Abstract
Helminth exposure appears to protect hosts from inappropriate inflammatory responses such as those causing inflammatory bowel disease. A recently identified, strongly pro-inflammatory limb of the immune response is characterized by T cell IL17 production. Many autoimmune type inflammatory diseases are associated with IL17 release. Because helminths protect from these diseases, we examined IL17 production in helminth-colonized mice. We colonized mice with Heligmosomoides polygyrus, an intestinal helminth, and analyzed IL17 production by lamina propria mononuclear (LPMC) and mesenteric lymph node (MLN) cells. Colonization with H. polygyrus reduces IL17A mRNA by MLN cells and inhibits IL17 production by cultured LPMC and MLN cells. Helminth exposure augments IL4 and IL10 production. Blocking both IL4 and IL10, but not IL10 alone restores IL17 production in vitro. Colonization of colitic IL10-deficient mice with H. polygyrus suppresses LPMC IL17 production and improves colitis. Antibody-mediated blockade of IL17 improves colitis in IL10-deficient mice. Thus, helminth-associated inhibition of IL17 production is likely an important mechanism mediating protection from inappropriate intestinal inflammation.
Keywords: Parasites-Helminths, T lymphocytes, cytokines, mucosa, IL17
Introduction
Inflammatory bowel disease (IBD) is prevalent in highly developed industrialized countries. IBD is rare in less developed nations but emerges as nations develop socioeconomically (1). This pattern of prevalence is opposite to that of parasitic worm exposure (2). Transmission of parasitic worms (helminths) is prevented by modern hygienic practices so colonization is rare in developed countries. Helminths alter their host’s immune responses (3), which may limit development of pathologic-type inflammations like IBD (2,4).
Helminth exposure can prevent or reverse colitis in animal models of IBD. Mice and rats develop colitis when rectally challenged with trinitrobenzenesulfonic (TNBS) acid in 50% ethanol (5). These animals are protected from TNBS-type colitis when exposed to Schistosoma mansoni (6,7), Trichuris muris (2), Trichinella spiralis (8), Hymenolepis diminuta (9) or Heligmosomoides polygyrus (10). IL10−/− mice develop severe chronic Th1-driven colitis in response to normal gut flora (11). Colonization with T. muris or H. polygyrus inhibits development of spontaneous colitis in IL10−/− mice (2). Furthermore, colitis improves in IL10−/− mice given H. polygyrus after inflammation is previously established (12). Exposure to helminths also protects mice from other immune-mediated inflammations like experimental autoimmune encephalitis (EAE, (13,14)), reactive airway disease (15,16), and autoimmune diabetes (17).
Recently identified is a novel, strongly pro-inflammatory limb of the immune response characterized by IL17 production by Th17 cells (18). Inflammatory conditions once attributed to aberrant Th1 or Th2 responses may result from Th17 activity. IL17 is important in colitis. IL17 mRNA and protein expression is augmented in inflamed mucosa from patients with ulcerative colitis or Crohn’s disease but not in disease controls (19,20). Mice with TNBS colitis have increased mucosal IL17 production (21). IL17 receptor-deficient mice are resistant to TNBS colitis (21). Colonic IL17 mRNA expression is increased in immune deficient (Rag−/−) mice that develop colitis after reconstitution with CD4 T cells from IL10−/− mice and treatment with rIL23 (22). Development of this colitis is inhibited with anti-IL17 and anti-IL6 antibody blockade (22).
IL17 expression is increased in other immune-mediated diseases such as multiple sclerosis (23,24) and asthma (25,26). IL17 expression is up-regulated in murine EAE (27), a model of multiple sclerosis. IL17−/− mice are resistant to EAE (27). In mice, IL17 expression is increased in reactive airway disease, and IL17 receptor-deficient mice are resistant to this model of asthma (28).
Murine colitis, EAE, and reactive airway disease all result from excessive IL17 production. Because helminths protect mice from these inflammations, we investigated if colonization with the intestinal nematode H. polygyrus would affect expression of IL17. We find that intestinal lamina propria mononuclear cell and mesenteric lymph node cell IL17 production is strongly inhibited in H. polygyrus-colonized mice. Colonization results in augmented IL4 and IL10 production (10). We show that these cytokines, especially IL4, regulate IL17 release. This is the first report that helminths inhibit pro-inflammatory IL17 production, which may explain why populations with high prevalence of helminth infections have low prevalence of autoimmune disease.
