV infection; however a prevalence of 5% was recorded from G. pallidipes and G. morsitans lab flies in Kenya. The relative incidence of this virus in wild populations varies from one location to another. For example, SGHV prevalence studies in different wild tsetse populations from two sites in Tanzania (coastal and inland) did not reveal infection in 200 dissected flies and 30 flies analyzed by PCR (Malele et al., 2013). In Kenya, six out of eight feral populations of G. pallidipes, G. brevipalpis and G. austeni, had infection rates ranging from 0% to 77%. Further screening will be performed to verify the status of colonized laboratory flies, and screening of feral populations from the three countries will continue in order to identify if there are any virus-free populations. Genetic analysis of GpHV from different regions of East Africa suggested a high degree of conservation enhancing the feasibility of a generic strategy for virus control (Kariithi et al., 2013).
The fourth verifiable indicator was the development of management strategies to reduce virus loads in tsetse fly mass rearing (Abd-Alla et al., 2011a). Polyclonal antibodies were generated against eight GpHV virion proteins, including those encoded by ORF10, ORF96 and the four PIF genes to study their potential to neutralize GpHV infection and to block virus transmission in tsetse flies. As the ORF10 protein has been localized on the surface of the GpHV virion, the anti-ORF10 has potential to block transmission of GpHV and is, therefore, a good candidate to block transmission of the virus in tsetse fly rearings and production systems (Kariithi et al., 2010). The impact of several antiviral drugs on the viral infection in G. pallidipes is presently being examined as a potential blood supplement. Recently, an effective control of GpSGHV in laboratory condition using antiviral drug valacyclovir has been achieved (Abd-Alla et al., 2012).
Several experiments were carried out to better understand the virus transmission and biology (Abd-Alla et al., 2010b). After demonstrating the role of horizontal transmission through the membrane feeding system used in the tsetse fly laboratory colony, it was recommended to initiate a “clean feeding” system. One clean-feeding colony was established by feeding the fly cages first on the fresh membrane and blood and keeping these cages and their progeny fed in the same way. The remaining blood was used by the other “normal-feeding” colony. The “clean feeding” colony was maintained separately from the other colony and samples were taken regularly to assess the virus load and the prevalence of SGH. The qPCR results indicated significant decreases in the virus load in the “clean feeding” colony in comparison with the “normal feeding” colony. Fly dissections indicated that after three months the clean-feeding colony became a SGH-free while the normal-feeding colony continued to show about 10% SGH (unpublished data).
A fifth verifiable indicator was to demonstrate horizontal transmission of entomopathogenic fungal inoculum. Experiments conducted in a large field cage (Calkins and Webb, 1983) to simulate field conditions have confirmed the horizontal transmission of conidia between Metarhizium anisopliae-infected G. pallidipes and fungus-free flies when the flies were allowed to mate. All the fungus-treated “donor” male or female flies succumbed to fungal infection within 6–10 d with mycosis. First or second line “recipient” flies that succeeded in mating with the “donor” flies, also died from fungal infection with mycosis within 7–15 days after mating. Fungal infection also had an effect on the reproduction potential of flies. Female flies in the control treatment produced more puparia than the fungus treatments, except in the second line recipient where there was no difference between the treatments (Maniania and Ekesi, 2013).
Acknowledgments
We are thankful to all colleagues who enthusiastically participated in this Coordinated Research Project and who contributed to reviewing the papers presented in this volume.
Footnotes
Disclosures
The authors Adly Abd-Alla, Max Bergoin, Andrew G. Parker, Nguya K. Maniania, Just M. Vlak, Kostas Bourtzis, Drion. G. Boucias and Serap Aksoy report no conflicts of interest to be declared.
