Abstract
Phosphatase and tensin homolog (PTEN) loss or mutation consistently activates the phosphatidylinositol 3-kinase (PI3-K)/Akt signaling pathway, which contributes to the progression and invasiveness of prostate cancer. Furthermore, the PTEN/PI3-K/Akt and Ras/MAPK pathways cooperate to promote the epithelial-mesenchymal transition (EMT) and metastasis initiated from prostate stem/progenitor cells. For these reasons, the PTEN/PI3-K/Akt pathway is considered as an attractive target for both chemoprevention and chemotherapy. Herein we report that eupafolin, a natural compound found in common sage, inhibited proliferation of prostate cancer cells. Protein content analysis indicated that phosphorylation of Akt and its downstream kinases was inhibited by eupafolin treatment. Pull-down assay and in vitro kinase assay results indicated that eupafolin could bind with PI3-K and attenuate its kinase activity. Eupafolin also exhibited tumor suppressive effects in vivo in an athymic nude mouse model. Overall, these results suggested that eupafolin exerts antitumor effects by targeting PI3-K.
Keywords: eupafolin, phosphatidylinositol 3-kinase, Akt, prostate cancer, chemoprevention
Introduction
Phosphatase and tensin homolog (PTEN), a lipid phosphatase that negatively regulates the PI3-K/Akt pathway, is frequently inactivated in prostate cancer. The loss of PTEN or its mutation activates the PI3-K/Akt signaling pathway, which contributes to the tumorigenesis, progression and invasiveness of prostate cancer [1–3]. The PTEN/PI3-K/Akt pathway is also critical for prostate cancer stem-like cell maintenance, and PTEN knockdown, accompanied by p53 loss, led to an increase in sphere formation as well as increased clonogenic and tumorigenic potential [4]. Recent research results also indicated that PTEN loss and Ras/MAPK activation can cooperate to promote epithelial mesenchymal transition (EMT) and metastasis initiated from prostate stem/progenitor cells [5,6]. Furthermore, the PI3-K/Akt pathway and the androgen receptor (AR) exhibit cross talk in prostate cancer [7,8]. The synergy between Akt and AR signaling is sufficient to initiate and progress naive adult murine prostatic epithelium to frank carcinoma and override the effect of androgen ablation [9]. Interestingly, another gene alteration associated with prostate cancer tumorigenesis, TMPRSS2-ERG rearrangement, is significantly enriched in PTEN-depleted prostate cancer specimens, and transgenic overexpression of ERG in mouse prostate tissues promotes marked acceleration and progression of high-grade prostatic intraepithelial neoplasia (HGPIN) to prostatic adenocarcinoma in a PTEN heterozygous background [10–12]. In addition, PTEN loss will evoke Akt and PI3-K expression in prostate cancer [13–15]. Both PI3-K amplification and PTEN deletion consistently activate Akt, which plays a central role in a number of cell signaling pathways that are important in cancer cell survival [16].
The activation of Akt requires phosphorylation at Thr308 by 3-phosphoinositide- dependent kinase 1 (PDK1) and at Ser473 by mammalian target of rapamycin complex 2 (mTORC2). Akt activation can sequentially activate the downstream mTOR complex1 (mTORC1) and GSK3β, both of which play important roles in cancer development. mTOR1 is pivotal in prostate cancer nutrition metabolism [17]. Knockout of tuberous sclerosis complex 1 (Tsc1), a potent negative regulator of mTORC1, will activate a molecular signaling cascade producing prostatic neoplasia and focal carcinogenesis [18]. Besides a role in tumorigenesis, the Akt pathway also modulates hypoxia-inducible factor-1 alpha (HIF-1α) expression and contributes to tumor angiogenesis in human prostate cancer cells [19]. All this evidence indicates that targeting PI3-K signaling might be beneficial in prostate cancer treatment and even eliminate prostate cancer stem-like cells.