Methods
Mice and H. polygyrus infection
This study used C57BL/6 wild type (Jackson Laboratory) and C57BL/6 IL10−/− mice. IL10−/− mice were bred on site in SPF animal facilities. At 6 wks of age, mice were colonized with 150 H. polygyrus third stage larvae (L3) by oral gavage. Infective, ensheathed H. polygyrus L3 (U.S. National Helminthological Collection no. 81930) were obtained (from fecal cultures of eggs) by the modified Baermann method and stored at 4°C until used. Animals were housed and handled appropriately following NIH guidelines and as approved by our Animal Review Committees.
Measurement of IL17 transcripts
Total RNA was extracted from freshly isolated mesenteric lymph node cells (29) then reverse transcribed with Moloney-monkey leukemia virus (400 Units) using oligo-dT as primer. For real-time PCR analysis each three concentrations of each sample (.2 μg, 0.04 μg, 0.008μg RNA) were amplified for IL17A using 5′-GGCCAAGGACTTCCTCCAGA-3′ and 5′-TTTCCCTCCGCATTGACACA-3 for primers and 5′-FAM-CAGACTACCTCAA-CCGTTCCACGTCAC -3′ as probe. The results were normalized to HPRT amplified using 5′-TGAAGAGCTACTGTAATGATCAGTCAAC-3′ and 5′-GCAAGCTTGCAACCTTAACCAT-3′ as primers and 5′-TET-TGCTTTCCCTGGTTAAGCAGTACAGCCC-3′ as probe. Amplification was performed using Taqman universal master mix (Applied BioSystems) and analyzed with a ABI PRISM 7700 Sequence Detection System.
LPMC isolation
Terminal ileal intestinal tissue was washed extensively with RPMI, and all visible Peyer’s patches were removed with a scissors. The intestine was opened longitudinally, cut into 5 mm pieces and then incubated in 0.5 mM EDTA in calcium and magnesium free Hanks’ for 20 min at 37°C with shaking to release intraepithelial lymphocytes and epithelial cells. This was repeated after thorough washing. Tissue then was incubated 20 min at 37°C in 20 ml RPMI containing 10% FCS, 25 mM HEPES buffer, 2 mM L-glutamine, 5x10−5 M β-mercaptoethanol, 1mM sodium pyruvate, 100 U/ml penicillin, 5 mg/ml gentamycin, and 100 mg/ml streptomycin (all GIBCO) and 1 mg/ml collagenase (Sigma #co130). At the end of the incubation, the tissue was subjected to further mechanical disruption using a 1 ml syringe. To remove debris, the LPMC preparations were washed through a pre-wet gauze layered in a funnel with RPMI. Then, the LPMC were washed once and were sieved through a pre-wet 2 cm nylon wool column gently packed into a 10 ml syringe. After washing, cells (up to 2 x107) were layered onto a column of Percoll with a 30:70% gradient. Cells were spun at 2200xG at room temperature for 20 min. The LPMC collected from the 30:70 interface were washed and maintained on ice until used. Cell viability was 90% as determined by eosin Y exclusion.
Cell culture
For cytokine analysis, cells were cultured for 48h in 96 well microtiter plates (Corning) with 200 μl of medium (5 x 105 cells/well) at 37°C. The culture medium was RPMI-1640 containing 10% FCS, 25 mM HEPES buffer, 2 mM L-glutamine, 5x10−5 M β-mercaptoethanol, 1mM sodium pyruvate, 100 U/ml penicillin, 5 mg/ml gentamycin, and 100 mg/ml streptomycin (all GIBCO). For most experiments, the cells were cultured alone or with anti-CD3 (2C11, ATCC, 1 μg/ml) and anti-CD28 (PV1, ATCC, 1 μg/ml). Cytokine blocking experiments used anti-IL10R mAb at 2.5 μg/ml (1B1.3, BD Pharmingen) and anti-IL4 mAb at 2.5 μg/ml (11B11, ATCC). Cytokine addition experiments used recombinant murine IL4 and/or IL10 each at 50ng/ml (PreproTech).
ELISA and intracellular flow cytometry
Commercial ELISA was used to measure the concentration of IL17A in culture supernatants. The capture antibody was MAB721 (R&D Systems) and detection antibody was biotynylated BAF421 (R&D Systems). Color development used streptavidin-HRP (Zymed San Francisco, CA) and TMB substrate (Endogen, Woburn, MA), and plates were read at 490nm. Sensitivities of the ELISAs were 30 pg/ml.