References
- Abd-Alla A, Bossin H, Cousserans F, Parker A, Bergoin M, Robinson A. Development of a non-destructive PCR method for detection of the salivary gland hypertrophy virus (SGHV) in tsetse flies. J Virol Methods. 2007;139:143–149. doi: 10.1016/j.jviromet.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Abd-Alla AMM, Cousserans F, Parker AG, Jehle JA, Parker NJ, Vlak JM, Robinson AS, Bergoin M. Genome analysis of a Glossina pallidipes salivary gland hypertrophy virus (GpSGHV) reveals a novel large double-stranded circular DNA virus. J Virol. 2008;82:4595–4611. doi: 10.1128/JVI.02588-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Alla A, Cousserans F, Parker A, Bergoin M, Chiraz J, Robinson A. Quantitative PCR analysis of the salivary gland hypertrophy virus (GpSGHV) in a laboratory colony of Glossina pallidipes. Virus Res. 2009a;139:48–53. doi: 10.1016/j.virusres.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Abd-Alla AMM, Vlak JM, Bergoin M, Maruniak JE, Parker AG, Burand JP, Jehle JA, Boucias DG. Hytrosaviridae: a proposal for classification and nomenclature of a new insect virus family. Arch Virol. 2009b;154:909–918. doi: 10.1007/s00705-009-0398-5. [DOI] [PubMed] [Google Scholar]
- Abd-Alla AMM, Boucias DG, Bergoin M. Hytrosaviruses: structure and genomic properties. In: Asgari S, Johnson KN, editors. Insect Virology. Caister Academic Press; Norfolk: 2010a. pp. 103–121. [Google Scholar]
- Abd-Alla AMM, Kariithi H, Parker AG, Robinson AS, Kiflom M, Bergoin M, Vreysen MJB. Dynamics of the salivary gland hypertrophy virus in laboratory colonies of Glossina pallidipes (Diptera: Glossinidae) Virus Res. 2010b;150:103–110. doi: 10.1016/j.virusres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Abd-Alla AMM, Parker AG, Vreysen MJB, Bergoin M. Tsetse salivary gland hypertrophy virus: hope or hindrance for tsetse control? PLoS Negl Trop Dis. 2011a;5:e1220. doi: 10.1371/journal.pntd.0001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Alla AMM, Salem TZ, Parker AG, Wang Y, Jehle JA, Vreysen MJB, Boucias D. Universal primers for rapid detection of Hytrosaviruses. J Virol Methods. 2011b;171:280–283. doi: 10.1016/j.jviromet.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Abd-Alla AM, Adun H, Parker AG, Vreysen MJ, Bergoin M. The antiviral drug valacyclovir successfully suppresses salivary gland hypertrophy virus (SGHV) in laboratory colonies of glossina pallidipes. PLoS One. 2012;7:e38417. doi: 10.1371/journal.pone.0038417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ER, Hamilton PB, Malele II, Gibson WC. The identification, diversity and prevalence of trypanosomes in field caught tsetse in Tanzania using ITS-1 primers and fluorescent fragment length barcoding. Infect, Genet Evolut. 2008;8:439–444. doi: 10.1016/j.meegid.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Adams ER, Hamilton PB, Rodrigues AC, Malele II, Delespaux V, Teixeira MMG, Gibson W. New Trypanosoma (Duttonella) vivax genotypes from tsetse flies in East Africa. Parasitology. 2010;137:641–650. doi: 10.1017/S0031182009991508. [DOI] [PubMed] [Google Scholar]
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- Akoda K, Van Den Abbeele J, Marcotty T, De Deken R, Sidibe I, van den Bossche P. Nutritional stress of adult female tsetse flies (Diptera: Glossinidae) affects the susceptibility of their offspring to trypanosomal infections. Acta Trop. 2009a;111:263–267. doi: 10.1016/j.actatropica.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Akoda K, van den Bossche P, Lyaruu EA, De Deken R, Marcotty T, Coosemans M, Van Den Abbeele J. Maturation of a Trypanosoma Brucei infection to the infectious metacyclic stage is enhanced in nutritionaly stressed tsetse flies. J Med Entomol. 2009b;46:1446–1449. doi: 10.1603/033.046.0629. [DOI] [PubMed] [Google Scholar]