Eupafolin is a flavonoid found in the common sage herb or Eupatorium perfoliatum L. Both of these plants have been used by Chinese or native Indians as traditional medicine [20,21]. Eupafolin exhibits anti-inflammatory, antioxidant and antitumor cell proliferation effects [22,23]. Here, we found that eupafolin inhibits prostate cancer cell proliferation and anchorage-independent growth. Pull-down and in vitro kinase assay results indicated that eupafolin could bind with PI3-K in vitro and inhibit PI3-K activation in a dose-dependent manner both in vitro and in vivo. Eupafolin attenuates tumor growth and the phosphorylation of Akt in a PC3 xenograft mouse model. Overall, eupafolin inhibits prostate cancer by suppressing PI3-K- mediated Akt signaling.
Materials and Methods
Materials
Eupafolin (> 95% purity) was purchased from Indofine (Hillsborough, NJ). Chemical reagents for molecular biology and buffer preparation, including Tris, NaCl, and SDS were obtained from Sigma-Aldrich (St. Louis, MO). Eagle’s MEM was from Invitrogen (Carlsbad, CA). Glutathione-Sepharose 4B and CNBr-Sepharose 4B were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). The luciferase assay substrate was obtained from Promega (Madison, WI). Antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), Cell Signaling Technology (Beverly, MA), or Upstate Biotechnology, Inc. (Charlottesville, VA).
Cell culture
PC3, DU 145, and LNCaP prostate cancer cells and normal RWPE-1 prostate cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). PC3, DU 145 and LNCaP Cells were propagated in F-12K, F-12 or RPMI-1640 medium (Cellgro, Manassas, VA) containing 10% fetal bovine serum (FBS, Gibco, Grand Island, NY) and 100 IU penicillin/ml and 100 μg/ml streptomycin (Cellgro) at 37 °C in a humidified incubator with 5% CO2. RWPE-1 cells were cultured in keratinocyte serum free medium (Gibco, NY). Cells were cytogenetically tested and authenticated before the cells were frozen. Each vial of frozen cells was thawed and maintained in culture for a maximum of 8 weeks.
MTS assay
PC3, DU 145, LNCaP and RWPE-1 cells were seeded at a density of 3 × 103 cells per well in 96-well plates in 100 μL medium. After 12 h (PC3, DU 145) or 48 h (LNCaP, RWPE-1) of culture, the appropriate concentrations of eupafolin were added to each well. After incubation for another 24, 48, 72 or 96 h, 20 μl of the CellTiter 96 Aqueous One Solution (Promega) were added to each well and cells were incubated for another 2 h at 37 °C. Absorbance was measured at 490 and 690 nm using the Thermo Multiskan plate-reader (Thermo Fisher scientific, Waltham, MA).
Western blotting
For Western blot analysis, cells (2 × 106) were seeded in a 10-cm dish and incubated for 24 h. The cells were then treated with eupafolin (0, 5, 10, 15, or 20 μM) for 24 h. The cells were harvested and protein concentration was determined. Proteins (30 μg) were subjected to 6 or 10% SDS-PAGE. After transferring the proteins, the PVDF membranes were incubated with a specific primary antibody at 4 °C overnight. Protein bands were visualized by a chemiluminescence detection kit (Amersham Pharmacia Biotech) after hybridization with a horseradish peroxidase-conjugated secondary antibody.
In vitro PI3-K kinase assay
The kinase assay was performed as described [24]. Briefly, active PI3-K (100 ng, EMD Millipore, Billerica, MA) was incubated with DMSO or various concentrations of eupafolin (0, 5, 10, 15, or 20 μM) for 15 min and then mixed with phosphatidylinositol sodium salt (20 μl of 0.5 mg/ml MP Biomedical, Solon, OH) at 30 °C for 20 min. Reactions were performed in a kinase buffer containing 50 μM unlabeled ATP with or without 10 μCi of [γ-32P] ATP. Reactions were terminated and resolved by thin layer chromatography (Merck, Whitehouse Station, NJ) and visualized by autoradiography.