For intracellular flow cytoplasmic staining, MLN cells were cultured and stimulated overnight with PMA and ionomycin as previously described (12). For the last 4 hours of culture, brefeldin A (Golgi Plug, BD Pharmingen) was added to the cells. The cells were then washed twice and adjusted to 107 cells/ml in FACS buffer (HBSS containing 1% FCS and .02% sodium azide). The cell suspensions then were dispensed into microcentrifuge tubes each containing 106 cells in 100 μl FACS buffer and 1 μg 2.4G2 antibody (anti-FcγR, ATCC) to block non-specific binding of antibodies to Fc receptors. The cells were then stained with saturating amounts of anti-CD4-Cy5 (RM2511) (CalTag, Burlingame, CA), anti-Thy 1.2-FITC (TS) (Sigma), conjugated antibodies for 30 min at 4° C. To identify cytokine secreting cells, cells were co-stained with anti-IL17-PE (BD Pharmingen) using Cytofix/Cytoperm Kit (BD Pharmingen) according to manufacturer’s instructions. Following staining, cells were washed twice and re-suspended for analysis on a Becton Dickinson FACS 440 flow cytometer (Mountain View, CA).
Induction and evaluation of colitis
To induce colitis, 5–6 wk old IL10−/− mice were given piroxicam (Sigma, St. Louis, MO) mixed into their feed (NIH-31M) for 2 wks. They received 60 mg piroxicam/250g of food during wk 1 and 80 mg piroxicam/250g food during wk 2. Mice were then placed on normal rodent chow without piroxicam. The colitis was evaluated 16 days after stopping piroxicam. For some experiments, piroxicam-treated IL10−/− mice were colonized with H. polygyrus between 24–48 hrs after stopping the piroxicam. To grade intestinal inflammation, colons were removed at the indicated time point, rolled, fixed and embedded in paraffin. The inflammation was scored from 0–4 using the following criteria: Grade 0 – No change from normal tissue. Grade 1 – Patchy mononuclear cell infiltrates in the lamina propria. Grade 2 – More uniform mononuclear cell inflammation involving both the epithelium and lamina propria; accompanied by epithelial hyperplasia and slight depletion of mucous from goblet cells. Grade 3 – Some epithelial and muscle hypertrophy with patchy lymphocytic infiltrates extending into the muscle layers, mucus depletion, occasional crypt abscesses and epithelial erosions. Grade 4 – Severe, transmural inflammation with prominent thickening of both the epithelial and muscle layers, mucus depletion, frequent crypt abscesses and ulcerations (12). In some experiments, IL10−/− colitic mice were treated with two i.p. injections of blocking anti-IL17 mAb (BD TC11 18H10) given one wk apart at 0.5mg/mouse or with an isotype control antibody.
Statistical analysis
Data are means ± SE of multiple determinations. Difference between two groups was compared using Student’s t-test. p values <0.05 were considered significant.
Results
Colonization with H. polygyrus reduces IL17A mRNA and protein expression by mesenteric lymph node cells
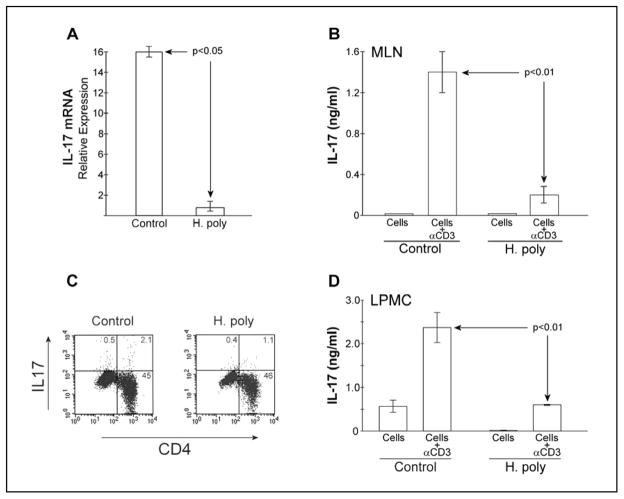
Colonization with H. polygyrus protects mice from TNBS colitis (10). IL17 expression increases in TNBS colitis and inhibition of IL17 signaling reduces colonic inflammation in that model (21). Therefore, we examined the effect of colonization with H. polygyrus on IL17 production. Control mice were treated similarly, but received no H. polygyrus larvae. RNA extracted from freshly isolated MLN cells contains transcripts for IL17A. Expression of IL17 mRNA is significantly reduced in MLN cells isolated from mice colonized with H. polygyrus as measured by real-time PCR (Fig 1A).