- Akoda K, van den Bossche P, Marcotty T, Kubi C, Coosemans M, Dedeken R, Van Den Abbeele J. Nutritional stress affects the tsetse fly’s immune gene expression. Med Vet Entomol. 2009c;23:195–201. doi: 10.1111/j.1365-2915.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- Aksoy S. Tsetse – a haven for microorganisms. Parasitol Today. 2000;16:114–118. doi: 10.1016/s0169-4758(99)01606-3. [DOI] [PubMed] [Google Scholar]
- Aksoy S. Sleeping sickness elimination in sight: time to celebrate and reflect, but not relax. PLoS Negl Trop Dis. 2011;5:1–3. doi: 10.1371/journal.pntd.0001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy S, Maudlin I, Dale C, Robinson AS, O’Neill SL. Prospects for control of African trypanosomiasis by tsetse vector manipulation. Trends Parasitol. 2001;17:29–35. doi: 10.1016/s1471-4922(00)01850-x. [DOI] [PubMed] [Google Scholar]
- Aksoy S, Weiss B, Attardo G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness 8. Adv Exp Med Biol. 2008a;627:35–48. doi: 10.1007/978-0-387-78225-6_3. [DOI] [PubMed] [Google Scholar]
- Aksoy S, Weiss B, Attardo GM. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. In: Aksoy Serap., editor. Transgenesis and the Management of Vector-Borne Disease (Advances in Experimental Medicine and Biology) Springer Science-Business Media, LLC Landes Bioscience; New York: 2008b. pp. 35–48. < www.spinger.com>. [DOI] [PubMed] [Google Scholar]
- Alam U, Medlok J, Brelsfoard C, Pais R, Lohs C, Balmand S, Carnogursky J, Heddi A, Takac P, Galvani A, Aksoy S. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathog. 2012;7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif B, Pavlik L. Insect cell culture: Virus replication and applications in biotechnology. J Invertebr Pathol. 2013;112 (Suppl 1):S138–S141. doi: 10.1016/j.jip.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J Insect Physiol. 2008;54:1–7. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RD, Maudlin I, Milligan PJM, Molyneux DH, Welburn SC. The possible role of Rickettsia-like organisms in trypanosomiasis epidemiology. Parasitology. 1990;100:209–217. doi: 10.1017/s0031182000061217. [DOI] [PubMed] [Google Scholar]
- Balmand S, Lohs C, Aksoy S, Heddi A. Tissue distribution and transmission routes for the tsetse fly endosymbionts. J Invertebr Pathol. 2013;112 (Suppl 1):S116–S122. doi: 10.1016/j.jip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CB, O’Neill SL, Mason P, Mandelco L, Woese CR, Tesh RB, Richards FF, Aksoy S. Genetic transformation and phylogeny of bacterial symbionts from tsetse 1. Insect Mol Biol. 1993a;1:123–131. doi: 10.1111/j.1365-2583.1993.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Beard CB, O’Neill SL, Tesh RB, Richards FF, Aksoy S. Modification of arthropod vector competence via symbiotic bacteria. Parasitol Today. 1993b;9:180–183. doi: 10.1016/0169-4758(93)90142-3. [DOI] [PubMed] [Google Scholar]