In vitro mTORC2 kinase assay
Purified Akt1 fusion proteins (1 μg) were used for an in vitro kinase assay. mTORC2 was pulled down with a Rictor antibody as described by Sarbassov, et al. [25]. Reactions were performed in a kinase buffer containing 100 mM potassium acetate, 25 mM HEPES, 1 mM MgCl2 and 50 μM ATP at 30 °C for 30 min. Reactions were terminated and proteins were analyzed by Western blotting using a specific primary antibody.
Anchorage-independent cell growth
PC3 cells (8×103/ml) were exposed to eupafolin (0–20 μM) in 1 ml of 0.33% basal medium Eagle agar supplemented with 10% FBS. The cultures were maintained in an incubator for 14 days, and the cell colonies were counted by a microscope with the Image-Pro Plus software (v.6) program (Media Cybernetics, Silver Spring, MD) as described by Colburn, et al. [26].
Pulldown assays
For pulldown assays, eupafolin-Sepharose 4B beads (100 μl, 50% slurry) were mixed with commercial PI3-K or a cellular supernatant fraction of PC3 cells (500 μg) overnight in a reaction buffer (50 mM Tris pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2 μg/ml bovine serum albumin, 0.02 mM phenylmethylsulfonyl fluoride (PMSF), and 1μg protease inhibitor mixture). The beads were washed 5 times with washing buffer (50 mM Tris pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, and 0.02 mM PMSF) and proteins bound to the beads were analyzed by Western blotting.
ATP and eupafolin competition assay
PI3-K (100 ng) was incubated with 100 μl eupafolin-Sepharose 4B or Sepharose 4B beads in a reaction buffer (same as the pulldown assay reaction buffer) for 12 h at 4 °C, and ATP was added at either 0.01 or 0.1 mM to a final volume of 500 μl, followed by incubation for 30 min. The samples were washed and proteins were detected by Western blotting.
Molecular modeling
Computer modeling of eupafolin with PI3-K beta was performed using the Schrödinger Suite 2011 software program [27]. First, an X-ray diffraction structure of PI3-K beta with a resolution of 3.3 Å (PDB ID 2Y3A, chain A) [28] was obtained from the RCSB Protein Data Bank [29]. PI3-K beta was prepared under the standard procedure of the Protein Preparation Wizard in Schrödinger Suite 2011 and hydrogen atoms were added at pH 7 and all water molecules were removed. Finally, the ATP binding site-based receptor grid was generated for docking. Eupafolin and LY294002 were prepared under the program of LigPrep of Schrödinger for docking by default parameters. Then eupafolin- and LY294002-protein docking was accomplished using the program Glide and default parameters under the extra precision (XP) mode. Herein we obtained the best-docking representative structure.
Cyclin D1 transcription activity assay
The pA3 luc-cyclin D1 plasmid was generously provided by Dr. Chris Albanese (Albert Einstein Cancer Center, Department of Developmental and Molecular Biology, Albert Einstein College of Medicine, Bronx, New York). This reporter gene was constructed from the −1715 to +134 region of the human cyclin D1 promoter region [30]. PC3 cells (5 × 104) were seeded into 12-well plates for 24 h before transfection. The pA3 luc-cyclin D1 plasmid (400 ng) was transfected into each well. The cells were cultured for another 48 h and then disrupted for firefly luciferase analysis. In addition, the reporter gene vector pRL-SV40 (Promega) was co-transfected into each cell line, with the transfection efficiencies normalized to the Renilla luciferase activity generated by this vector.
