Abstract
Background
Selecting nonviral carriers for in vivo gene delivery is often dependent on determining the optimal carriers from transfection assays in vitro. The rationale behind this in vitro strategy is to cast a net sufficiently wide to identify the few effective carriers of plasmids for in vivo studies. Nevertheless, many effective in vivo carriers may be overlooked by this strategy because of the marked differences between in vitro and in vivo assays.
Methods
After solid-phase synthesis of linear and branched histidine/lysine (HK) peptides, the two peptide carriers were compared for their ability to transfect MDA-MB-435 tumor cells in vitro and then in vivo.
Results
By contrast to their transfection activity in vitro, the linear H2K carrier of plasmids was far more effective in vivo compared to the branch H2K4b. Surprisingly, negatively-charged polyplexes formed by the linear H2K peptide gave higher transfection in vivo than did those with a positive surface charge. To examine the distribution of plasmid expression within the tumor from H2K polyplexes, we found widespread expression by immunohistochemical staining. With a fluorescent tdTomato expressing-plasmid, we confirmed a pervasive distribution and gene expression within the tumor mediated by the H2K polyplex.
Conclusions
Although mechanisms underlying the efficiency of gene expression are probably multifactorial, unpacking of the H2K polyplex within the tumor appears to have a significant role. Further development of these H2K polyplexes represents an attractive approach for plasmid-based therapies of cancer. © 2014 The Authors. The Journal of Gene Medicine published by John Wiley & Sons, Ltd.
Keywords: cancer, gene therapy, nonviral, plasmid
Introduction
With recent advances of genomic information for the root causes of disease, there has been increased interest in nucleic acid therapeutics, including small interfering (si)RNA and gene therapy. Nucleic acid therapeutics require the delivery of highly negatively-charged nucleic acids from relatively low molecular weight siRNA to hundreds of times larger molecular weight plasmids. Although several groups including ours 1–3 have shown efficient nonviral delivery to tumor xenografts with siRNA, efforts with nonviral delivery of plasmids to tumor xenografts have been thwarted by inefficient delivery. If there was an efficient delivery system for plasmids, this delivery system might represent a transformation for treating tumors and be an important step toward successful ‘gene’ therapy in the clinic.
Our laboratory has focused primarily on developing histidine and lysine peptide carriers with varied complexity to deliver plasmids in vitro and in vivo. Positively-charged lysines bind and protect DNA, partially neutralizing its charge 4, whereas histidines bind DNA, buffer endosomal pH and thus presumably aid in release of nucleic acids from endosomes. The development of linear and branched HK peptides by using solid phase synthesis has allowed the systematic modification of amino acid sequences and branching, providing ideal conditions for structure-function studies (Figure1). Using in vitro assays, we found that linear peptides were ineffective compared to higher branch peptides as carriers of plasmids. Indeed, the four branch peptide, H2K4b, resulted in a transfection efficiency of malignant cells almost 6 logs greater than that of the linear peptide, H2K. Although the combination of liposomes and linear H2K synergistically increased transfection in primary nontransformed cells, the linear H2K by itself was still a poor carrier in these cells 4. These findings with HK peptides were dissimilar to those performed with linear poly(ethylene imine), which gave high transfection results in vitro and compared favorably to branch poly(ethylene imine) 5,6. Because of the results from the in vitro transfection assays, we selected the branched peptide to deliver the nucleic acids in vivo. Our approach for selection of an effective carrier in vivo is similar to that of most other researchers in the nucleic acid delivery field: selecting an effective in vitro carrier for the in vivo studies. Moreover, the carriers 7–9 that show promise are often modified with polyethylene glycol and tumor-targeting ligands for studies in vivo.
Figure 1.
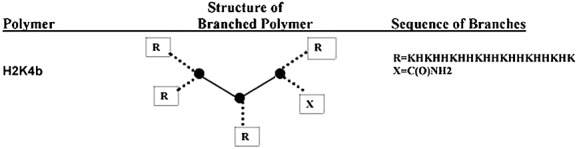
Schematic of HK peptides.
Although many effective carriers for studies in vivo have been identified based on results obtained in vitro, the development of nonviral carriers for clinical studies has still been painstakingly slow. One reason for the delay in the discovering promising gene delivery carriers may be the lack of in vitro assays that reflect results obtained in vivo. Although the discrepancy between in vitro and in vivo assays has been widely recognized, the exclusion of carriers based on their lack of efficacy in vitro may result in not testing viable and effective carriers in vivo. This was the case with the linear H2K until recently. Very few nonviral studies have considered this potential problem 10 and, to our knowledge, this has not been investigated for polyplexes.
In the present study, we compared the branch H2K4b carrier with what was considered to be the negative control H2K carrier for systemic delivery of luciferase plasmids to tumor xenografts in vivo. Surprisingly, transfection efficiency of tumor xenografts with the linear peptide, H2K, was significantly greater than with the branched H2K4b peptide. With biophysical methods including gel retardation and isothermal calorimetry, we then examined the properties and mechanisms of H2K polyplexes, particularly those with a reduced N/P ratio and negative surface charge, that enhance transfection in vivo.
Materials and methods
Animals
Female athymic mice (4 − 8 weeks old, body weight; 20 g) were purchased from NCI Frederick (Frederick, MD, USA). All experiments were performed in accordance with regulations by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Cell lines
Three malignant breast cancer cell lines [MDA-MB-435, MDA-MB-231 and 4 T1 (murine)], a glioma (C6), a glioblastoma (N2A) and lung cell lines (H460) were cultured and maintained in Dulbecco's minimal essential medium (DMEM) containing 10% fetal calf serum (FCS) and 20 mM glutamine.
Plasmids
The pCpG-Luc plasmid was made by ligating the luciferase gene (digested from pMOD-Luc plasmid; InvivoGen, San Diego, CA, USA) into the multiple cloning site of pCpG-mcs (InvivoGen) 7. pCpG-tdTomato was made by ligating the tdTomato transgene with BglII and NheI sites (GeneScript, Piscataway, NJ, USA) into the multiple cloning site of the pCpG vector.