- Beard CB, Durvasula RV, Richards FF. Bacterial symbiosis in arthropods and the control of disease transmission. Emer Infect Dis. 1998;4:581–591. doi: 10.3201/eid0404.980408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi A, Bassetti F, Gabrieli P, Beadell J, Falchetto M, Scolari F, Gomulski LM, Regazzini E, Ouma JO, Caccone A, Okedi LM, Attardo GM, Guglielmino CR, Aksoy S, Malacrida AR. Polyandry is a common event in wild populations of the tsetse fly Glossina fuscipes fuscipes and may impact population reduction measures. PLoS Negl Trop Dis. 2011;5:e1190. doi: 10.1371/journal.pntd.0001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucias DG, Deng F, Hu Z, Garcia-Maruniak A, Lietze VU. Analysis of the structural proteins from the Musca domestica hytrosavirus with an emphasis on the major envelope protein. J Invertebr Pathol. 2013;112 (Suppl 1):S44–S52. doi: 10.1016/j.jip.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Bourtzis K. Wolbachia-based technologies for insect pest population control. In: Aksoy S, editor. Transgenesis and the Management of Vector-Borne Disease (Advances in Experimental Medicine and Biology) Springer Science+Business Media. LLC; 233 Springer Street, New York, New York 10013, USA, New York: 2008. pp. 104–113. < www.springer.com>. [DOI] [PubMed] [Google Scholar]
- Bourtzis K, O’Neill S. Wolbachia infections and arthropod reproduction. Bioscience. 1998;48:287–293. [Google Scholar]
- Budd LT. Economic Analysis. Vol. 2. Natural Resources International Limited; Aylesford, UK: 2000. DFID-funded tsetse and trypanosome research and development since 1980. [Google Scholar]
- Caljon G, De Vooght L, Abbeele JVD. Options for the delivery of antipathogen molecules in arthropod vectors. J Invertebr Pathol. 2013;112 (Suppl 1):S75–S82. doi: 10.1016/j.jip.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Calkins CO, Webb JC. A cage and support framework for behavioral tests of fruit flies in the field. Fla Entomol. 1983;66:514. [Google Scholar]
- Chen X, Sond L, Aksoy S. Concordant evolution of a symbiont with its host insect species: Molecular phylogeny of Genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J Mol Evol. 1999;48:49–58. doi: 10.1007/pl00006444. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Ruel TD, Zhou W, Moloo SK, Majiwa P, O’Neill SL, Aksoy S. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med Vet Entomol. 2000;14:44–50. doi: 10.1046/j.1365-2915.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- De Vooght L, Caljon G, Coosemans M, Van Den Abbeele J. Functional analysis of the Twin-Arginine Translocation pathway in Sodalis glossinidius, a bacterial symbiont of the tsetse fly. Appl Env Microbiol. 2011;77:1132–1134. doi: 10.1128/AEM.02379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vooght L, Caljon G, Stijlemans S, Coosemans M, Van Den Abbeele J. Expression and extracellular release of a functional anti-trypanosome Nanobody in Sodalis glossinidius, a bacterial symbiont of the tsetse fly. Microb Cell Fact. 2012:11. doi: 10.1186/1475-2859-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudoumis V, Tsiamis G, Wamwiri F, Brelsfoard C, Alam U, Aksoy E, Dalaperas S, Abd-Alla A, Ouma J, Takac P, Aksoy S, Bourtzis K. Detection and characterization of Wolbachia infections in laboratory and natural populations of different species of tsetse (genus Glossina) BMC Micobiology. 2012;12:1–13. doi: 10.1186/1471-2180-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudoumis V, Alam U, Aksoy E, Abd-Alla AMM, Tsiamis G, Brelsfoard C, Aksoy S, Bourtzis K. Tsetse-Wolbachia symbiosis: Comes of age and has great potential for pest and disease control. J Invertebr Pathol. 2013;112 (Suppl 1):S94–S103. doi: 10.1016/j.jip.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyaru JC, Ouma JO, Malele II, Matovu E, Masiga DK. Landmarks in the evolution of technologies for identifying trypanosomes in tsetse flies. Trends Parasitol. 2010;26:388–394. doi: 10.1016/j.pt.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Farikou O, Njiokou F, Mbida JAM, Njitchouang GR, Djeunga HN, Asonganyi T, Simarro PP, Cuny G, Geiger A. Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes - an epidemiological approach in two historical human african trypanosomiasis foci in Cameroon. Infect, Genet Evol. 2010a;10:115–121. doi: 10.1016/j.meegid.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Farikou O, Njiokou F, Simo G, Asonganyi T, Cuny G, Geiger A. Tsetse fly blood meal modification and trypanosome identification in two sleeping sickness foci in the forest of southern Cameroon. Acta Trop. 2010b;116:81–88. doi: 10.1016/j.actatropica.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Farikou O, Njiokou F, Cuny G, Geiger A. Microsatellite genotyping reveals diversity within populations of Sodalis glossinidius, the secondary symbiont of tsetse flies. Vet Microbiol. 2011a;150:207–210. doi: 10.1016/j.vetmic.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Farikou O, Thevenon S, Njiokou F, Allal F, Cuny G, Geiger A. Genetic diversity and population structure of the secondary symbiont of tsetse flies, Sodalis glossinidius, in sleeping sickness foci in Camerooon. PLoS Negl Trop Dis. 2011b;5:e1281. doi: 10.1371/journal.pntd.0001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Maruniak A, Maruniak JE, Farmerie W, Boucias DG. Sequence analysis of a non-classified, non-occluded DNA virus that causes salivary gland hypertrophy of Musca domestica, MdSGHV. Virology. 2008;377:184–196. doi: 10.1016/j.virol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Maruniak A, Abd-Alla AMM, Salem TZ, Parker AG, van Oers MM, Maruniak JE, Kim W, Burand JP, Cousserans F, Robinson AS, Vlak JM, Bergoin M, Boucias DG. Two viruses that cause salivary gland hypertrophy in Glossina pallidipes and Musca domestica are related and form a distinct phylogenetic clade. J Gen Virol. 2009;90:334–346. doi: 10.1099/vir.0.006783-0. [DOI] [PubMed] [Google Scholar]
- Geden CJ, Lietze VU, Boucias DG. Seasonal prevalence and transmission of salivary gland hypertrophy virus of house flies (Diptera: Muscidae) J Med Entomol. 2008;45:42–51. doi: 10.1603/0022-2585(2008)45[42:spatos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Geden C, Garcia-Maruniak A, Lietze VU, Maruniak J, Boucias DG. Impact of house fly salivary gland hyperthrophy virus (MdSGHV) on a heterologous host, Stomoxys calcitrans. J Med Entomol. 2011a;46:1128–1135. doi: 10.1603/me11021. [DOI] [PubMed] [Google Scholar]
- Geden CJ, Steenberg T, Lietze VU, Boucias DG. Salivary gland hyperthrophy virus of house flies in Denmark: prevalence, host range, and comparison with a Florida isolate. J Vector Ecol. 2011b;36:231–238. doi: 10.1111/j.1948-7134.2011.00163.x. [DOI] [PubMed] [Google Scholar]
- Geiger A, Ravel S, Mateille T, Janelle J, Patrel D, Cuny G, Frutos R. Vector competence of Glossina palpalis gambiensis for Trypanosoma brucei sl and genetic diversity of the symbiont Sodalis glossinidius. Mol Biol Evol. 2007;24:102–109. doi: 10.1093/molbev/msl135. [DOI] [PubMed] [Google Scholar]
- Geiger A, Fardeau ML, Grebaut P, Vatunga G, Josenando T, Herder S, Cuny G, Truc P, Ollivier B. First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis papalis) midgut. Infect, Genet Evol. 2009;9:1364–1370. doi: 10.1016/j.meegid.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Geiger A, Fardeau ML, Falsen E, Ollivier B, Cuny G. Serratia glossinae sp nov., isolated from the midgut of the tsetse fly Glossina palpalis gambiensis. Int J Syst Evol Microbiol. 2010;60:1261–1265. doi: 10.1099/ijs.0.013441-0. [DOI] [PubMed] [Google Scholar]
