Abstract
Neuroblastoma is a common pediatric tumor characterized by clinical heterogeneity. Because it is derived from sympathetic neuroblasts, the NTRK family of neurotrophin receptors plays an integral role in neuroblastoma cell survival, growth, and differentiation. Indeed, high expression of NTRK1 is associated with favorable clinical features and outcome, whereas expression of NTRK2 and its ligand, brain-derived neurotrophic factor (BDNF), are associated with unfavorable features and outcome. AZ64 (Astra Zeneca) is a potent and selective inhibitor of the NTRK tyrosine kinases that blocks phosphorylation at nanomolar concentrations. To determine the preclinical activity of AZ64, we performed intervention trials in a xenograft model with NTRK2-overexpressing neuroblastomas. AZ64 alone significantly inhibited tumor growth compared to vehicle-treated animals (p = 0.0006 for tumor size). Furthermore, the combination of AZ64 with conventional chemotherapeutic agents, irinotecan and temozolomide (irino–temo), showed significantly enhanced anti-tumor efficacy compared to irino–temo alone [(p < 0.0001 for tumor size, p < 0.0005 for event-free survival (EFS)]. We also assessed the combination of AZ64 and local radiation therapy (RT) on a neuroblastoma hindlimb xenograft model, and the efficacy of local RT was significantly increased when animals were treated simultaneously with AZ64 (p < 0.0001 for tumor size, p = 0.0006 for EFS). We conclude that AZ64 can inhibit growth of NTRK-expressing neuroblastomas both in vitro and in vivo. More importantly, it can significantly enhance the efficacy of conventional chemotherapy as well as local RT, presumably by inhibition of the NTRK2/BDNF autocrine survival pathway.
Keywords: TrkA, TrkB, AZ64, Neuroblastoma, Inhibition, Signaling, Differentiation
Background
Neuroblastoma is a common pediatric tumor of the sympathetic nervous system, and it provides an excellent model to study the consequences of NTRK signaling and inhibition in cancer [5]. Neuroblastomas are characterized by clinical heterogeneity, from spontaneous regression in infants to relentless progression in older children. The prognosis for older patients with advanced disease remains poor, with 3-year event-free survival (EFS) probabilities of 30–40 % [21, 39, 41]. Neuroblastomas can be classified into distinct molecular subsets based on genetic alterations and biologic features [4], and the expression of specific NTRK receptors likely contributes to these distinct behaviors [5, 34, 47–49, 56, 59, 64].
Neurotrophin signaling through the TRK family of receptor tyrosine kinases plays a critical role in the development and maintenance of the central and peripheral nervous systems. Activation of TRK receptors regulates cell survival, proliferation, migration, differentiation, and apoptosis. TRK receptors exert this influence by modulating the responses of neurons to neurotrophic factors in a temporally and spatially regulated manner. TrkA (NTRK1) is the only receptor for nerve growth factor (NGF), TrkB (NTRK2) binds both brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT4), and TrkC (NTRK3) is the primary receptor for neurotrophin-3 (NT3), although NT3 can also bind to and activate TrkA and TrkB [2, 3, 30, 31].
TrkA expression in neuroblastomas mediates neuronal differentiation, growth arrest, and inhibition of angiogenesis in response to NGF [14, 36]. In contrast, unfavorable neuroblastomas usually express TrkB and its ligand BDNF, which together comprise an autocrine/paracrine survival pathway [1, 40, 49]. These tumors typically have gross chromosomal aberrations including amplification of the MYCN proto-oncogene. The TrkB/BNDF pathway promotes cell survival, protects cells from injury, and inhibits chemotherapy-mediated cell death [27, 29, 43]. Although a number of genes are likely involved in the development and clinical behavior of favorable and unfavorable neuroblastomas, the pattern of TRK gene expression (especially expression of TrkA or TrkB) clearly plays a role.
Previously, we showed that inhibition of the TRK signaling pathway with indolocarbazole derivatives from Cephalon, Inc. (CEP-751, CEP-2563, CEP-701/lestaurtinib) [19, 44, 45, 58] could inhibit growth of neuroblastoma cells in vitro and in vivo [17, 18, 27, 28]. Indeed, there is evidence of lestaurtinib activity in a phase 1 trial as a single agent in patients with recurrent/refractory disease [46]. However, this agent is not moving forward in clinical trials, so other TRK inhibitors need to be investigated. AZ64 (Astra Zeneca, Inc.) is an active, orally available, small molecule kinase inhibitor with nanomolar potency against TrkA, TrkB, and TrkC. We wanted to test the effect of AZ64, alone or in combination with other treatments, in neuroblastoma cell lines growing in vitro and in a xenograft mouse model to determine its potential utility as a targeted therapy for this disease.
Materials and methods
Compounds
AZ64 (Astra Zeneca) AZ64 was developed as a potent and selective inhibitor of Trk receptor tyrosine kinases, with a Ki for TrkA and TrkB of <2.0 ± 0.25 nM. The kinase specificity and selectivity for AZ64 was evaluated against a panel of 177 kinases and showed a high degree of specificity to Trk with only 11 enzymes having inhibition at 500 nm. Subsequent testing with AZ64 further demonstrated selectivity with IC50 values of 0.2 nM against TrkA and 2 nM against TrkB, and IC50 values corresponding to 30 nM for FGFR1 and 33 nM for CDK2/cyclinE, respectively. These results suggest AZ64 is a potent and selective Trk inhibitor with at least a ~15-fold selectivity window against other known RTK targets and strongly suggests Trk receptors are the primary drug target of AZ64 at physiologically relevant concentrations.
AZ64 was formulated in 0.5 % HPMC. 0.5 % Methyl cellulose powder (Methocel K4 M prep, Dow Chemicals) was slowly added to the 0.1 % tween 80 solution in water, stirred overnight, and stored at 4 °C (0.5 % HPMC). A hand sonicator (Sonic Dismembrator model 100, Fisher Scientific) was used to get the compound into a fine particle suspension in 0.5 % HPMC. Fresh batches of suspension were made for each dosing. Animals were dosed by oral gavage at 100 mg/kg twice daily (Monday to Friday) and once daily on Saturday and Sunday. Vehicle alone or saline was used as the control.