The plasmids were prepared for systemic injection as described previously, with a few modifications 11. GT115 bacteria (InvivoGen) containing the plasmids were grown in Superbroth to mid-log phase. The plasmids were then purified with an EndoFree Maxi kit (Qiagen, Valencia, CA, USA). An analytical gel of each plasmid (cut and uncut) was examined to confirm that there was no contamination with other nucleic acids.
Peptides
The linear H2K and branch H2K4b peptides, with predominant repeating groups, -HHK-, were synthesized on a Rainin Voyager synthesizer (PTI, Tucson, AZ, USA) by the biopolymer core facility at the University of Maryland, as described previously 12. Each of four terminal branches of H2K4b emanates from the three-lysine core. The linear H2K and the branch sequences of H2K4b have the sequence: KHKHHKHHKHHKHHKHHKHK. The H2K and H2K4b peptides were first analyzed by high-performance liquid chromatography (HPLC) (Beckman, Fullerton, CA, USA) and were not purified further if HPLC showed that the purity of the polymer was 95% or greater. Further analyses of the peptides were performed by matrix-assisted laser desorption/ionization-time of flight mass spectroscopy on a Voyager system (Applied Biosystems, Foster City, CA, USA) and, in selected cases, by amino acid analysis (AAA Laboratory Service, Boring, OR, USA).
In vitro transfection and luciferase reporter assay
Initially, 1.5 × 105 cells were plated in 24-well plates in the presence of 500 µl of DMEM with 10% serum; after 24 h, the cells reached 70% confluency. Unless otherwise indicated, increasing amounts of the peptide (0.125, 0.25, 0.5, 1.0, 2.0 and 4.0 µg) in 50 µl were mixed with 1.0 µg of the plasmid [CPG-Luciferase (Luc) or CPG-tdTomato in 42 µl of Opti-MEM or water] and the mixture was allowed to stand for 30 min (1/1, w/w ratio for HK/DNA is approximately equal to 1.3/1, N/P ratio). The transfection protocol was performed as described previously with few modifications 13. In brief, 50 µl of the transfection complex were added to cells for 24 h. The cells were then lysed with 100 µl of 1 × passive lysis buffer (Promega Corp., Madison, WI, USA). The protein concentration was measured by using the BCA protein assay kit (Pierce, Rockford, IL, USA). Luciferase activity was measured and expressed as relative light units by the direct current Turner 20/20 luminometer (Turner Design, Sunnyvale, CA, USA) as described previously 13 with four measurements performed for each concentration.
Similarly, transfections with H2K4b and H2K were carried out with two different three-dimensional (3-D) cell cultures (3D InsertTM-PS Scaffolds; Celltreat Scientific, Shirley, MA, USA, or Lipidure-Coat, NOP Corporation, Tokyo Japan). The MDA-MB-435 cells (1.5 × 105 cells) were added to each well containing 0.5 ml of DMEM with 10% FCS. Twenty-four hours later, after spheroids had developed, different ratios (1/8 to 8/1) of H2K or H2K4b to pCpGluc plasmids were added to the wells containing the cells. After an additional 24 h, the luciferase activity was measured.
In vitro uptake of HK polyplex
After the DNA was labeled with Sytox-orange, HK plasmid polyplexes at various ratios were prepared as described in the in vitro transfection section above. The labeled polyplexes were incubated with MDA-MB-435 cells for 4 h, the cells were then fixed with 4% formalin, and the nuclei stained with chromatin dye Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) in phosphate-buffered saline for 5 min. Images were obtained with Nikon TE2000-S (Nikon, Tokyo, Japan).
Particle size and zeta potential
For each preparation of plasmid polyplexes, the size and zeta potential were determined with the Zetasizer (Malvern, Westborough, MA, USA) prior to their injection. The size was reported as the average size obtained from unimodal analysis of dynamic light scattering of the particles at a 90° angle with use of software provided by the manufacturer of the instrument. Each particle size and zeta potential data point represents the mean ± SD of three measurements.
Gel retardation assay
Various amounts of HK peptides were mixed with 1 µg of plasmid and incubated for 30 min at room temperature. Specifically, the following HK/plasmid ratios (w/w) were prepared in water or in 50 mM NaCl: 1/8, 1/4, 1/2, 1/1, 2/1 and 4/1. After 30 min, the HK polyplex was loaded onto the gel (1% agarose containing ethidium bromide) and electrophoresis was carried out at a constant voltage of 75 V for 45 min in TAE buffer. With certain specified ratios, the formed HK polyplex was incubated for an additional 15 min in 2.5 M NaCl. Similarly, the effect of serum (10% FCS or mouse serum) on the stability of H2K and H2K4b polyplexes was evaluated by gel electrophoresis. After the HK plasmids (ratios ½ and 4/1) were prepared in OptiMEM and FCS was added (final 10% FCS) for up to 2 h, the polyplexes were analyzed by gel electrophoresis. A gel retardation assay was also performed on each in vivo HK polyplex preparation before injection into the mice. Gel retardation assays similar to those described above were also carried out with HK polyplexes after exposing them to increasing concentrations of serum to assess their stability.
Isothermal titration calorimetry
Peptides (25 μM, 5 µl per injection) and DNA (3.75 nM, 1.45 ml) were dissolved in phosphate buffer (10 mM, pH 7.3). The thermodynamic profile of HK peptides binding to DNA was obtained with the use of VP-ITC (MicroCal, Inc., Northampton, MA, USA) at 37 °C. The sample cell was filled with DNA (1450 µl), and the syringe contained peptide solution (250 µl). Each injection (5 µl; a total of 56 injections) was carried out for 10 s at 360-s intervals. The dilution heat was determined with the titration of peptides into buffer without DNA. Molar enthalpy and final figures were generated using Origin, version 5.0 (http://www.originlab.com). The binding study of each HK polymer was examined in duplicate.