- Geiger A, Fardeau ML, Njiokou F, Joseph M, Asonganyi T, Ollivier B, Cuny G. Bacterial diversity associated with populations of Glossina spp. from Cameroon and distribution within the Campo sleeping sickness focus. Microbial Ecol. 2011;62:632–643. doi: 10.1007/s00248-011-9830-y. [DOI] [PubMed] [Google Scholar]
- Hamilton PB, Adams ER, Malele II, Gibson WC. A novel, high-throughput technique for species identification reveals a new species of tsetse-transmitted trypanosome related to the Trypanosoma brucei subgenus, Trypanozoon. Infect, Genet Evol. 2008;8:26–33. doi: 10.1016/j.meegid.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes P. Tsetse-transmitted trypanosomes-Their biology, disease impact and control. J Invertebr Pathol. 2013;112 (Suppl 1):S11–S14. doi: 10.1016/j.jip.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Ioannidis P, Dunning Hotopp JC, Sapountzis P, Siozios S, Tsiamis G, Bordenstein SR, Baldo L, Werren JH, Bourtzis K. New criteria for selecting the origin of DNA replication in Wolbachia and closely related bacteria. BMC Genomics. 2007;8:182. doi: 10.1186/1471-2164-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle JA, Abd-Alla AMM, Wang Y. Phylogeny and evolution of Hytrosaviridae. J Invertebr Pathol. 2013;112 (Suppl 1):S62–S67. doi: 10.1016/j.jip.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Kaiwa N, Hosokawa T, Kikuchi Y, Nikoh N, Meng XY, Kimura N, Ito M, Fukatsu T. Primary gut symbiont and secondary, Sodalis-allied symbiont of the Scutellerid stinkbug Cantao ocellatus 1. Appl Environ Microbiol. 2010;76:3486–3494. doi: 10.1128/AEM.00421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariithi HM, Ince AI, Boeren S, Vervoort J, Bergoin M, van Oers MM, Abd-Alla A, Vlak JM. Proteomic analysis of Glossina pallidipes Salivary Gland Hypertrophy Virus virions for immune intervention in tsetse fly colonies. J Gen Virol. 2010;91:3065–3074. doi: 10.1099/vir.0.023671-0. [DOI] [PubMed] [Google Scholar]
- Kariithi HM, Ince IA, Boeren S, Abd-Alla AMM, Parker AG, Aksoy S, Vlak JM, Oers MMv. The salivary secretome of the tsetse fly Glossina pallidipes (Diptera: Glossinidae) infected by salivary gland hypertrophy virus. PLoS Negl Trop Dis. 2011;5:e1371. doi: 10.1371/journal.pntd.0001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariithi HM, Ahmadi M, Parker AG, Franz G, Ros VID, Haq I, Elashry AM, Vlak JM, Bergoin M, Vreysen MJB, Abd-Alla AMM. Prevalence and genetic variation of salivary gland hypertrophy virus in wild populations of the tsetse fly Glossina pallidipes from southern and eastern Africa. J Invertebr Pathol. 2013;112 (Suppl 1):S123–S132. doi: 10.1016/j.jip.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Lietze VU, Abd-Alla AMM, Vreysen MJB, Geden CJ, Boucias DG. Salivary gland hypertrophy viruses: a novel group of insect pathogenic viruses. Annu Rev Entomol. 2010;56:63–80. doi: 10.1146/annurev-ento-120709-144841. [DOI] [PubMed] [Google Scholar]
- Lietze VU, Abd-Alla AM, Boucias DG. Two hytrosaviruses, MdSGHV and GpSGHV, induce distinct cytopathologies in their respective host insects. J Invertebr Pathol. 2011a;107:161–163. doi: 10.1016/j.jip.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Lietze VU, Geden CJ, Doyle MA, Boucias DG. Disease dynamics and persistence of MdSGHV-infections in laboratory house fly (Musca domestica) populations. Appl Environ Microbiol. 2011b;78:311–317. doi: 10.1128/AEM.06500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietze VU, Salem TZ, Prompiboon P, Boucias DG. Tissue tropism of the Musca domestica salivary gland hypertrophy virus. Virus Res. 2011c;155:20–27. doi: 10.1016/j.virusres.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malele II, Kinung’hi SM, Nyingilili HS, Matemba LE, Sahani JK, Mlengeya TD, Wambura M, Kibona SN. Glossina dynamics in and around the sleeping sickness endemic Serengeti ecosystem of northwestern Tanzania. J Vector Ecol. 2007;32:263–268. doi: 10.3376/1081-1710(2007)32[263:gdiaat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Malele I, Nyingilili H, Msangi A. Factors defining the distribution limit of tsetse infestation and the implication for livestock sector in Tanzania. Afr J Agric Res. 2011a;6:2341–2347. [Google Scholar]