Irinotecan (irino) was given at a dose of 0.63 mg/kg daily by oral gavage Monday to Friday of each week. Temozolomide (temo) was given at a dose of 7.5 mg/kg daily by oral gavage Monday through Friday of each week. The same doses were used when combined with AZ64. Both irino and temo were resuspended in saline for the oral gavage. All chemotherapy agents other than AZ64 were obtained through the pharmacy at the Children’s Hospital of Philadelphia (CHOP). The doses used above were based on published studies and modified based on our own experience with these drugs in our xenograft model system [27, 28].
Cell lines
For the xenograft tumor studies, we used SY5Y-TrkB (BR6), a subclone of SY5Y transfected with TrkB that expresses this receptor at high levels [27]. This line does not express detectable levels of the TrkAIII isoform [60]. In vitro studies were confirmed with SY5Y-TrkA (P23A), a subclone of SY5Y transfected with TrkA, as well as the SY5Y parental line as a Trk-negative control (SY5Y) [27, 49]. Cells were grown in RPMI-1640 medium containing 10 % fetal bovine serum with or without 0.3 mg/ml G418 and maintained in 150 cm3 Corning culture flasks in a humidified atmosphere of 95 % air and 5 % CO2. Cells were harvested using 0.2 % tetrasodium EDTA in phosphate-buffered saline (PBS).
In vitro experiments
To determine the effect of AZ64 on the Trk-null SY5Y, TrkA-expressing SY5Y-TrkA, and the TrkB-expressing SY5Y-TrkB, cells were grown in 10-cm3 dishes to 70–80 % confluency in standard culture medium and harvested for protein extraction. We analyzed Trk expression by Western blot using an anti-phospho-Trk antibody (Phospho-TrkA, Tyr-490 antibody; Cell Signaling Technologies, Danvers, MA) or an anti-pan-Trk antibody (Santa Cruz Biotechnology, Inc.; Santa Cruz, CA). We exposed cells to NGF (for TrkA) or BDNF (for TrkB) for 15 min in the absence or presence of increasing concentrations of AZ64 to determine the concentration that achieved 50 % inhibition of receptor phosphorylation (IC50). RT-CES analysis was done to determine the effect of AZ64 on growth. 2 × 104 cells were plated per well of the RT-CES E-plates. After about 24 h, cells were treated with different concentrations of AZ64 for 1 h, followed by addition of 100 ng/ml of ligand (NGF or BDNF). Cell growth was monitored for 6 days. Similar experiments were carried out under low serum conditions, where 24 h after plating, the medium was changed to 2 % serum RPMI, and cells were treated as mentioned above and growth monitored. All the in vitro experiments were repeated at least 3 times.
Animals
Six-week-old athymic nu/nu mice were obtained from Charles River Laboratories. Mice were maintained at five per cage under humidity- and temperature-controlled conditions in a light/dark cycle that was set at 12-h intervals. We assessed animals for toxicity by examining behavior daily, weight gain/loss 2–3 × a week, appearance of skin, and blood counts before treatment and after 1 and 4 weeks of treatment. We examined tissues in treated mice for gross signs of toxicity (e.g., brain, peripheral nervous system, liver, and pancreas) at the time of killing and tumor removal. Abnormal tissues based on size or appearance were examined microscopically, if found. The Institutional Animal Care Committee of the Joseph Stokes, Jr. Research Institute at CHOP approved the animal studies described herein.
In vivo experiments
For the xenograft studies, animals were injected in the flank with 1 × 107 SY5Y-TrkB cells in 0.3 ml of Matrigel (BD Bioscience, Palo Alto, CA). Tumors were measured 2 times a week in 3 dimensions, and the volume was calculated as follows: [(d1×d2×d3) × p/6]. Body weights were determined twice a week, and the dose of compound was adjusted accordingly. Treatment with AZ64 was started about 10 days after tumor inoculation when the average SY5Y-TrkB tumor size was 0.2 cm3. For local radiation therapy (RT) experiments, 5 × 106 SY5Y-TrkB cells were injected in the right hindlimb, and treatment was started when the average tumor size was 0.1 cm3. External beam RT was delivered as a single dose of 4 Gy, a treatment regimen previously determined to inhibit growth but not eradicate the tumor.
Statistical analysis
Comparisons of tumor size results were analyzed by two-sample Student t test. Comparison of tumor size change over time was analyzed by linear mixed models. For event-free survival (EFS) life-table analysis, an event was defined as tumor size that exceeded 3 cm3 or any evidence of animal discomfort resulting from the tumor or the treatment. Kaplan–Meier curves were estimated and compared between groups by log-rank test. All analyses were conducted using SAS-9 or Stata-8.