Cell cycle analysis
Subconfluent cultures of PC3 cells were serum-starved for 16 h to synchronize at G0, and then pretreated with eupafolin for 2 h followed by stimulating with medium containing 10% FBS and 20 μM eupafolin for 16 h. The cells were trypsinized, washed with ice-cold DPBS, and fixed with ice-cold 70% ethanol at −20 °C overnight. Cells were then washed twice with DPBS, incubated for 30 min in the dark at room temperature with 0.5 mg/ml RNase A and 200 μg/ml propidium iodide in DPBS, and finally subjected to flow cytometry analysis using the FACS Calibur flow cytometer. Data were analyzed using ModFit LT (Verity Software House, Inc., Topsham, ME).
In vivo tumor growth assay
All animal studies were conducted in accordance with guidelines approved by the KRIBB-IACUC (Korea Research Institute of Bioscience & Biotechnology - Institutional Animal Care and Use Committee). Athymic nude mice (BALB/c nude mice, 6 wk old) were purchased from Orient Bio Inc (Jungwon-gu, Gyeonggi-Do, Republic of Korea). The animals were housed in climate-controlled quarters with a 12-h light/12-h dark cycle. Animals were randomly assigned to the following groups: vehicle group (n = 15); 10 mg/kg eupafolin-treated group (n = 15); 50 mg/kg eupafolin-treated group (n = 15); and 50 mg/kg eupafolin control group (no cells injected; n = 15). Each mouse was administered eupafolin (10 or 50 mg/kg body weight in 100 μl of 10% PEG400 in autoclaved PBS as vehicle) or only vehicle 3 times per week by intraperitoneal injection. After 3 days of treatment, PC3 cells (1 × 106) were injected subcutaneously into the right flank of mice in each group (except control group). Following injection, mice were continuously administered with eupafolin or vehicle. Mice in the 50 mg/kg eupafolin control group were not injected with cells but maintained for comparison of body weight and tumor development. Mice were weighed and tumors measured by caliper three times per week. Tumor volume was calculated from measurements of 2 diameters of the individual tumor according to the following formula: tumor volume (mm3) = (length × width × width/2). Mice were monitored until day 28 and at that time mice were euthanized and tumors extracted.
Statistical analysis
All quantitative data are expressed as means ± S.E. or S.D. as indicated. A one-way ANOVA was used for statistical analysis. A probability of p < 0.05 was used as the criterion for statistical significance.
Results
Eupafolin inhibits prostate cancer cell proliferation and anchorage-independent cell growth
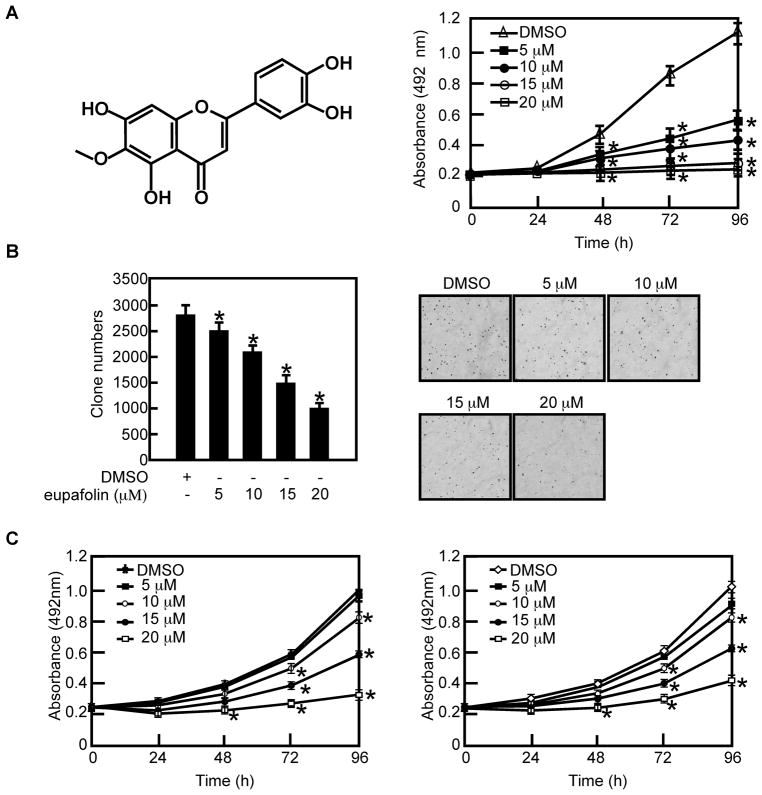