- Malele II, Magwisha HB, Nyingilili HB, Mamiro KA, Rukambile EJ, Daffa JW, Lyaruu EA, Kapange LA, Kasilagila GK, Lwitiko NK, Msami HM, Kimbita EN. Multiple Trypanosoma infections are common amongst Glossina species in the new farming areas of Rufiji district. Tanzania Parasit Vectors. 2011b;4:217. doi: 10.1186/1756-3305-4-217. (25 Ref.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malele II, Manangwa O, Nyingilili HH, Kitwika WA, Lyaruu EA, Msangi AR, Ouma JO, Nkwangulila G, Abd-Alla AMM. Prevalence of SGHV among tsetse species of economic importance in Tanzania and their implication for SIT application. J Invertebr Pathol. 2013;112 (Suppl 1):S133–S137. doi: 10.1016/j.jip.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Maniania NK, Ekesi S. The use of entomopathogenic fungi in the control of tsetse flies. J Invertebr Pathol. 2013;112 (Suppl 1):S83–S88. doi: 10.1016/j.jip.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Maudlin I, Welburn SC, Mehlitz D. The relationship between rickettsia-like-organisms and trypanosome infections in natural populations of tsetse in Liberia 3. Trop Med Parasitol. 1990;41:265–267. [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria 9. Nat Rev Microbiol. 2012;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- Muturi CN, Ouma JO, Malele II, Ngure RM, Rutto JJ, Mithofer KM, Enyaru J, Masiga DK. Tracking the feeding patterns of tsetse flies (Glossina genus) by analysis of bloodmeals using mitochondrial cytochromes genes. PLoS One. 2011;6:1–6. doi: 10.1371/journal.pone.0017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogge G. Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experientia. 1976;32:995–996. doi: 10.1007/BF01933932. [DOI] [PubMed] [Google Scholar]
- Novakova E, Hypsa V. A new Sodalis lineage from bloodsucking fly Craterina melbae (Diptera, Hippoboscoidea) originated independently of the tsetse flies symbiont Sodalis glossinidius 1. FEMS Microbiol Lett. 2007;269:131–135. doi: 10.1111/j.1574-6968.2006.00620.x. [DOI] [PubMed] [Google Scholar]
- O’Neill SL, Gooding RH, Aksoy S. Phylogenetically distant symbiotic microorganisms reside in Glossina midgut and ovary tissues. Med Vet Entomol. 1993;7:377–383. doi: 10.1111/j.1365-2915.1993.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Pais R, Lohs C, Wu Y, Wang JW, Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol. 2008;74:5965–5974. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompiboon P, Lietze VU, Denton JSS, Geden CJ, Steenberg T, Boucias DG. Musca domestica salivary gland hypertrophy virus, a globally distributed insect virus that infects and sterilizes female houseflies. Appl Environ Microbiol. 2010;76:994–998. doi: 10.1128/AEM.02424-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio RV, Hu Y, Aksoy S. Strategies of the home-team: symbioses exploited for vector-borne disease control. Trends Microbiol. 2004;12:325–336. doi: 10.1016/j.tim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Rio RVM, Symula RE, Wang J, Lohs C, Wu YN, Synder AK, Bjornson RD, Oshima K, Biehl BS, Perna NT, Hattori M, Aksoy Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: Glossinidae) obligate symbiont Wigglesworthia. mBio. 2012;3:e00240-11. doi: 10.1128/mBio.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridaki A, Bourtzis K. Wolbachia-induced reproductive parasitism and applications. Entomol Hellenica. 2009;18:3–16. [Google Scholar]