Polyplex formation in vivo
To the plasmid DNA (40.5 µg in 150 µl of water), increasing amounts of HK (5.06, 10.1, 20.2, 40.5, 81 and 162 µg in 100 µl of water) were added quickly and mixed by pipetting. Forty-five minutes after mixing, the polyplex (approximately 200 µl) was administered by intravenous injection. The remainder of the polyplex preparation was analyzed by gel electrophoresis, size and their zeta potential.
In vivo transfection
When tumors in mice were approximately 100 mm3 in size unless otherwise indicated, the mice were treated by intravenous injection in the tail vein with plasmid polyplexes prepared as described above. After 24 h, the tumor and major organs were homogenized and luciferase activity was measured with a direct current TD 20/20 luminometer (Turner Design).
Immunohistochemical detection of luciferase
Tumors were fixed with Streck Cell Preservative (Streck, Omaha, NE, USA) for 48 h and processed as paraffin-embedded tissue sections. Immunostaining was performed in accordance with the manufacturer's instructions (Vector, Versatile ABC, Burlingame, CA, USA). Briefly, tumor sections were deparaffinized in xylene and rehydrated in graded ethanol. Antigens were retrieved by maintaining tissue in 100 mM citrate buffer (Sigma-Aldrich, St Louis, MO, USA) (pH 6.0) at 95 °C for 30 min. Endogenous peroxidase was blocked by 3% H2O2 in 100% methanol for 10 min and nonspecific binding was reduced with 5% rabbit serum for 30 min at room temperature. For detection of luciferase, tumor sections were incubated with rabbit polyclonal antibody (targeting luciferase; dilution 1:500; Abcam, Cambridge, MA, USA) at 4 °C overnight. After incubation with a secondary anti-rabbit biotin-labeled antibody for 30 min and then with peroxidase-labeled streptavidin (Vector) for an additional 30 min, chromogen diaminobenzidine was added to tumor sections for 5 min for color development. The tumor sections were then dehydrated and mounted with glass coverslips. Three independent experiments were performed.
Tumor localization and distribution
Fluorescently Sytox-orange-labeled plasmids in complex with HK peptides were used to validate the polyplex delivery to various tissues. After implanted MDA-MB-435 tumor xenografts reached 100 mm3, HK peptides in complex with labeled plasmids were administered by intravenous injection and mice were euthanized 4 h later. Frozen sections were prepared from several organs (lungs, liver, kidneys) and tumor xenografts. Images of these tissues were obtained with a TE2000-S microscope. Five independent experiments were performed.
pCpG-tdTomato detection
Twenty-four hours after the HK-pCpG-td (tandem dimer)Tomato polyplexes (w/w, HK/plasmid, 1/2 ratio) were injected intravenously, mice were euthanized and frozen sections of MDA-MB-435 tumor xenografts were prepared. Images of the tumors were obtained with a TE2000-S microscope.
Statistical analysis
All results, reported as the mean ± SEM, represent at least four separate data measurements unless otherwise indicated. Results were analyzed using a t-test with p < 0.05 considered statistically significant.
Results
Effective transfection with the branched H2K4b peptide in vitro
With the use of a standard transfection assay with 10% FBS, the H2K4b peptide in complex with a plasmid expressing luciferase gave very high transfection, particularly at higher peptide/DNA ratios where the polyplex was positively charged (Figure2). By contrast, cells transfected with the linear H2K polyplex had negligible luciferase expression. Similar transfection trends were observed when HK polyplexes were prepared in either OptiMEM (Figure2a) or water (Figure2b) (polyplexes were markedly smaller in size, particularly at higher ratios, when prepared in water). Similarly, 3-D or reverse transfection assays with the H2K polyplex showed no luciferase expression (data not shown).
Figure 2.
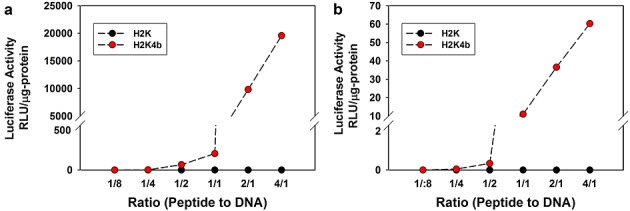
In vitro transfection. After MDA-MB-435 cells reached 70% confluency in 24-well plates, increasing amounts (0.125, 0.25, 0.5, 1.0, 2.0 and 8.0 µg) of peptides (H2K or H2K4b) were mixed with 1.0 µg of the plasmid (CPG-Luc) in Opti-MEM (a) or water (b) and the mixture was allowed to stand for 30 min. After the transfection complex was added to cells for 24 h, the cells were lysed and luciferase activity was measured. Duplicate measurements were made at each concentration in four separate experiments. Data from the luciferase activity is expressed as the mean A 1/1, w/w ratio is approximately equivalent to a 1.3/1, N/P ratio.
From studies similar to these, we initially concluded that H2K simply did not bind DNA to form polyplexes; consequently, H2K was not tested as an in vivo carrier for many years. Recent work has shown that, although H2K does show decreased DNA binding, it can indeed form polyplexes of various sizes, and thus the poor transfection apparently was not a result of the polyplex size or surface charge (see Supporting information, Table S1).
The in vitro transfection results were consistent with the intracellular delivery of Sytox-orange labeled plasmids in complex with HK peptides (see Supporting information, Figure S1). At higher ratios for H2K4b plasmid polyplexes, where increased cellular levels of labeled plasmids were observed, increased luciferase levels were also observed. For all ratios of H2K/plasmid, labeled plasmid polyplexes were not seen intracellularly and, not surprisingly, exogenous transfected luciferase levels were either not detected or were extremely low.