Figure 1.
IL17 mRNA expression, cytokine production, and Th17 frequency is reduced in H. polygyrus-colonized mice. A) Total RNA was isolated from freshly isolated unstimulated MLN cells of C57BL/6 WT naïve (control) or 2 week helminth-colonized mice. IL17A mRNA content was determined by real-time PCR using HPRT mRNA as reference. Data are from 3 separate experiments. B) MLN cells were isolated from B6WT mice colonized for 2 weeks with H. polygyrus. Control MLN cells were from helminth-naïve B6WT mice. MLN cells were cultured for 48hr at 5x105 cells/well with or without anti-CD3 stimulation. Culture supernatant IL17A content was measured by ELISA. Data are means ± SE from 3 separate experiments. C) MLN cells from helminth-naïve and 2 week colonized B6WT mice were isolated and cultured with PMA/ionomycin then Golgi-plug. Then cells were fixed, labeled and prepared for intra-cytoplasmic flow cytometry. Cells were stained for IL17 and CD4 expression. Results presented are representative of 3 experiments. D) LPMC were isolated from B6WT mice colonized for 2 weeks with H. polygyrus or helminth-naïve B6WT mice and cultured for 48 hr at 5x105 cells/well in the absence or presence of anti-CD3/anti-CD28 stimulation. Culture supernatant IL17 content was measured by ELISA. Data are means ± SE from 2 separate experiments
We also examined MLN cell expression of IL17 after T cell receptor stimulation with anti-CD3 mAb. MLN cells were isolated from helminth-naïve (control) or colonized mice and cultured for 48 hrs in the absence or presence of anti-CD3 mAb. Culture supernatants were tested for IL17 content by ELISA. MLN cells from H. polygyrus-colonized mice made significantly less IL17 as compared to naïve mice (Fig 1B).
Colonization with H. polygyrus reduces the frequency of IL17-expressing MLN T cells
IL17 is produced by a distinct lineage of CD4+ T cells (Th17). Inhibition of IL17 production could result from a decrease in the number of IL17-expressing T cells in the MLN population. MLN cells were isolated from helminth naïve and colonized mice, then stimulated overnight with PMA and ionomycin to maximally activate T cells. Secretion of IL17 was blocked by addition of brefeldin A for the last 4 hrs of stimulation. IL17-expression was evaluated by intra-cytoplasmic flow. The frequency of CD4+IL17+ T cells was reduced by approximately 50% in H. polygyrus-colonized as compared to naïve mice (Fig 1C).
Colonization with H. polygyrus reduces IL17 expression by intestinal lamina propria mononuclear cells
Heligmosomoides polygyrus resides in the duodenum and proximal jejunum of mice. Colonization produces no histological change in the more distal ileum, but does alter IFNγ, IL4, IL13, and IL10 production by lymphocytes isolated from this site (10). We examined if helminth exposure altered IL17 production by ileal lamina propria mononuclear cells (LPMC). LPMC were isolated for naïve or helminth-colonized mice and cultured for 48hr in the absence or presence of monoclonal anti-CD3 and anti-CD28 mAb. Culture supernatants were tested for IL17 content by ELISA. LPMC from H. polygyrus-colonized mice made significantly less IL17 as compared to naïve mice (Fig 1D).