Eupafolin (Figure 1) is a flavonoid found in Eupatorium perfoliatum L and is known for its anti-inflammatory activity [22,23]. To examine whether eupafolin can affect prostate cancer cell proliferation, we first determined the cytotoxicity of eupafolin on PC3 cells. Results indicated that 55% of cells survived after treatment with 100 μM eupafolin for 48 h (Supplementary Fig. 1). Based on these results, we selected 0, 5, 10, 15 and 20 μM concentrations of eupafolin to determine its effect on PC3 cells. Our results indicated that eupafolin could inhibit PC3 cell proliferation (Figure 1A, right panel) and anchorage independent cell growth (Figure 1B) in a dose-dependent manner. To investigate whether eupafolin can inhibit the proliferation of other prostate cancer cell lines, DU 145 (Figure 1C, left panel) and LNCaP cells (Figure 1C, right panel) were chosen and treated with eupafolin. The results indicated eupafolin could also suppress proliferation these cell types in a dose-dependent manner (Figure 1C). However, PC-3 cells were most sensitive to eupafolin at low dose (5 μM) compared to the other cell lines. Therefore we chose PC-3 cells to perform further mechanistic studies. The effect of eupafolin on normal RWPE-1 prostate cells was also determined and we found that eupafolin also slightly inhibited RWPE-1 at 20μM but had almost no effect at 5–10μM (Supplementary Fig. 2).
Fig. 1.
Eupafolin inhibits proliferation and anchorage-independent growth of prostate cancer cells. A, left panel, the chemical structure of eupafolin. Right panel, eupafolin suppresses PC3 cell proliferation in a dose-dependent manner. PC3 cells (3 × 103 cells/well) were treated with the indicated doses of eupafolin for the specified times. The absorbance was measured as described in “Materials and Methods”. Data are shown as means ± S.D. and the asterisk (*) indicates a significant (p < 0.05) decrease in proliferation of cells treated with eupafolin compared to untreated control cells. B, eupafolin suppresses anchorage-independent growth of PC3 cells. Bar graphs depict the inhibitory effect of eupafolin on PC3 cells (left panel). Colony numbers are shown as means ± S.D. from 3 independent experiments. The asterisk (*) indicates a significant (p < 0.05) decrease in colony numbers in cells treated with eupafolin compared to the DMSO-treated group. Representative photographs of colony formation are shown in the right panels. C, eupafolin suppresses DU 145 (left panel) and LNCaP (right panel) prostate cancer cell proliferation. Cells (3 × 103 cells/well) were treated with the indicated doses of eupafolin for the specified times. The absorbance was measured as described in “Materials and Methods”. Data are shown as means ± S.D. and the asterisk (*) indicates a significant (p < 0.05) decrease in proliferation in cells treated with eupafolin compared to untreated control cells.