Effective delivery of DNA to tumor xenografts with the linear H2K peptide
After MDA-MB-435 cells were implanted in athymic mice and tumors had grown in the mid-axillary line to approximately 100 mm3 in size, various ratios of HK peptides in complex with luciferase-expressing plasmids (peptide/DNA, w/w, 1/8–4/1; N/P, approximately 1/6–5.2/1) were injected into the tail veins of the mice. By contrast to the results obtained in vitro, the linear H2K treated mice, particularly at low peptide to DNA ratios, had higher luciferase values in tumor xenografts compared to those in the H2K4b group (Figure3). Although the expressed luciferase levels were reduced with smaller tumors of 50 mm3, the H2K remained significantly more effective as carriers of plasmids compared to H2K4b (data not shown). The zeta potentials at these lower peptide to DNA ratios (w/w, 1/8 to 1/2; N/P, approximately 1/6 to 1/1.54) were negative (ranging from –18 to –25 mV). By use of dynamic light scattering, the size differences between H2K and H2K4b polyplexes were not significant except at the 1/1 ratio (Figure S2; see also Supporting information, Table S2). These results challenge not only the in vitro results, but also the widespread belief that positively-charged polyplexes will be the most effective in vivo. Similar to MDA-MB-435 xenografts, H2K polyplexes at low peptide to DNA ratios gave high luciferase values in several tumor xenografts (brain, lung, human and mouse breast cancers) (see Supporting information, Figure S3).
Figure 3.
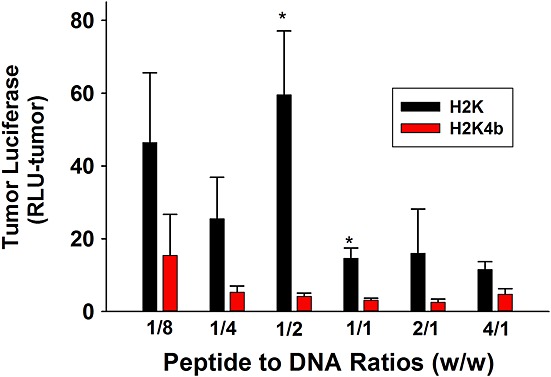
In vivo transfection of tumor xenografts. When tumors in mice were approximately 100 mm3 in size, the mice were injected intravenously in the tail vein with plasmid polyplexes prepared as described in Materials and methods. After 24 h, the tissues and major organs were homogenized and luciferase activity was measured with a direct current TD 20/20 luminometer. *p < 0.05, H2K versus H2K4b.
Greater specificity of the H2K polyplex toward tumor xenografts
In addition to the greater tumor expression from the H2K polyplex, we were also interested in gene expression in other organs. Because the lung is the first vascular organ that the polyplex traverses after intravenous administration, we were particularly interested in comparing gene expression of the polyplexes in the tumor and lungs (Figure4a). The tumor to lung was higher at all peptide to DNA ratios with the H2K polyplex group compared to the H2K4b treatment group. Moreover, the gene expression in the tumor for the H2K polyplex was far greater than expression of luciferase in the lungs. By contrast, the lung expression was significantly greater than tumor expression of luciferase with the H2K4b polyplex. This difference in tumor to lung expression was particularly marked at the lower ratios where the HK polyplexes exhibited a negative zeta potential. In addition to the lung, other major organs showed lower expression than the tumor with the H2K polyplex (Figure4b).
Figure 4.
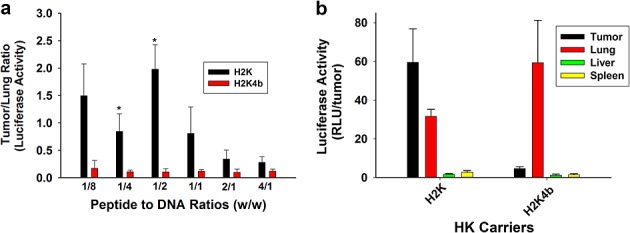
Increased specificity of H2K polyplexes for tumor xenografts. Tumor-bearing mice were treated by intravenous injection with plasmid polyplexes prepared as described in Materials and methods. After 24 h, the tissues and major organs were homogenized and luciferase activity was measured with a direct current TD 20/20 luminometer. Several ratios of HK to plasmid (1/8 to 4/1) were used to compare tumor and lung luciferase expression from H2K or H2K4b polyplexes (a). Because H2K showed higher tumor and tumor/lung expression, particularly at a w/w ratio of 1:2 (HK to plasmids), this ratio was used to show luciferase activity in several major organs (b). *p < 0.05, H2K versus H2K4b.
Pervasive distribution of H2K polyplexes within tumor matrix
We then investigated whether the increased luciferase expression in the tumor was a result of higher expression within localized areas of the tumor or was more widespread. Immunohistochemical studies of luciferase indicated that H2K plasmid polyplexes were found to be widespread thoughout the tumor xenograft. By contrast, there was significantly less staining for luciferase in the tumor when the H2K4b polyplex was administered to the tumor-bearing mice. Immunohistochemical staining was repeated twice, confirming that luciferase expression was more intense and widespread throughout the tumor in the H2K plasmid polyplex group (Figure5a). To confirm this result and to examine whether the most intense luciferase staining was primarily located near the tumor blood vessels, double staining for vessels (CD31) and luciferase was performed (Figure5b). In addition to luciferase staining more intensely with the H2K polyplex group, immunostaining staining for this treatment group appeared to be uniform and, somewhat surprisingly, did not appear more intense around the tumor vessels. Lastly, we confirmed that H2K polyplex gene expression was higher and more widespread than that of the H2K4b polyplex by use of a fluorescent gene product (tdTomato). In place of the luciferase-expressing plasmid, the tdTomato plasmid in complex with H2K carrier similarly showed greater and more widespread gene expression compared to the H2K4b carrier (Figure5c).
Figure 5.