IL10 blockade does not release IL17 production by MLN from helminth-colonized mice
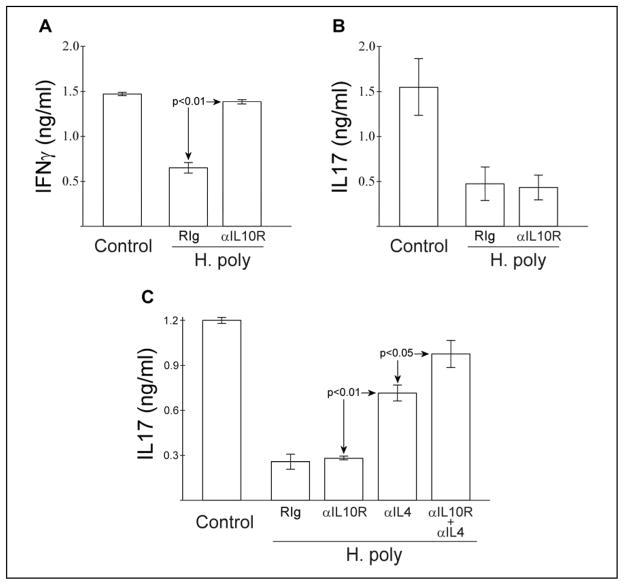
Like IL17, MLN and LPMC IFNγ production is strongly inhibited in mice colonized with H. polygyrus. Colonization dramatically increases IL10 production by cultured lymphocytes stimulated with anti-CD3. Previously, we found that blocking in situ IL10 signaling in vitro with monoclonal anti-IL10R antibody (1B1.3) releases inhibition of IFNγ (10). Thus, helminth-induced IL10 actively inhibits IFNγ release. We tested if similar blockade of IL10R would augment IL17 release. MLN cells were isolated as above from helminth-colonized mice and were stimulated in the presence of blocking anti-IL10R antibody or isotype control (Fig 2A, B). Unlike IFNγ, IL17 production was not affected by IL10 blockade alone.
Figure 2.
Simultaneous blockade of IL10 and IL4 signaling restores in vitro MLN cell IL17 production. B6WT MLN cells were isolated and cultured as in Figure 1B with anti-CD3 stimulation. Some cultures included blocking anti-IL10R (1B1.3, 2.5μg/ml), anti-IL4 (11B11, 2.5μg/ml) or isotype control (RIg) antibody. IL17A production was determined by ELISA. Data mean ± SE from 2 (A,B) or 3 (C) separate experiments.
Blockade of IL4 and IL10 synergize to restore IL17 production by MLN cells from helminth-colonized mice
In addition to IL10, helminth exposure increases lymphocyte IL4 production (10). IL4 can suppress Th17 activity (30). We examined if the in situ produced IL4, which is enhanced in colonized mice suppresses IL17. MLN cells were isolated as above from helminth naïve or colonized mice and were stimulated in the presence of blocking anti-IL4 antibody (11B11). Addition of anti-IL4 partially restored IL17 production (Fig 2C). Including anti-IL10R antibody with anti-IL4 further enhanced IL17 release. Anti-IL4, anti-IL10R, or a combination of anti-IL4 and anti-IL10R did not increase IL17 production by MLN cells from helminth-naïve mice (data not shown).
Colonization with H. polygyrus suppresses IL17 production from IL10−/− mice
Helminth-associated suppression of IL17 is not released by blocking IL10 in culture. However blocking IL10 enhances IL17 production if IL4 is also blocked. We examined if colonization with H. polygyrus would effectively suppress IL17 production in IL10−/− mice. MLN cells were isolated from naïve or helminth colonized IL10−/− mice and cultured as above. MLN cells from H. polygyrus-colonized IL10−/− mice made significantly less (p<0.01) IL17 as compared to naïve mice (Fig 3A). Addition of neutralizing anti-IL4 mAb to these cultures significantly increased in vitro IL17 production. Addition of anti-IL4 mAb to cell cultures obtained from helminth-naïve mice does not increase IL17 production (data not shown).
Figure 3.
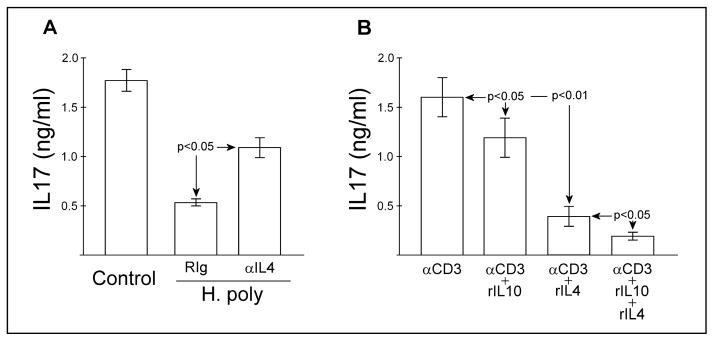
Heligmosomoides polygyrus colonization inhibits IL17 in IL10−/− mice. and IL17 blockade improves established IL10−/− colitis. A) MLN cells were isolated from helminth-naïve or H. polygyrus-colonized IL10−/− mice and cultured as in Figure 1B with anti-CD3 stimulation. Some cultures included blocking anti-IL4 (11B11, 2.5μg/ml) or isotype control (RIg) antibody. IL17 production was determined by ELISA. Data mean ± SE from 2 separate experiments. B) MLN cells were isolated from helminth-naïve IL10−/− mice and cultured as in Fig 1B with anti-CD3 stimulation in the absence and presence of recombinant IL10 and/or IL4. IL17A production was determined by ELISA. Data mean ± SE from 3 separate experiments.