Eupafolin inhibits Akt and activation of its downstream targets
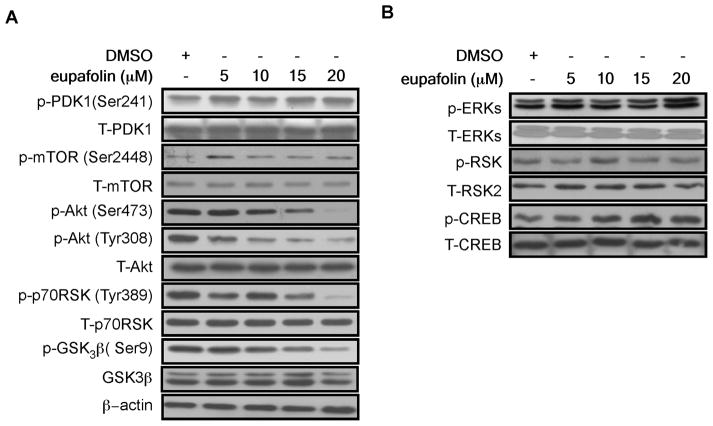
To further investigate the mechanism of the anti-proliferation effect of eupafolin, PC3 cells were chosen and treated with different concentrations of eupafolin for 24 h. Determination of the signal transduction pathway closely related with cell survival showed that phosphorylation of Akt at Ser473 and Thr308 was strongly inhibited by eupafolin in a dose-dependent manner. The phosphorylation of GSK3β (Ser9) and p70S6K (Thr398), downstream kinases of Akt, was also inhibited by eupafolin treatment (Figure 2A). However, PDK1, mTOR, MEK/ERKs and downstream kinases, RSK2 and CREB, were not affected (Figure 2B).
Fig. 2.
Eupafolin suppresses PI3-K/Akt signaling. A, PC3 prostate cancer cells were treated with eupafolin for 24 h at the indicated concentration. The protein content of phosphorylated and total proteins associated with the PI3-K signaling pathway was visualized by Western blotting with specific primary and HRP-conjugated secondary antibodies. B, PC3 cells were treated as in A. The protein content of phosphorylated and total proteins associated with the ERKs signaling pathway was visualized by Western blotting with specific primary and HRP-conjugated secondary antibodies.
Eupafolin causes cell cycle arrest at G1
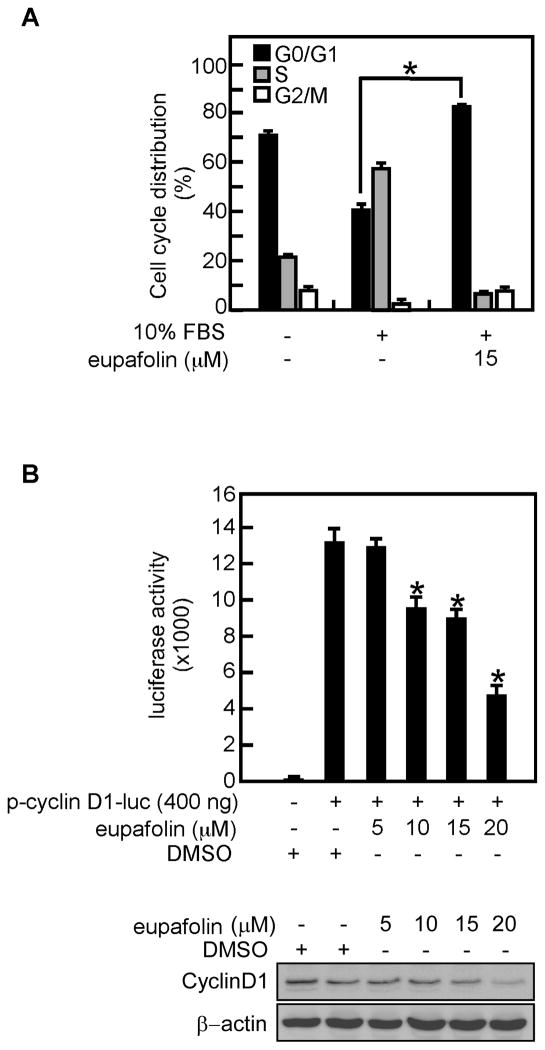
Cell proliferation is closely associated with cell cycle progression. To determine the effect of eupafolin on PC3 cell cycle progression, cells were serum-starved for 16 h to synchronize at G0 and pretreated with eupafolin for 2 h followed by stimulation with medium containing 10% FBS and eupafolin. The results indicated that compared with control, eupafolin caused PC3 cell cycle arrest at G1 (Figure 3A). Cycle cell progression is regulated by cyclins and Akt and its downstream kinase GSK3β regulate transcription of cyclin D1 [31]. Results of a cyclin D1 reporter gene assay showed that eupafolin inhibited cyclin D1 transcription activity in a dose-dependent manner (Figure 3B upper panel). The protein level of cyclin D1 was also decreased after eupafolin treatment (Figure 3B lower panel).