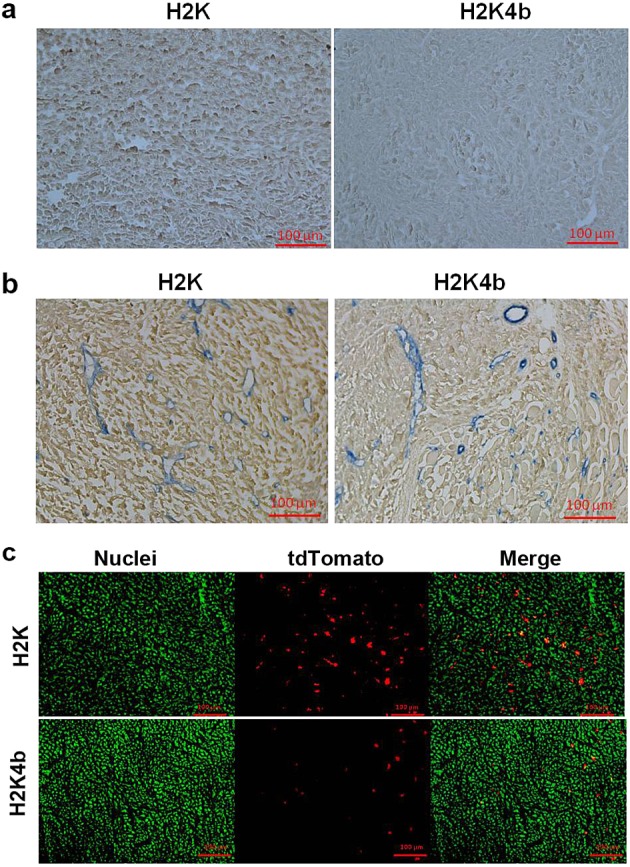
Widespread expression of exogenous gene expression within tumor xenografts. Twenty-four hours after mice with tumor xenografts were administered with HK polyplexes (1/2; w/w ratio), the tumors were processed to determine the degree of exogenous gene expression within tumor xenografts. Immunohistochemical staining of luciferase (a) and luciferase and CD31 (b). Instead of luciferase expression, tdTomato expression in frozen sections of the tumor xenografts was examined (c). Representative figures are from experiments performed three times.
Enhanced delivery of H2K polyplexes to tumors
To determine whether increased delivery of H2K polyplexes was a result of the higher transfection of tumor xenografts, we fluorescently labeled the plasmids with Sytox orange to determine the amount of different polyplexes within the tumor xenograft. Four hours after the tumor-bearing mice were treated by injection with the HK polyplexes, the mice were euthanized. H2K polyplexes were consistently found at higher levels in the MDA-MB-435 tumor xenografts than were H2K4b polyplexes (Figure6). Thus, higher transfection of the tumor by the H2K polyplex was the result, at least in part, of increased delivery. Increased background fluorescence in the lungs, liver and kidneys prevented determination of the amount of polyplexes accumulated in these tissues.
Figure 6.
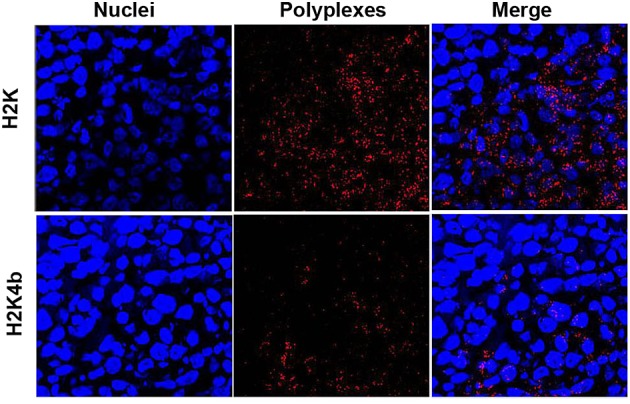
Enhanced delivery of H2K polyplexes to tumor xenografts. Fluorescently Sytox-orange-labeled plasmids in complex with HK peptides were used to quantify the amount polyplex delivered to tumor xenografts. After implanted MDA-MB-435 tumor xenografts reached 100 mm3, HK peptides in complex with labeled plasmids were administered by intravenous injections, and mice were euthanized 4 h later. Frozen sections were prepared from tumor xenografts and nuclei were stained with 4',6-diamidino-2-phenylindole. Images of these tissues were obtained with fluorescent microscopy. Upper panel: H2K; lower panel: H2K4b.
Decreased binding of H2K with DNA
Isothermal titration calorimetry (ITC) quantifies differences in binding, so that molecular signature correlates of in vivo efficacy may be obtained. At low peptide to DNA ratios, ITC shows a higher negative enthalpy for the H2K4b toward DNA compared to that of H2K (Figure7). This indicates that H2K4b has a higher affinity for DNA. Similarly, on a weight by weight basis, H2K4b retards DNA in the absence and presence of serum (10% FBS) more effectively than does H2K (see Supporting information, Figure S4), indicating a higher affinity of the four-branched H2K4b for DNA. Further evidence that H2K has a lower affinity for DNA is suggested by the rapid degradation of its polyplexes when exposed to trypsin (data not shown) or supernatant from medium of cells (see Supporting information, Figure S5). We also determined whether NaCl could disrupt the formed HK polyplexes and release DNA. After exposure to NaCl (2. 5 m), DNA was completely released from H2K polyplexes, whereas most of the DNA was retained with H2K4b polyplexes (data not shown).
Figure 7.