Results
Effect of AZ64 on TrkB activation in vitro
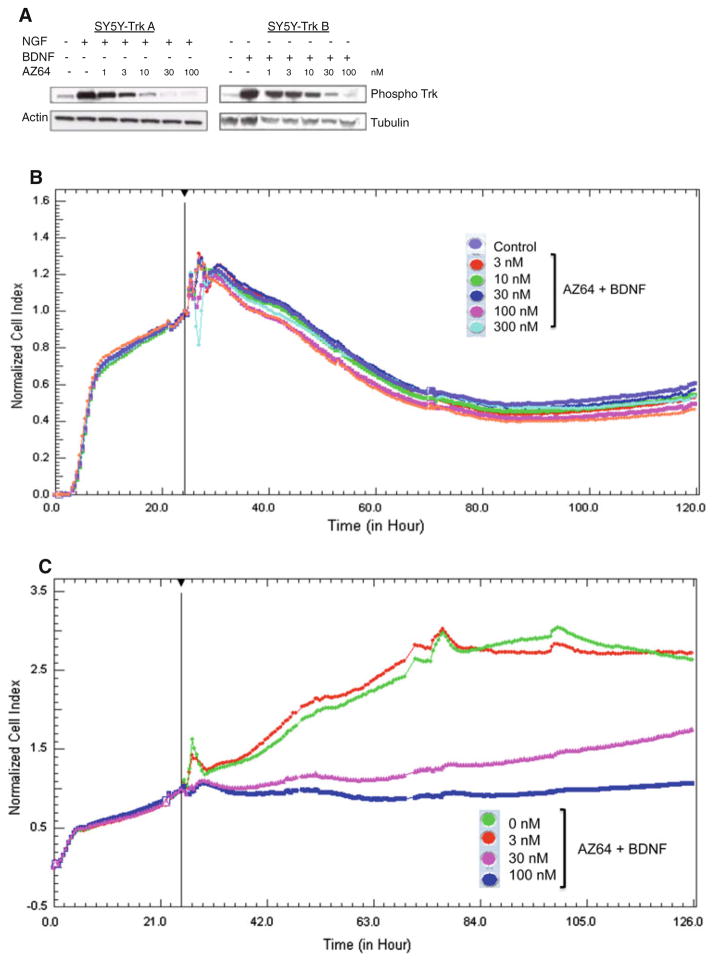
We first tested the ability of AZ64 to inhibit Trk phosphorylation induced by exogenous NGF or BDNF in the SY5Y-Trk-transfected neuroblastoma lines. There was very little Trk phosphorylation seen at steady state in the absence of exogenous NGF/BDNF (Fig. 1a). However, there was intense phosphorylation of Trk by 15 min after the addition of exogenous ligands in the absence of AZ64. Increasing concentrations of AZ64 progressively inhibited this ligand-induced Trk phosphorylation. We saw substantial inhibition of phosphorylation at 3–30 nM AZ64, with almost complete inhibition between 30 and 100 nM. Increasing concentrations of AZ64 did not affect the growth of parental SY5Y cells. No significant difference was seen between the control and treatment groups (Fig. 1b). SY5Y-TrkB cells showed more inhibition with lower concentrations of AZ64 in 2 % serum media versus the complete media (Fig. 1c). The IC50 for SY5Y-TrkB cells was between 30 and 100 nM in media with 2 % serum.
Fig. 1.
a Effect of AZ64 on Trk phosphorylation induced by ligands. SY5Y cells were transfected with either TrkA (SY5Y-TrkA) or TrkB (SY5Y-TrkB), and cells were exposed to ligands (NGF or BDNF) in the absence and presence of increasing concentrations of AZ64. Maximal inhibition of ligand-induced autophosphorylation was seen by 30–100 nM. 1A. b Effect of AZ64 on growth of SY5Y cells (Trk-null) in medium with 2 % serum. Cells were cultured in RT-CES growth plates in the presence of BDNF. AZ64 from 3 to 300 nM had no effect on the growth of SY5Y cells in presence of BDNF, as assessed by RT-CES analysis. c Effect of AZ64 on growth of SY5Y-TrkB cells in medium with 2 % serum. Cells were cultured in RT-CES growth plates in the presence of BDNF and increasing concentrations of AZ64. There was a dose-dependent inhibition of growth, with an IC50 between 30 and 100 nM
Effect of single-agent AZ64 on SY5Y-TrkB neuroblastoma xenografts
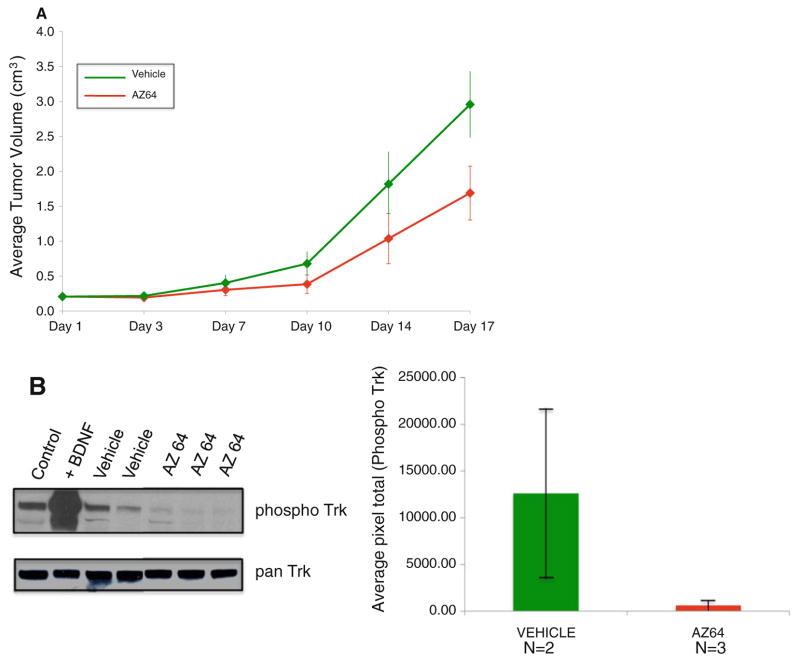
We tested the ability of AZ64 to inhibit SY5Y-TrkB cells growing as xenografts in athymic nu/nu mice. We injected 1 × 107 cells subcutaneously in the flank of nude mice. We initiated treatment when the tumors measured 0.2 cm3, usually 10–14 days from inoculation. We treated mice (10 per group) with either AZ64 or vehicle twice a day (Monday to Friday) and once a day on Saturday and Sunday, and tumors were measured twice a week. There was a significant difference in the tumor growth rate for AZ64 versus vehicle, when AZ64 was used as a single-agent therapy (p = 0.0006) (Fig. 2a). Furthermore, analysis of TrkB phosphorylation status of the tumors shows substantial inhibition of Trk phosphorylation in tumors from mice treated with AZ64 (Fig. 2b, c).