Fig. 3.
Eupafolin causes cell cycle arrest at G1. A, PC3 prostate cancer cells were serum-starved for 16 h to synchronize at G0 phase and then pretreated with eupafolin for 2 h followed by stimulation with 10% FBS including eupafolin for 16 h. The cell cycle distribution was measured by flow cytometry with propidium iodide. Data are expressed as the percentage of cells in G1/G0, S, or G2/M and are shown as means ± S.D. of values from triplicate experiments. B, eupafolin inhibits cyclin D1 transcriptional activity. The inhibitory effect of eupafolin on cyclin D1 activity was measured by introducing cyclin D1 reporter plasmids into PC3 cells followed by treatment with various concentrations of eupafolin. Luciferase activity was measured and data are shown as means ± S.D. of values from triplicate experiments (upper panel). The protein content of cyclin D1was determined by Western blotting (lower panel). The asterisk (*) indicates a significant (p < 0.05) difference in cell cycle distribution (A) or luciferase activity (B) in treated versus untreated cells.
Eupafolin binds with PI3-K in vitro and ex vivo
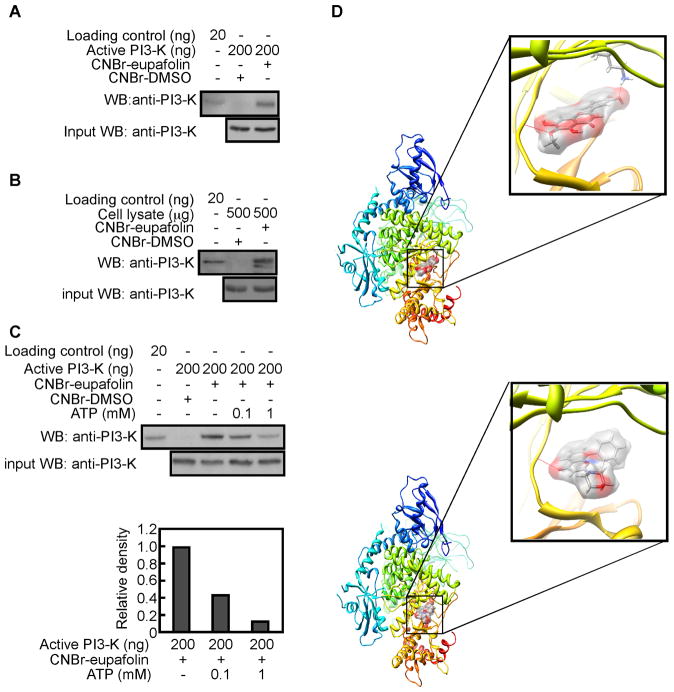
PI3-K plays an important role in Akt activation. We speculated that PI3-K might be a molecular target of eupafolin. To test this idea, we performed an ex vivo pulldown assay using eupafolin-conjugated Sepharose 4B beads and active PI3-K. Results revealed that eupafolin-conjugated beads, but not Sepharose 4B beads alone, could bind with PI3-K (Figure 4A). In addition, eupafolin could bind with endogenous PI3-K when eupafolin-conjugated beads were mixed with a PC3 cell lysate (Figure 4B). Further examination demonstrated that eupafolin competed with ATP for binding with PI3-K (Figure 4C). To better understand how eupafolin interacts with PI3-K beta, we performed a computer modeling study using the Glide docking program of Schrödinger Suite 2011. In the computer-docking model, eupafolin was shown to bind well at the ATP binding pocket of PI3-K beta (Figure 4D, upper panel). Comparison of the binding modes of eupafolin and LY294002, a well-known PI3-K inhibitor, using the same Glide program showed that the binding affinity was similar (−9.386 and −7.338 kcal/mol) for eupafolin and LY294002, respectively (Figure 4D, lower panel). This suggests that eupafolin might be a potential inhibitor of PI3-K beta. Note that some images were generated with the UCSF Chimera program [32].