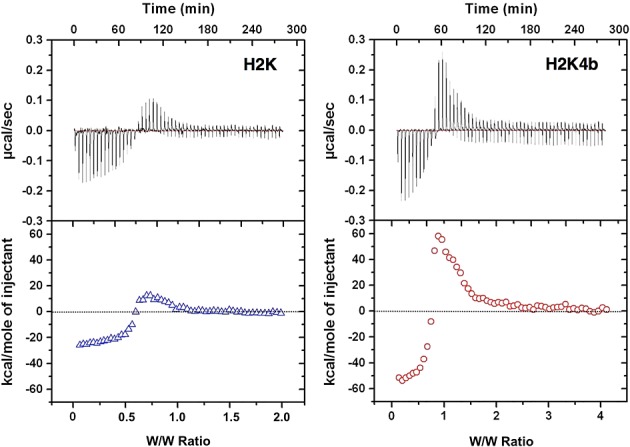
Isothermal titration calorimetry of polyplexes. Representative ITC raw data and integrated heat release of H2K and H2K4b titrated with plasmid DNA (10 mM phosphate buffer, pH 7.0). Compared with H2K, the four-branched peptide shows a greater initial exothermic reaction, indicating higher binding capacity.
When the H2K polyplex ratio (1/2) was incubated with medium exposed to cells for 48 h, the polyplex was degraded within 30 min (see Supporting information, Figure S5). Furthermore, the 4/1 ratio showed significant signs of degradation at the 4-h time point. By contrast, both ratios of H2K4b polyplex showed no evidence of degradation at the 30-min time point, and only the 1/2 ratio showed degradation at 4 h. This is consistent with a loosely packed H2K polyplex compared to a more densely pack H2K4b polyplex. Thus, we then centrifuged polyplexes upon cells to decrease the transit time and exposure of the HK polyplexes to the enzymatic degradation of the medium. Centrifugation of H2K polyplexes increased transfection, particularly at higher w/w ratios, although transfection was still quite low (see Supporting information, Figure S6).
Discussion
In the present study, we determined that negatively-charged linear H2K polyplexes were far more effective for systemic plasmid delivery to tumor xenografts, despite their lack of activity in vitro. The high gene expression and distribution throughout the tumor from H2K polyplexes represent major advances toward the use of plasmids expressing therapeutic genes or small hairpin RNA in the treatment of cancer. Because linear HK carriers in vivo have sufficient DNA binding for systemic transport of the polyplex to the tumor from an intravenous administration, yet still apparently enable rapid unpacking of the polyplex within the tumor, this encourages future studies aiming to examine a range of linear peptide structures that vary in length and sequence patterns. Because lysines and histidines interact with nucleic acids through ionic and non-ionic interactions, respectively, 3, different patterns within the peptide are expected to affect binding affinity of peptide with the DNA, stability of the polyplex, and transfection in vivo.
Although the most important result is this discovery of an effective plasmid delivery system for tumors in vivo, the marked discrepancy between in vitro and in vivo studies with the two peptide carriers, indicates that new in vitro assays are required so as not to miss effective carriers. With H2K polyplexes, several in vitro transfection assays (e.g. reverse transfection assays, standard transfections with and without serum, and transfection of 3-D spheroids culture) were tested but they all failed, demonstrating only negligible to minimal increases in luciferase activity. Plasmid polyplexes of various sizes were also formed with H2K by varying the w/w ratio but, again, transfection results with small and large polyplexes were still quite poor. Centrifugation of H2K polyplexes, particularly larger ones, did increase transfection but only minimally, indicating that the relatively long transit time was not a significant factor bringing about differences between the polyplexes in vitro. Although enzymatic degradation probably does not play a major role in reduced transfection in vitro, the sensitivity of H2K polyplexes to enzymatic degradation does indicate the presence of loosely packed polyplexes, consistent with rapid unpacking of H2K polyplexes within the tumor matrix.
We are unaware of studies in which systemically delivered polyplexes with a reduced N/P ratio (<1) giving a negative surface charge result in efficient transfection of tumor xenografts 8,14–18. The rationale for using polyplexes with a high N/P ratio has been based on charge neutralization, condensation and stabilization of the polyplexes, as well as the finding that higher ratios usually translate into higher gene expression and transfection efficiency in vitro 15,16,19–22. Moreover, interaction of positively-charged polyplexes with the negatively-charged membranes of tumor cells (e.g. increase content of glycosaminoglycans) is considered to be important for their uptake and endocytosis in vitro and in vivo 23. With the addition of a surface steric layer (e.g. polyethylene glycol, alginate) and/or a tissue-targeting ligand, interaction of positively-charged polyplexes with the membranes may be less important, although it is still likely to have an auxiliary role in uptake in vitro and in vivo 8,24–26. Although negatively-charged polyplexes with reduced ratios or with negatively-charged coatings 8,27 have rarely been tested in vivo and have not been previously administered systemically, other nanoparticles with a negative surface charge 23,28–32, including gold particles coated with siRNA, neutral or cationic liposomes coated with hyaluronic acid 33,34, and/or anionic liposomes, have demonstrated efficacy in vitro and/or in vivo, some of which have a prolonged biological half life 35. Alternatively, negatively-charged nanoparticles in the presence of serum may have enhanced uptake because of the corona of serum proteins absorbed to the negatively-charged particles 36. Not surprisingly, proteins absorbed to the negatively-charged gold particles were cationic, reducing the negative charge on the particle 36. Compared to negatively-charged polyplexes, cationic polyplexes may be more susceptible to degradation in vivo by ionic competition from proteins of the serum or from basement membranes 37,38. A recent study indicated that cationic polyplexes, particularly those less than 100 nm in size, accumulated within the negatively-charged mesangium of the kidneys with subsequent degradation of the polyplex 39. Whether biologically charged proteins disrupt H2K or H2K4b polyplexes with varied N/P ratios and modify their half-life is unknown, although pharmacokinetic and biodistribution studies will help resolve some of these questions.