Fig. 2.
a Effect of AZ64 as a single agent on SY5Y-TrkB xenografts. There was a significant difference in the inhibition of tumor growth with AZ64 treatment compared to a vehicle control (p = 0006). b Trk phosphorylation status in xenograft tumors. Control SY5Y-TrkB cells show a moderate level of steady-state phosphorylation that increases dramatically within 10 min after exposure to 100 nM BDNF in vitro (first two lanes). Xenograft tumors from mice treated with vehicle (N = 2) also show moderate levels of steady-state phosphorylation, but xenografts treated with AZ64 (N = 3) showed almost complete inhibition of TrkB phosphorylation. The bar graph to the right shows quantitation of TrkB phosphorylation, normalized to total TrkB expression of the two xenografted tumors treated with vehicle and the three xenografted tumors treated with AZ64. The error bars show standard deviations
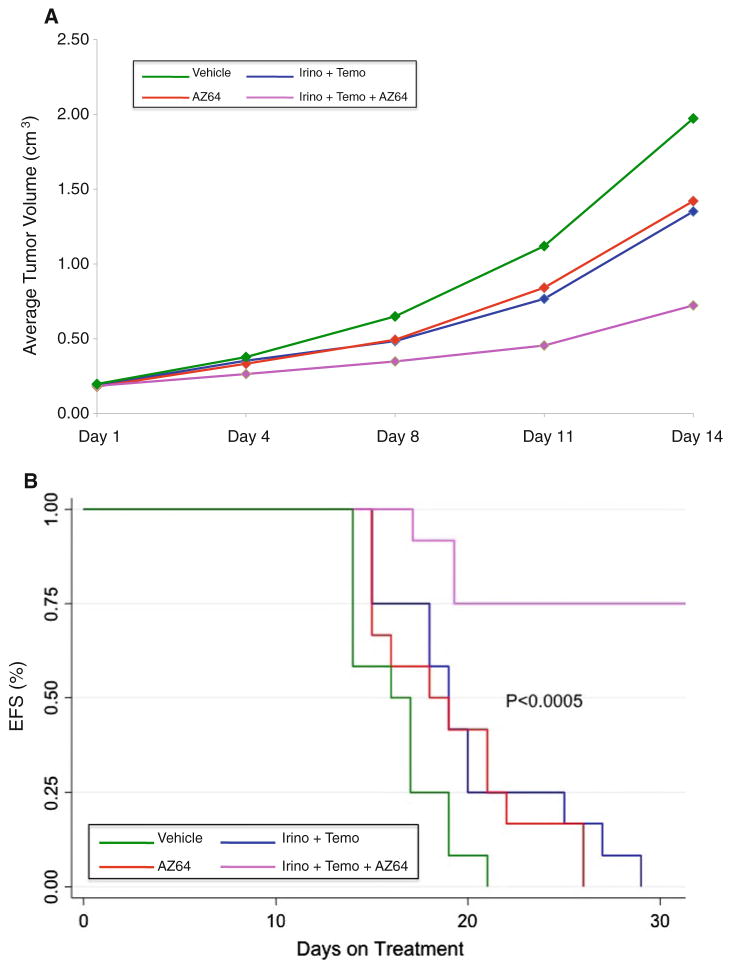
Effect of AZ64 in combination with irino–temo on SY5Y-TrkB xenografts
The combination of irino–temo was recently developed to treat for recurrent neuroblastomas. To determine whether AZ64 co-treatment enhanced the effect of conventional chemotherapy agents, we treated groups of mice with either vehicle, AZ64, irino–temo, or both irino–temo and AZ64. Tumors treated with the combination of irino–temo plus AZ64 grew significantly more slowly than with either chemotherapy or AZ64 alone (p < 0.0001; Fig. 3a). This resulted in a 75 % EFS by day 28 in the combination group, which was significantly better than all other groups (p < 0.0005; Fig. 3b). Thus, co-treatment with AZ64 significantly enhanced the effect of paired-agent chemotherapy. Furthermore, this was achieved without additional toxicity.
Fig. 3.
a Effect of AZ64 alone or in combination with irino–temo on SY5Y-TrkB xenografts. Treatment of xenografts with AZ64 had a similar effect on inhibiting growth as treatment with irino–temo, but they were not significantly different from each other (p = 0.38). However, the combination of AZ64 plus irino–temo caused a much more dramatic and significant inhibition of tumor growth compared to either AZ64 or irino–temo alone (p < 0.0001 for both). b Survival analysis. Mice were killed once their tumor size reached or exceeded 3 cm3. There was a significant and similar prolongation of survival for mice treated with either AZ64 or irino–temo alone compared to the vehicle control. However, the combination of AZ64 plus irino–temo significantly improved the EFS compared to that of animals treated with either treatment alone (p < 0.005). All animals in the single-agent arms had to be killed by 28 days, whereas 75 % of mice in the combination group were still alive at 28 days
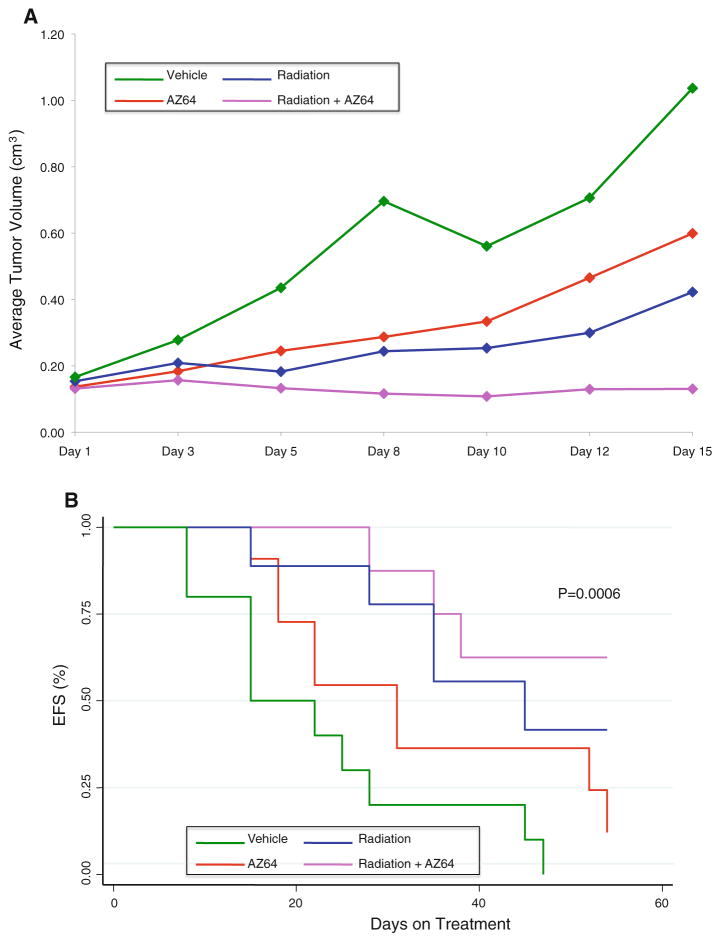
Effect of AZ64 in combination with local radiation therapy (RT) on SY5Y-TrkB xenografts
To determine whether AZ64 co-treatment enhanced the effect of local RT, we treated groups of mice with either vehicle, AZ64, RT, or both RT and AZ64. Tumors treated with the combination of RT plus AZ64 grew significantly more slowly than with either RT or AZ64 alone (p < 0.0001; Fig. 4a). The combination of RT + AZ64 resulted in a significantly better EFS by day 40 than vehicle or either treatment alone (p = 0.0006; Fig. 4b). Thus, co-treatment with AZ64 significantly enhanced the effect of local RT, without additional toxicity.
Fig. 4.