- Saridaki A, Bourtzis K. Wolbachia: more than just a bug in insects’ genitals. Curr Opin Microbiol. 2010;13:67–72. doi: 10.1016/j.mib.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Sasanya JJ, Abd-Alla AMM, Parker AG, Cannavan A. Analysis of the antiviral drugs acyclovir and valacyclovir-hydrochloride in tsetse flies (Glossina pallidipes) using LC-MSMS. J Chromatogr B Analyt Technol Biomed Sci. 2010;878:2384–2390. doi: 10.1016/j.jchromb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Schneider DI, Garschall KI, Parker AG, Abd-Alla AMM, Miller WJ. Global Wolbachia prevalence, titer fluctuations and their potential of causing cytoplasmic incompatibilities in tsetse flies and hybrids of Glossina morsitans subgroup species. J Invertebr Pathol. 2013;112 (Suppl 1):S104–S115. doi: 10.1016/j.jip.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari F, Siciliano P, Gabrieli P, Gomulski LM, Bonomi A, Gasperi G, Malacrida AR. Safe and fit genetically modified insects for pest control: from lab to field applications. Genetica. 2011;139:41–52. doi: 10.1007/s10709-010-9483-7. [DOI] [PubMed] [Google Scholar]
- Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Ann Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- Simarro PP, Louis FJ, Jannin J. Sleeping sickness, forgotten illness: what are the consequences in the field? Med Trop. 2003;63:231–235. [PubMed] [Google Scholar]
- Snyder AK, McMillen CM, Wallenhorst P, Rio RV. The phylogeny of Sodalis-like symbionts as reconstructed using surface-encoding loci. FEMS Microbiol Lett. 2011;317:143–151. doi: 10.1111/j.1574-6968.2011.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreysen MJB, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, Juma KG, Dyck VA, Msangi AR, Mkonyi PA, Feldmann HU. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J Econ Entomol. 2000;93:123–135. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- Wang J, Brelsfoard C, Wu Y, Aksoy S. Intercommunity effects on microbiome and GpSGHV density regulation in tsetse flies. J Invertebr Pathol. 2013;112 (Suppl 1):S32–S39. doi: 10.1016/j.jip.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jehle JA. Nudiviruses and other large, double-stranded circular DNA viruses if ubvertebrates: new insights on an old topic. J Invertebr Pathol. 2009;101:187–193. doi: 10.1016/j.jip.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Burand JP, Jehle JA. Nudivirus genomics: diversity and classification. Virol Sin. 2007;22:128–136. [Google Scholar]
- Wang Y, Bininda-Emonds ORP, Oers MMv, Vlak JM, Jehle JA. Nudiviruses give insights into the evolution of nuclear arthropod-specific large circular double-stranded DNA viruses. Virus Genes. 2011;42:444–456. doi: 10.1007/s11262-011-0589-5. [DOI] [PubMed] [Google Scholar]
- Weiss BL, Wang J, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011;9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Maltz M, Aksoy S. Obligate symbionts activate immune system development in the tsetse fly. J Immunol. 2012 doi: 10.4049/jimmunol.1103691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernegreen JJ. Genome evolution in bacterial endosymbionts of insects. Nat Rev Genet. 2002;3:850–861. doi: 10.1038/nrg931. [DOI] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]