The antibody blockade experiments suggest that in situ generated IL4 can suppress IL17 production. We tested this by adding recombinant murine IL10 and/or IL4 to anti-CD3 stimulated MLN cell cultures obtained from helminth-naïve IL10−/− mice (Fig 3B). Addition of rIL10 partially (26±8%) but significantly reduces IL17 production in these cultures. Addition of rIL4 reduces IL17 production more completely (73±9%). Addition of both IL4 and IL10 inhibited IL17 production by 87±4%. Addition of IL10 and IL4 to MLN cell cultures obtained from helminth-naïve wild type mice showed similar significant (p<0.05) suppression of IL17 (34±8% and 72±11%, respectively).
IL10−/− mice spontaneously develop colitis that is associated with increased mucosal IL17 production (22). Synchronous uniform colitis can be induced in IL10−/− mice by treating them with low dose piroxicam (31), a non-selective inhibitor of cyclooxygenase. Low dose piroxicam does not cause colitis in wild-type mice and the colitis induced in IL10−/− mice persists after piroxicam is withdrawn. Like spontaneous colitis, piroxicam-induced colitis is associated with a dramatic increase in LMPC IL17 production (Fig 4A). Colitis was scored on a 0 to 4 point scale as described in Methods. The colitis score was 3.5 ± 0.4 (SE) for the piroxicam-treated helminth-naïve (Control) group. After mice were rendered colitic with piroxicam, we colonized some with H. polygyrus. Colonization with H. polygyrus reverses established colitis in IL10−/− mice (12). After 2 wks of worm colonization, we isolated LPMC from helminth-naïve and H. polygyrus-exposed mice. The colitis score was 0.6 ± 0.3 (SE, p<0.01) for the piroxicam-treated helminth-exposed group. LPMC IL17 production returned to low levels in mice carrying the helminth (Fig 4A). Also, LPMC from non-colitic (no piroxicam treatment) IL10−/− mice made significantly less IL17 after H. polygyrus exposure than LPMC from non-colitic helminth naïve mice.
Figure 4.
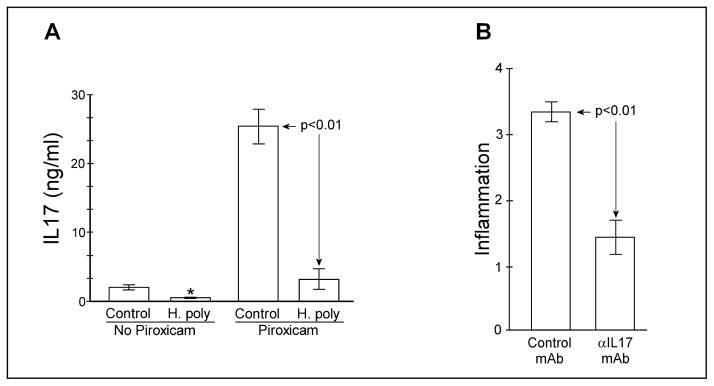
Heligmosomoides polygyrus colonization inhibits IL17 in colitic mice and IL17 blockade improves established IL10−/− colitis. A) Some IL10−/− mice were rendered colitic with piroxicam then colonized with H. polygyrus. Controls were given sham gavage. Other IL10−/− mice did not receive piroxicam. After 2 weeks, LPMC were isolated and cultured for 48hr at 5x103 cells/well. IL17A was measured by ELISA. Data are mean ± SE of 2 experiments. For the piroxicam untreated groups, Control vs. H.poly (*) p<0.05. B) IL10−/− mice were rendered colitic with piroxicam then treated with two injections of blocking anti-IL17 mAb (TC11 18H10, BD Pharmingen) given one week apart at 0.5mg/mouse or with isotype control antibody. One week after the last injection colons were removed for histology. Colitis was scored using a 0–4 point scale by readers blinded to the experimental group. Data are mean ± SE from 2 experiments.
Blockade of IL17A reverses established colitis in IL10−/− mice
Colonization with H. polygyrus reverses colitis and reduces LPMC IL17 production in IL10−/− mice. This suggests that helminth-associated inhibition of IL17 production may permit resolution of the persistent inflammation. To test this hypothesis, we treated colitic IL10−/− mice with blocking anti-IL17A mAb (Fig 4B). IL17 blockade significantly reduced inflammation in IL10−/− mice with established colitis.