a Effect of AZ64 alone or in combination with local radiation on SY5Y-TrkB xenografts. Neuroblastoma xenografts were grown on the hindlimbs of mice and killed when they reached 1 cm3. Treatment with AZ64 had a similar effect on inhibiting growth as treatment with local radiation, and both were significantly different than the vehicle control (p < 0.0001). However, the combination of AZ64 plus radiation caused a much more dramatic and significant inhibition of tumor growth compared to either AZ64 or radiation alone (p = 0.0043 and p < 0.0001, respectively). b Survival analysis. There was a modest prolongation of survival for mice treated with AZ64 alone compared to the vehicle control, and there was a greater prolongation of survival with local radiation. However, the combination of AZ64 plus radiation significantly improved the EFS compared to that of animals treated with either treatment alone (p = 0.0006)
Discussion
We and others demonstrated previously that NTRK receptors likely play a role in the heterogeneous clinical behaviors of neuroblastomas (reviewed in [6]). NTRK1/TrkA is expressed in favorable tumors that are likely to regress, differentiate, or respond well to modest therapy [34, 48, 50, 59]. On the other hand, NTRK2/TrkB is expressed in unfavorable tumors, particularly those with MYCN amplification [49]. These tumors coexpress the TrkB ligand, BDNF, creating an autocrine survival pathway that promotes drug resistance, angiogenesis, invasion, and metastasis [14, 27, 29, 40]. These data suggest that targeted therapy that inhibits NTRK signaling pathways, especially the NTRK2/TrkB signaling pathway, could be an effective therapeutic strategy, either alone or in combination with conventional treatments.
Previously, we examined the potential role of lestaurtinib, a selective inhibitor of NTRK and FLT3 receptors, in the treatment of NTRK-expressing neuroblastoma cells in vitro and in a xenograft model [17, 18, 27, 28, 51]. Lestaurtinib also showed efficacy and limited toxicity in a phase 1 clinical trial conducted through the new approaches to neuroblastoma therapy (NANT) consortium [46]. However, lestaurtinib is not moving forward into further clinical trials, so we wanted to explore the suitability of alternative NTRK1 inhibitors in our preclinical model. AZ64 is a novel and potent Trk-selective inhibitor provided by Astra Zeneca. This compound inhibits Trk family kinases at nanomolar concentrations, and AZ64 can be administered orally, which makes it appealing for clinical treatment. Another group has tested AZ64 in head and neck models [65]. Also, a related compound, AZ-23/AZ623, was found to inhibit neuroblastoma growth and was synergistic with topotecan [61, 66], but it was not being developed for clinical trials. No one has tested the efficacy of AZ64 in neuroblastomas, or the ability of a Trk inhibitor to enhance the efficacy of irino–temo, the chemotherapy regimen currently used for recurrent/refractory neuroblastoma patients. Also, no one had determined whether a Trk inhibitor could enhance the efficacy of local RT in a mouse xenograft model.
We showed that AZ64 dramatically inhibited the autophosphorylation of TrkA and TrkB (after ligand exposure), and it inhibited the growth of SY5Y-TrkB cells growing in vivo as xenografts in athymic nu/nu mice. We also showed that AZ64 significantly enhanced the efficacy of conventional chemotherapy (irino–temo) and local RT, without additional toxicity. These effects are presumably the result of blocking an important TrkB/BDNF autocrine survival pathway that is commonly found in advanced stage tumors, especially with MYCN amplification [49]. Thus, our results suggest that selective inhibition of the TrkB pathway could be an important adjunct to neuroblastoma treatment, and it is likely to be most effective when combined with other agents or modalities of proven value in neuroblastoma therapy. The fact that both lestaurtinib and AZ64 were effective as single agents in neuroblastoma xenografts and enhanced the efficacy of chemotherapy (and RT for AZ64) strongly suggests that their effect is mediated through the inhibition of the NTRK pathway, and not some off-target effect.