It is not clear why the mechanisms differed between linear H2K and branched H2K4b polyplexes in their accumulation and transfection of tumor xenografts. Although enhanced permeation and retention is a primary mechanism for the accumulation of polyplexes within tumors 40, this mechanism cannot readily explain the differences between the H2K and H2K4b polyplexes. Gene expression with H2K polyplexes in tumor xenografts was approximately ten-fold greater than H2K4b, whereas gene expression with H2K polyplexes in the lungs was two-fold lower. H2K polyplexes expressed luciferase in the tumor to a greater degree partly as a result of their increased delivery and pervasive distribution within tumor tissues. Although delivery differences between the polyplexes to the tumor were particularly relevant, the penetration and transfection efficiency of H2K polyplexes were still surprising in the tumor xenografts. The pre-injection size of the two polyplexes also cannot readily explain their differences in transfection of the tumor in that the H2K4b polyplexes were slightly smaller in size. Similarly, the surface charges of the two polyplexes were equally negative and thus cannot explain their distribution differences within the tumor. Although there is no direct evidence, we consider that greater penetration of H2K polyplexes within the tumor may be based on reduced binding of H2K with DNA. Reduced binding may augment greater penetration of the polyplex through its break-up into smaller components upon reaching the acidic tumor environment. Interestingly, a linear alanine-lysine peptide (A2K), which has reduced binding to DNA compared to the H2K carrier, is predominantly delivered with its DNA cargo to the lung, rather than the tumor tissue (tumor/lung approximately 1/14). The intermediate binding of H2K with DNA may explain why the H2K polyplex maintains stability within the bloodstream but releases DNA within the target organ.
There have been few studies to date that examined properties of nonviral polyplexes and correlations between their in vitro and in vivo transfection efficiencies. One investigation studied a large data set of lipidoids that varied in size, zeta potential, percentage encapsulation of siRNA, and specific cells transfected 10. Although most properties were not predictive of an in vitro and in vivo correlation, the particular cell and percentage encapsulation did have predictive value. Moreover, the ability of in vitro transfection results to predict in vivo results increased markedly with pegylated forms of lipidoids. When pegylation (with or without ligand) of the nanoparticle leads to reduce transfection or silencing in vitro (as with HK peptides) 41, alternative assays such as molecular characterization assays that may be more predictive of effective carriers in vivo must be considered.
Although the substantial increase in transfection of tumor xenografts in a murine model with H2K polyplexes is likely to be multifactorial, we suspect that binding of H2K with DNA has a central role. Based on the reduced binding and disruption of H2K polyplexes into smaller polyplexes within the acidic tumor matrix (Figure8), the schematic shows one possible mechanism for the increased transfection of tumor xenografts by H2K polyplexes. Assays such as fluorescence resonance energy transfer have been developed to localize unpacking of polyplex in vitro 42 and their application to the in vivo setting may aid in the development of more effective carriers. Moreover, the use of microfluidics alone with various blood components to mimic the shear forces of the vasculature 43 or in combination with other assays (binding measurement with ITC and endosomal lysis assays) has promise to identify effective in vivo carriers. The development of these assays may transform the initial evaluation of nonviral carriers of nucleic acids for systemic delivery that is independent of reporter-gene expression in vitro and facilitate development of nonviral carriers for the clinic.
Figure 8.
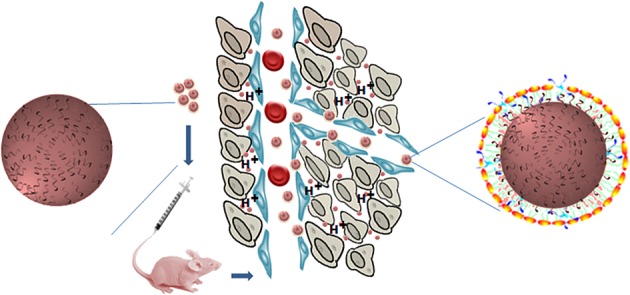
Increased penetration of H2K polyplexes within tumor matrix. On the basis of reduced binding of H2K with plasmid DNA, this schematic shows the potential effects of serum on binding to and then disrupting H2K polyplexes into smaller polyplexes once inside the acidic tumor matrix.
Acknowledgments
The authors thank P. Talalay for careful reading and invaluable editorial assistance on an earlier version of this manuscript. This work was supported by the National Institutes of Health (R01-CA136938). The authors declare that they have no conflicts of interest
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web site.
References
- Bartlett DW, Davis ME. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34:322–333. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumour by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126:77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou ST, Hom K, Zhang D, et al. Enhanced silencing and stabilization of siRNA polyplexes by histidine-mediated hydrogen bonds. Biomaterials. 2013;35:846–855. doi: 10.1016/j.biomaterials.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QR, Zhang L, Stass SA, et al. Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 2001;29:1334–1340. doi: 10.1093/nar/29.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choosakoonkriang S, Lobo BA, Koe GS, et al. Biophysical characterization of PEI/DNA complexes. J Pharm Sci. 2003;92:1710–1722. doi: 10.1002/jps.10437. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Moro E, Pettenazzo A, et al. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Ther. 1997;4:1100–1106. doi: 10.1038/sj.gt.3300503. [DOI] [PubMed] [Google Scholar]
- Leng Q, Scaria P, Ioffe OB, et al. A branched histidine/lysine peptide, H2K4b, in complex with plasmids encoding antitumour proteins inhibits tumour xenografts. J Gene Med. 2006;8:1407–1415. doi: 10.1002/jgm.982. [DOI] [PubMed] [Google Scholar]
- Jiang G, Min S-H, Oh EJ, et al. DNA/PEI/alginate polyplex as an efficient in vivo gene delivery system. Biotechnol Bioproc Engin. 2007;12:684–689. [Google Scholar]
- Malek A, Merkel O, Fink L, et al. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol Appl Pharmacol. 2009;236:97–108. doi: 10.1016/j.taap.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Whitehead KA, Matthews J, Chang PH, et al. In vitro-in vivo translation of lipid nanoparticles for hepatocellular siRNA delivery. ACS Nano. 2012;6:6922–6929. doi: 10.1021/nn301922x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesoon-Wood LA, Kim WH, Kleinman HK, et al. Systemic gene therapy with p53 reduces growth and metastases of a malignant human breast cancer in nude mice. Hum Gene Ther. 1995;6:395–405. doi: 10.1089/hum.1995.6.4-395. [DOI] [PubMed] [Google Scholar]