Discussion
Inflammatory bowel disease and other pathologic inflammations like MS and asthma are associated with elevated IL17 expression (19,20,23–26). IL17 expression is increased in TNBS-colitis (21) and in Rag-deficient mice rendered colitic by reconstitution with IL10−/− CD4+ T cells and exogenous IL23 treatment (22). IL10−/− mice develop colitis spontaneously (32) and an identical colitis can be initiated in these mice by brief treatment with piroxicam, with the colitis persisting after piroxicam is discontinued (31). We found that mucosal IL17 production is dramatically increased (~10 fold) in colitic IL10−/− mice as compared to age-matched, non-colitic mice. Lamina propria T cells are the source of this IL17 (data not shown). Blockade of IL17 with anti-IL17 mAb improves established colitis in this model. This extends previous observations that abrogation of IL17 prevents initial development of colitis in the TNBS (21) and Rag-reconstitution (22) models and shows that maintenance of colitis requires ongoing IL17 signaling.
Dysregulated immune responses produce pathologic inflammation like that of IBD. Immune-mediated diseases such as IBD, MS, and asthma have become prevalent in highly developed industrialized countries but remain rare in less developed nations (3). This suggests that an environmental factor absent in highly industrialized countries protects individuals in the population from developing immune-mediated disease. One such environmental factor may be the absence of exposure to parasitic worms (2). Helminth carriage was previously universal (33) and has become rare in industrialized countries due to sewage treatment, highly regulated food industries, cement side walks, and other modern hygienic innovations.
Previously we showed that colonization of colitic IL10−/− mice with H. polygyrus results in resolution of the intestinal inflammation (12). Exposure to helminths reduces inflammation in TNBS-type colitis (2,6,8,9) and in murine models of MS (13,14) and asthma (15,16). These inflammations are associated with increased IL17 production. Therefore we investigated if colonization with H. polygyrus inhibits mucosal IL17 expression in wild type and IL10−/− mice.
We found that colonization with H. polygyrus resulted in a 16-fold decrease in MLN cell IL17 mRNA expression as compared to that of helminth naïve mice. This experiment evaluated transcripts from freshly isolated and otherwise unstimulated lymphocytes and reflects the baseline cytokine skewing of the colonized animal. Unstimulated MLN cells from either colonized or naïve mice did not release IL17 in culture. However, when stimulated with anti-CD3 mAb, MLN cells do release IL17, and mice harboring H. polygyrus showed a 3-fold decrease in production of this cytokine as measured by ELISA. This suggested that IL17-producing T cells remained present in the MLN population of helminth-colonized mice, and could be recruited to express IL17, but not to the level shown by cells from naïve animals. Flow cytometric analysis of PMA and ionomycin-stimulated CD4+ T cells showed that the frequency of IL17-expressing cells (Th17) was decreased by nearly 50% in the MLN population of H. polygyrus-colonized mice.
Intestinal LPMC produced more IL17 than did similarly cultured MLN cells. Unlike MLN cells, LPMC from helminth-naïve wild type mice made IL17 without additional stimulation. This unstimulated production was abrogated in LPMC from helminth-colonized mice. Treatment with anti-CD3 and anti-CD28 mAb augmented LPMC IL17 production. Like MLN cells, anti-CD3-stimulated LPMC from H. polygyrus-exposed mice produced about one third the amount of IL17 as that made by lymphocytes from helminth-naïve mice. This reduction in capacity to make IL17 could make the intestinal mucosa more resistant to pathologic inflammation.
Helminths induce MLN cell and LPMC IL10 production in their hosts, which could regulate IL17 production. Previously, we found that blocking IL10 reversed helminth-inhibited IL12/23p40 and IFNγ release (10). Therefore, we tested whether in situ IL10 production mediated the suppression of IL17 production in cultured MLN cells from helminth-colonized mice. Unlike IFNγ, IL17 production was not enhanced by blocking IL10 signaling. This suggests that helminthic modulation of IL17 production occurs through a mechanism distinct from that of IFNγ in wild type mice.