A variety of other cancers have rearrangements or aberrant expression of Trk genes. Rearrangement of a Trk gene was first identified in a colon cancer line, but Trk rearrangements have been identified in papillary thyroid carcinomas [7, 9, 22–24, 53], pediatric sarcomas and leukemias [32, 33, 35, 55], and secretory breast cancers [16, 38, 62]. However, these gene rearrangements probably result in oncogenic activation. Aberrant expression of apparently unrearranged Trk genes plays a role in the pathogenesis of NB as well as other tumor types. We showed previously that TrkB is expressed in unfavorable Wilms tumors, similar to NBs [15]. Also, TrkC is expressed in favorable medulloblastomas in a manner very analogous to TrkA expression in NBs [25, 26, 57]. Trk genes are also aberrantly expressed in medullary thyroid carcinomas [42], prostate cancers [20, 37, 52, 63], breast cancers [8, 10–13], and others [54]. Thus, rearrangement or aberrant expression of Trk genes clearly plays an important role in a variety of cancers. Therefore, targeted therapy against NTRK family receptors should be beneficial for the treatment of a variety of pediatric and adult cancers, not just neuroblastoma.
Acknowledgments
This work was supported in part by grants from the NIH (CA-094194, CA-097323; GMB), the Richard and Nancy Wolfson Young Investigator Fund (JEM), the St. Baldrick’s Foundation (CRV), and the Audrey E. Evans Endowed Chair in Molecular Oncology (GMB).
Contributor Information
Radhika Iyer, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA.
Carly R. Varela, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA. The Department of Pediatrics, The University of Pennsylvania, Philadelphia, PA 19104, USA
Jane E. Minturn, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA. The Department of Pediatrics, The University of Pennsylvania, Philadelphia, PA 19104, USA
Ruth Ho, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA.
Anisha M. Simpson, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA
Jennifer E. Light, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA
Audrey E. Evans, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA. The Department of Pediatrics, The University of Pennsylvania, Philadelphia, PA 19104, USA
Huaqing Zhao, Biostatistics and Data Management Core, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Kenneth Thress, AstraZeneca, Pharmaceuticals, 35 Gatehouse Drive, Waltham, MA 02451, USA.
Jeffrey L. Brown, AstraZeneca, Pharmaceuticals, 35 Gatehouse Drive, Waltham, MA 02451, USA
Garrett M. Brodeur, Email: brodeur@email.chop.edu, Division of Oncology, Children’s Hospital of Philadelphia, Colket Translational Research Building, Rm. 3018, 3501 Civic Center Blvd., Philadelphia, PA 19104-4302, USA. The Department of Pediatrics, The University of Pennsylvania, Philadelphia, PA 19104, USA
References
- 1.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. [see comments] Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 2.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 3.Barbacid M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann N Y Acad Sci. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur GM, Minturn JE, Ho R, Simpson AM, Iyer R, Varela CR, Light JE, Kolla V, Evans AE. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244–3250. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodeur GM, Nakagawara A, Yamashiro DJ, Ikegaki N, Liu XG, Azar CG, Lee CP, Evans AE. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol. 1997;31(1–2):49–55. doi: 10.1023/a:1005729329526. [DOI] [PubMed] [Google Scholar]
- 7.Butti MG, Bongarzone I, Ferraresi G, Mondellini P, Borrello MG, Pierotti MA. A sequence analysis of the genomic regions involved in the rearrangements between TPM3 and NTRK1 genes producing TRK oncogenes in papillary thyroid carcinomas. Genomics. 1995;28:15–24. doi: 10.1006/geno.1995.1100. [DOI] [PubMed] [Google Scholar]
- 8.Davidson B, Reich R, Lazarovici P, Ann Florenes V, Nielsen S, Nesland JM. Altered expression and activation of the nerve growth factor receptors TrkA and p75 provide the first evidence of tumor progression to effusion in breast carcinoma. Breast Cancer Res Treat. 2004;83:119–128. doi: 10.1023/B:BREA.0000010704.17479.8a. [DOI] [PubMed] [Google Scholar]
- 9.Delvincourt C, Patey M, Flament JB, Suarez HG, Larbre H, Jardillier JC, Delisle MJ. Ret and trk proto-oncogene activation in thyroid papillary carcinomas in French patients from the Champagne-Ardenne region. Clin Biochem. 1996;29:267–271. doi: 10.1016/0009-9120(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 10.Descamps S, Pawlowski V, Revillion F, Hornez L, Hebbar M, Boilly B, Hondermarck H, Peyrat JP. Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res. 2001;61:4337–4340. [PubMed] [Google Scholar]
- 11.Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864–17870. doi: 10.1074/jbc.M010499200. [DOI] [PubMed] [Google Scholar]
- 12.Dolle L, Adriaenssens E, El Yazidi-Belkoura I, Le Bourhis X, Nurcombe V, Hondermarck H. Nerve growth factor receptors and signaling in breast cancer. Curr Cancer Drug Targets. 2004;4:463–470. doi: 10.2174/1568009043332853. [DOI] [PubMed] [Google Scholar]
- 13.Dolle L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene. 2003;22:5592–5601. doi: 10.1038/sj.onc.1206805. [DOI] [PubMed] [Google Scholar]
- 14.Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 2002;62:1802–1808. [PubMed] [Google Scholar]