- Leng Q, Scaria P, Zhu J, et al. Highly branched HK peptides are effective carriers of siRNA. J Gene Med. 2005;7:977–986. doi: 10.1002/jgm.748. [DOI] [PubMed] [Google Scholar]
- Leng Q, Mixson AJ. Modified branched peptides with a histidine-rich tail enhance in vitro gene transfection. Nucleic Acids Res. 2005;33:e40. doi: 10.1093/nar/gni040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E, Cotten M, Foisner R, et al. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991;88:4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Hosaka S, Kawa S, et al. Potential tumour-targeting peptide vector of histidylated oligolysine conjugated to a tumour-homing RGD motif. Cancer Gene Ther. 2001;8:783–787. doi: 10.1038/sj.cgt.7700362. [DOI] [PubMed] [Google Scholar]
- Behr JP, Demeneix B, Loeffler JP, et al. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989;86:6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou SM, Erbacher P, Remy JS, et al. Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J Gene Med. 2000;2:128–134. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<128::AID-JGM95>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Schiffelers RM, Ansari A, Xu J, et al. Cancer siRNA therapy by tumour selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luten J, van Steenis JH, van Someren R, et al. Water-soluble biodegradable cationic polyphosphazenes for gene delivery. J Control Release. 2003;89:483–497. doi: 10.1016/s0168-3659(03)00127-5. [DOI] [PubMed] [Google Scholar]
- Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug Chem. 1999;10:406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- Son KK, Tkach D, Patel DH. Zeta potential of transfection complexes formed in serum-free medium can predict in vitro gene transfer efficiency of transfection reagent. Biochim Biophys Acta. 2000;1468:11–14. doi: 10.1016/s0005-2736(00)00312-6. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Bellocq NC, Davis ME. Effects of structure of beta-cyclodextrin-containing polymers on gene delivery. Bioconjug Chem. 2001;12:280–290. doi: 10.1021/bc0001084. [DOI] [PubMed] [Google Scholar]
- Balazs DA, Godbey W. Liposomes for use in gene delivery. J Drug Deliv. 2011;2011:326497. doi: 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogris M, Steinlein P, Carotta S, et al. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS PharmSci. 2001;3:E21. doi: 10.1208/ps030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GF, Fella C, Pelisek J, et al. towards synthetic viruses: endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol Ther. 2005;11:418–425. doi: 10.1016/j.ymthe.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wolschek MF, Thallinger C, Kursa M, et al. Specific systemic nonviral gene delivery to human hepatocellular carcinoma xenografts in SCID mice. Hepatology. 2002;36:1106–1114. doi: 10.1053/jhep.2002.36372. [DOI] [PubMed] [Google Scholar]
- Lei Y, Rahim M, Ng Q, et al. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J Control Release. 2011;153:255–261. doi: 10.1016/j.jconrel.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi NL, Giljohann DA, Thaxton CS, et al. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- Seferos DS, Prigodich AE, Giljohann DA, et al. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 2009;9:308–311. doi: 10.1021/nl802958f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafari MR, Reed CJ, Rostron C. Prospects of anionic nanolipoplexes in nanotherapy: transmission electron microscopy and light scattering studies. Micron. 2007;38:787–795. doi: 10.1016/j.micron.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Guo W, Lee RJ. Efficient gene delivery using anionic liposome-complexed polyplexes (LPDII) Biosci Rep. 2000;20:419–432. doi: 10.1023/a:1010338219401. [DOI] [PubMed] [Google Scholar]
- Xiao K, Li Y, Luo J, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Park EJ, Morishita Y, et al. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufay Wojcicki A, Hillaireau H, Nascimento TL, et al. Hyaluronic acid-bearing lipoplexes: physico-chemical characterization and in vitro targeting of the CD44 receptor. J Control Release. 2012;162:545–552. doi: 10.1016/j.jconrel.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Poon Z, Lee JB, Morton SW, et al. Controlling in vivo stability and biodistribution in electrostatically assembled nanoparticles for systemic delivery. Nano Lett. 2011;11:2096–2103. doi: 10.1021/nl200636r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giljohann DA, Seferos DS, Patel PC, et al. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007;7:3818–3821. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruponen M, Yla-Herttuala S, Urtti A. Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: physicochemical and transfection studies. Biochim Biophys Acta. 1999;1415:331–341. doi: 10.1016/s0005-2736(98)00199-0. [DOI] [PubMed] [Google Scholar]
- Tandia BM, Vandenbranden M, Wattiez R, et al. Identification of human plasma proteins that bind to cationic lipid/DNA complex and analysis of their effects on transfection efficiency: implications for intravenous gene transfer. Mol Ther. 2003;8:264–273. doi: 10.1016/s1525-0016(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Zuckerman JE, Choi CH, Han H, et al. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci U S A. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Shiramoto S, Miyashita M, et al. Tumour targeting based on the effect of enhanced permeability and retention (EPR) and the mechanism of receptor-mediated endocytosis (RME) Int J Pharm. 2004;277:39–61. doi: 10.1016/j.ijpharm.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Chou ST, Leng Q, Scaria P, et al. Selective modification of HK peptides enhances siRNA silencing of tumour targets in vivo. Cancer Gene Ther. 2011;18:707–716. doi: 10.1038/cgt.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi CA, Love KT, Sahay G, et al. FRET-labelled siRNA probes for tracking assembly and disassembly of siRNA nanocomplexes. ACS Nano. 2012;6:6133–6141. doi: 10.1021/nn3013838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia PM, Farokhzad OC, Karnik R, et al. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat Nanotechnol. 2010;7:623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