Helminths induce strong Th2 responses typified by IL4 production. IL4 can inhibit IL17 production by memory T cells (30). Therefore, we tested whether in situ IL4 production mediated the suppression of MLN cell IL17 production after helminth exposure. IL4 blockade partially restored IL17 production by MLN cells from helminth-colonized mice. Thus, IL17 suppression due to Th2 skewing may be a mechanism of helminth-mediated protection from IL17 driven inflammation. Blockade of IL4 did not completely restore IL17 production. Addition of anti-IL10R antibody synergized with anti-IL4 antibody to permit enhanced IL17 release. Thus helminth-induced IL4 and IL10 function in concert to maximally inhibit IL17.
Blockade of IL10 alone did not permit IL17 production by MLN cells from colonized wild type mice. Thus, IL10 is not necessary or sufficient in itself to regulate IL17 secretion. Consistent with this observation, helminth-exposure suppressed IL17 production by MLN and LPMC from non-colitic IL10−/− mice. Helminth exposure reverses established IL10−/− colitis (12). The resolution in colitis is associated with a nearly 10-fold decrease in LPMC IL17 production. The reduction in IL17 production was not due to the presence of fewer T cells in culture since LPMC were cultured at the same number of cells/well whether isolated from colitic or non-colitic mice. Improvement in inflammation could result in less IL17 production. In the absence of colitis, helminth colonization suppresses LP cell IL17 production in wild type and IL10−/− mice. Furthermore, we and others (22) find that blockade of IL17 with mAb suppresses colitis. This suggests that helminth-associated inhibition of mucosal IL17 contributes to resolution of colitis.
This is the first report that colonization with an intestinal helminth results in inhibition of IL17 production and a decrease in Th17 cells. We measured IL17 production by LPMC and MLN cells from mice actively colonized with H. polygyrus. These studies do not address whether mucosal IL17 production remains suppressed after colonization has resolved. These results suggest that suppression of T cell IL17 release is one of the mechanisms that protect helminth-colonized mice from inappropriate inflammation and reverses ongoing intestinal inflammation (12). IL4 in conjunction with IL10 are possible important helminth-induced regulatory cytokines for IL17.
Acknowledgments
Financial Support:
The National Institutes of Health (DK38327, DK58755, AI49382, DK07663, DK25295, DK034928, AI018919), the Department of Veterans Affairs Office of Research and Development, and the Crohn’s and Colitis Foundation of America, Inc. supported this research.
Reference List
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Elliott DE, Urban JFJ, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB Journal. 2000;14:1848–1855. doi: 10.1096/fj.99-0885hyp. [DOI] [PubMed] [Google Scholar]
- 3.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. International Journal for Parasitology. 2007;37:457–464. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 5.Te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflammatory Bowel Diseases. 2006;12:995–999. doi: 10.1097/01.mib.0000227817.54969.5e. [DOI] [PubMed] [Google Scholar]
- 6.Elliott D, Li J, Blum A, Metwali A, Qadir K, Urban JFJ, Weinstock JV. Exposure to Schistosome Eggs protects mice from TNBS-induced colitis. American Journal of Physiology. 2003;284:G385–G391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 7.Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, Van Marck EA, Pelckmans PA. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53:99–107. doi: 10.1136/gut.53.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins SM. Intestinal nematode infection ameliorates experimental colitis in mice. Infection & Immunity. 2002;70:5931–5937. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. Journal of Immunology. 2005;174(11):7368–75. doi: 10.4049/jimmunol.174.11.7368. [DOI] [PubMed] [Google Scholar]
- 10.Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF, Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T cell cytokine production in normal distal murine intestine. Infect Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection & Immunity. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. European Journal of Immunology. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 13.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. International Immunology. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 14.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infection & Immunity. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. Journal of Experimental Medicine. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 18.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 19.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen OH, Kirman I, Rudiger N, Hendel J, Vainer B. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scandinavian Journal of Gastroenterology. 2003;38:180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory Bowel Diseases. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 22.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. Journal of Clinical Investigation. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature Medicine. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 24.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Multiple Sclerosis. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 25.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respiratory Research. 2006;7:135–135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. Journal of Allergy & Clinical Immunology. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 27.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 28.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. Journal of Experimental Medicine. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 30.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 32.Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clinical Immunology & Immunopathology. 1995;76:S174–S178. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 33.Goncalves ML, Araujo A, Ferreira LF. Human intestinal parasites in the past: new findings and a review. Memorias do Instituto Oswaldo Cruz. 2003;98(Suppl 1):103–118. doi: 10.1590/s0074-02762003000900016. [DOI] [PubMed] [Google Scholar]