- 15.Eggert A, Grotzer MA, Ikegaki N, Zhao H, Cnaan A, Brodeur GM, Evans AE. Expression of the neurotrophin receptor TrkB is associated with unfavorable outcome in Wilms’ tumor. J Clin Oncol. 2001;19:689–696. doi: 10.1200/JCO.2001.19.3.689. [DOI] [PubMed] [Google Scholar]
- 16.Euhus DM, Timmons CF, Tomlinson GE. ETV6-NTRK3–Trking the primary event in human secretory breast cancer. Cancer Cell. 2002;2:347–348. doi: 10.1016/s1535-6108(02)00184-8. [DOI] [PubMed] [Google Scholar]
- 17.Evans AE, Kisselbach KD, Liu X, Eggert A, Ikegaki N, Camoratto AM, Dionne C, Brodeur GM. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Med Pediatr Oncol. 2001;36:181–184. doi: 10.1002/1096-911X(20010101)36:1<181::AID-MPO1043>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Evans AE, Kisselbach KD, Yamashiro DJ, Ikegaki N, Camoratto AM, Dionne CA, Brodeur GM. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin Cancer Res. 1999;5:3594–3602. [PubMed] [Google Scholar]
- 19.George DJ, Dionne CA, Jani J, Angeles T, Murakata C, Lamb J, Isaacs JT. Sustained in vivo regression of Dunning H rat prostate cancers treated with combinations of androgen ablation and Trk tyrosine kinase inhibitors, CEP-751 (KT-6587) or CEP-701 (KT-5555) Cancer Res. 1999;59:2395–2401. [PubMed] [Google Scholar]
- 20.George DJ, Suzuki H, Bova GS, Isaacs JT. Mutational analysis of the TrkA gene in prostate cancer. Prostate. 1998;36:172–180. doi: 10.1002/(sici)1097-0045(19980801)36:3<172::aid-pros5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, Pulsipher M, Grupp SA, Diller L. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 22.Greco A, Mariani C, Miranda C, Lupas A, Pagliardini S, Pomati M, Pierotti MA. The DNA rearrangement that generates the TRK-T3 oncogene involves a novel gene on chromosome 3 whose product has a potential coiled-coil domain. Mol Cell Biol. 1995;15:6118–6127. doi: 10.1128/mcb.15.11.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greco A, Mariani C, Miranda C, Pagliardini S, Pierotti MA. Characterization of the NTRK1 genomic region involved in chromosomal rearrangements generating TRK oncogenes. Genomics. 1993;18:397–400. doi: 10.1006/geno.1993.1482. [DOI] [PubMed] [Google Scholar]
- 24.Greco A, Miranda C, Pagliardini S, Fusetti L, Bongarzone I, Pierotti MA. Chromosome 1 rearrangements involving the genes TPR and NTRK1 produce structurally different thyroid-specific TRK oncogenes. Genes Chromosomes Cancer. 1997;19:112–123. [PubMed] [Google Scholar]
- 25.Grotzer MA, Janss AJ, Fung K, Biegel JA, Sutton LN, Rorke LB, Zhao H, Cnaan A, Phillips PC, Lee VM, Trojanowski JQ. TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol. 2000;18:1027–1035. doi: 10.1200/JCO.2000.18.5.1027. [DOI] [PubMed] [Google Scholar]
- 26.Grotzer MA, Janss AJ, Phillips PC, Trojanowski JQ. Neurotrophin receptor TrkC predicts good clinical outcome in medulloblastoma and other primitive neuroectodermal brain tumors. Klin Padiatr. 2000;212:196–199. doi: 10.1055/s-2000-10044. [DOI] [PubMed] [Google Scholar]
- 27.Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6466. [PubMed] [Google Scholar]
- 28.Iyer R, Evans AE, Qi X, Ho R, Minturn JE, Zhao H, Balamuth N, Maris JM, Brodeur GM. Lestaurtinib enhances the antitumor efficacy of chemotherapy in murine xenograft models of neuroblastoma. Clin Cancer Res. 2010;16:1478–1485. doi: 10.1158/1078-0432.CCR-09-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaboin J, Kim CJ, Kaplan DR, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3′-kinase pathway. Cancer Res. 2002;62:6756–6763. [PubMed] [Google Scholar]
- 30.Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 32.Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998;58:5046–5048. [PubMed] [Google Scholar]
- 33.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 34.Kogner P, Barbany G, Dominici C, Castello MA, Raschella G, Persson H. Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Res. 1993;53:2044–2050. [PubMed] [Google Scholar]
- 35.Liu Q, Schwaller J, Kutok J, Cain D, Aster JC, Williams IR, Gilliland DG. Signal transduction and transforming properties of the TEL-TRKC fusions associated with t(12;15)(p13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. EMBO J. 2000;19:1827–1838. doi: 10.1093/emboj/19.8.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucarelli E, Kaplan D, Thiele CJ. Activation of trk-A but not trk-B signal transduction pathway inhibits growth of neuroblastoma cells. Eur J Cancer. 1997;33:2068–2070. doi: 10.1016/s0959-8049(97)00266-9. [DOI] [PubMed] [Google Scholar]
- 37.MacGrogan D, Saint-Andre JP, Dicou E. Expression of nerve growth factor and nerve growth factor receptor genes in human tissues and in prostatic adenocarcinoma cell lines. J Neurochem. 1992;59:1381–1391. doi: 10.1111/j.1471-4159.1992.tb08451.x. [DOI] [PubMed] [Google Scholar]
- 38.Makretsov N, He M, Hayes M, Chia S, Horsman DE, Sorensen PH, Huntsman DG. A fluorescence in situ hybridization study of ETV6-NTRK3 fusion gene in secretory breast carcinoma. Genes Chromosomes Cancer. 2004;40:152–157. doi: 10.1002/gcc.20028. [DOI] [PubMed] [Google Scholar]
- 39.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res. 1995;55:1798–1806. [PubMed] [Google Scholar]
- 41.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 42.McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA. 1999;96:4540–4545. doi: 10.1073/pnas.96.8.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Middlemas DS, Kihl BK, Moody NM. Brain derived neurotrophic factor protects human neuroblastoma cells from DNA damaging agents. J Neurooncol. 1999;45:27–36. doi: 10.1023/a:1006342423175. [DOI] [PubMed] [Google Scholar]
- 44.Miknyoczki SJ, Chang H, Klein-Szanto A, Dionne CA, Ruggeri BA. The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin Cancer Res. 1999;5:2205–2212. [PubMed] [Google Scholar]
- 45.Miknyoczki SJ, Dionne CA, Klein-Szanto AJ, Ruggeri BA. The novel Trk receptor tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits antitumor efficacy against human pancreatic carcinoma (Panc1) xenograft growth and in vivo invasiveness. Ann N Y Acad Sci. 1999;880:252–262. doi: 10.1111/j.1749-6632.1999.tb09530.x. [DOI] [PubMed] [Google Scholar]
- 46.Minturn JE, Evans AE, Villablanca JG, Yanik GA, Park JR, Shusterman S, Groshen S, Hellriegel ET, Bensen-Kennedy D, Matthay KK, Brodeur GM, Maris JM. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother Pharmacol. 2011;68(4):1057–1065. doi: 10.1007/s00280-011-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawara A, Arima M, Azar CG, Scavarda NJ, Brodeur GM. Inverse relationship between trk expression and N-myc amplification in human neuroblastomas. Cancer Res. 1992;52:1364–1368. [PubMed] [Google Scholar]
- 48.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328:847–854. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14:759–767. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakagawara A, Brodeur GM. Role of neurotrophins and their receptors in human neuroblastomas: a primary culture study. Eur J Cancer. 1997;33:2050–2053. doi: 10.1016/s0959-8049(97)00280-3. [DOI] [PubMed] [Google Scholar]
- 51.Norris RE, Minturn JE, Brodeur GM, Maris JM, Adamson PC. Preclinical evaluation of lestaurtinib (CEP-701) in combination with retinoids for neuroblastoma. Cancer Chemother Pharmacol. 2011;68(6):1469–1475. doi: 10.1007/s00280-011-1623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pflug BR, Dionne C, Kaplan DR, Lynch J, Djakiew D. Expression of a Trk high affinity nerve growth factor receptor in the human prostate. Endocrinology. 1995;136:262–268. doi: 10.1210/endo.136.1.7828539. [DOI] [PubMed] [Google Scholar]
- 53.Pierotti MA, Bongarzone I, Borrello MG, Mariani C, Miranda C, Sozzi G, Greco A. Rearrangements of TRK proto-oncogene in papillary thyroid carcinomas. J Endocrinol Invest. 1995;18:130–133. doi: 10.1007/BF03349721. [DOI] [PubMed] [Google Scholar]
- 54.Pierotti MA, Greco A. Oncogenic rearrangements of the NTRK1/NGF receptor. Cancer Lett. 2006;232:90–98. doi: 10.1016/j.canlet.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 55.Rubin BP, Chen CJ, Morgan TW, Xiao S, Grier HE, Kozakewich HP, Perez-Atayde AR, Fletcher JA. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am J Pathol. 1998;153:1451–1458. doi: 10.1016/S0002-9440(10)65732-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryden M, Sehgal R, Dominici C, Schilling FH, Ibanez CF, Kogner P. Expression of mRNA for the neurotrophin receptor trkC in neuroblastomas with favourable tumour stage and good prognosis. Br J Cancer. 1996;74:773–779. doi: 10.1038/bjc.1996.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segal RA, Goumnerova LC, Kwon YK, Stiles CD, Pomeroy SL. Expression of the neurotrophin receptor TrkC is linked to a favorable outcome in medulloblastoma. Proc Nat Acad Sci USA. 1994;91:12867–12871. doi: 10.1073/pnas.91.26.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, Murphy KM, Dauses T, Allebach J, Small D. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki T, Bogenmann E, Shimada H, Stram D, Seeger RC. Lack of high-affinity nerve growth factor receptors in aggressive neuroblastomas. J Natl Cancer Inst. 1993;85:377–384. doi: 10.1093/jnci/85.5.377. [DOI] [PubMed] [Google Scholar]
- 60.Tacconelli A, Farina AR, Cappabianca L, Desantis G, Tessitore A, Vetuschi A, Sferra R, Rucci N, Argenti B, Screpanti I, Gulino A, Mackay AR. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell. 2004;6:347–360. doi: 10.1016/j.ccr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Thress K, Macintyre T, Wang H, Whitston D, Liu ZY, Hoffmann E, Wang T, Brown JL, Webster K, Omer C, Zage PE, Zeng L, Zweidler-McKay PA. Identification and preclinical characterization of AZ-23, a novel, selective, and orally bioavailable inhibitor of the Trk kinase pathway. Mol Cancer Ther. 2009;8:1818–1827. doi: 10.1158/1535-7163.MCT-09-0036. [DOI] [PubMed] [Google Scholar]
- 62.Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, Becker L, Carneiro F, MacPherson N, Horsman D, Poremba C, Sorensen PH. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 63.Walch ET, Marchetti D. Role of neurotrophins and neurotrophins receptors in the in vitro invasion and heparanase production of human prostate cancer cells. Clin Exp Metastasis. 1999;17:307–314. doi: 10.1023/a:1006652605568. [DOI] [PubMed] [Google Scholar]
- 64.Yamashiro DJ, Nakagawara A, Ikegaki N, Liu XG, Brodeur GM. Expression of TrkC in favorable human neuroblastomas. Oncogene. 1996;12:37–41. [PubMed] [Google Scholar]
- 65.Yilmaz T, Jiffar T, de la Garza G, Lin H, Milas Z, Takahashi Y, Hanna E, MacIntyre T, Brown JL, Myers JN, Kupferman ME. Theraputic targeting of Trk suppresses tumor proliferation and enhances cisplatin activity in HNSCC. Cancer Biol Ther. 2010;10:644–653. doi: 10.4161/cbt.10.6.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zage PE, Graham TC, Zeng L, Fang W, Pien C, Thress K, Omer C, Brown JL, Zweidler-McKay PA. The selective Trk inhibitor AZ623 inhibits brain-derived neurotrophic factor-mediated neuroblastoma cell proliferation and signaling and is synergistic with topotecan. Cancer. 2011;117:1321–1391. doi: 10.1002/cncr.25674. [DOI] [PubMed] [Google Scholar]