Fig. 4.
Eupafolin binds to PI3-K. A, eupafolin binds to PI3-K in vitro. Active PI3-K (200 ng) was subjected to a pulldown assay with eupafolin conjugated with CNBr-Sepharose 4B beads. Eupafolin binding to PI3-K was visualized by Western blotting with a PI3-K antibody. B, eupafolin binds with PI3-K ex vivo. PC3 cell lysates (500 μg) were pulled down with CNBr or CNBr-eupafolin-conjugated beads. The pulled down proteins were visualized using antibodies to detect PI3-K. C, eupafolin binds with PI3-K in competition with ATP. Active PI3-K (200 ng) was subjected to a pulldown assay with eupafolin conjugated with CNBr-Sepharose 4B beads. Then the beads were incubated with different concentrations of ATP (0, 0.1, or 1 mM). Eupafolin binding to PI3-K was visualized by Western blotting with anti-PI3-K. D, upper panel, eupafolin (shown as surface representation with transparency of 50%) binds to the ATP binding pocket of PI3-K beta. Lower panel, LY294002 (shown as surface representation with transparency of 50%) binds to the ATP binding pocket of PI3-K beta.
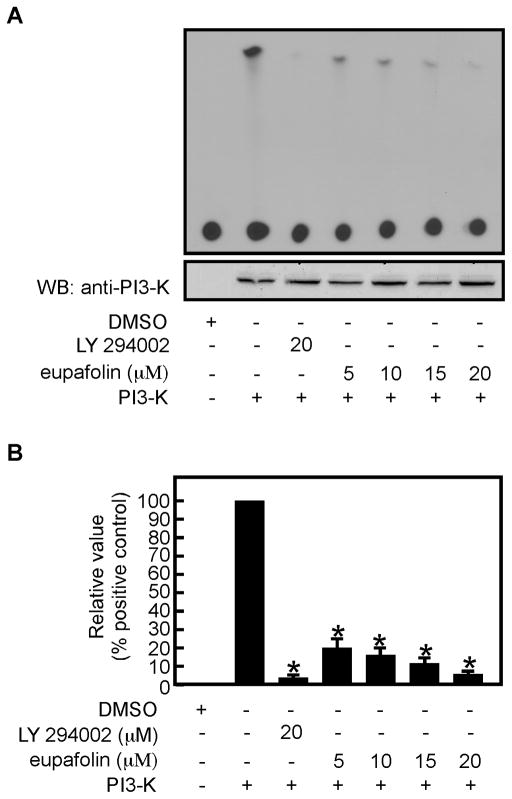
Eupafolin inhibits PI3-K activity in vitro
Based on the results showing that eupafolin could directly bind with PI3-K, we determined whether eupafolin could inhibit PI3-K kinase activity in vitro using phosphatidylinositol sodium salt as a substrate. We found that PI3-K activity was inhibited by eupafolin in vitro (Figure 5A, B). Next, we evaluated the effect of eupafolin on mTORC2 kinase activity using GST-Akt1 as a substrate. mTORC2 is a kinase complex that phosphorylates Akt at Ser473. The results showed that the phosphorylation of Akt1 at Ser473 was not affected by eupafolin (Supplementary Figure 3).
Fig. 5.
Eupafolin inhibits PI3-K kinase activity in vitro. A, the effect of eupafolin on PI3-K activity was measured by an in vitro kinase assay using PIP2 as the PI3-K substrate. 32P-labeled PIP3 was visualized by autoradiography as described in “Materials and Methods”. B, quantification of the effect of eupafolin on PI3-K kinase activity (from A).
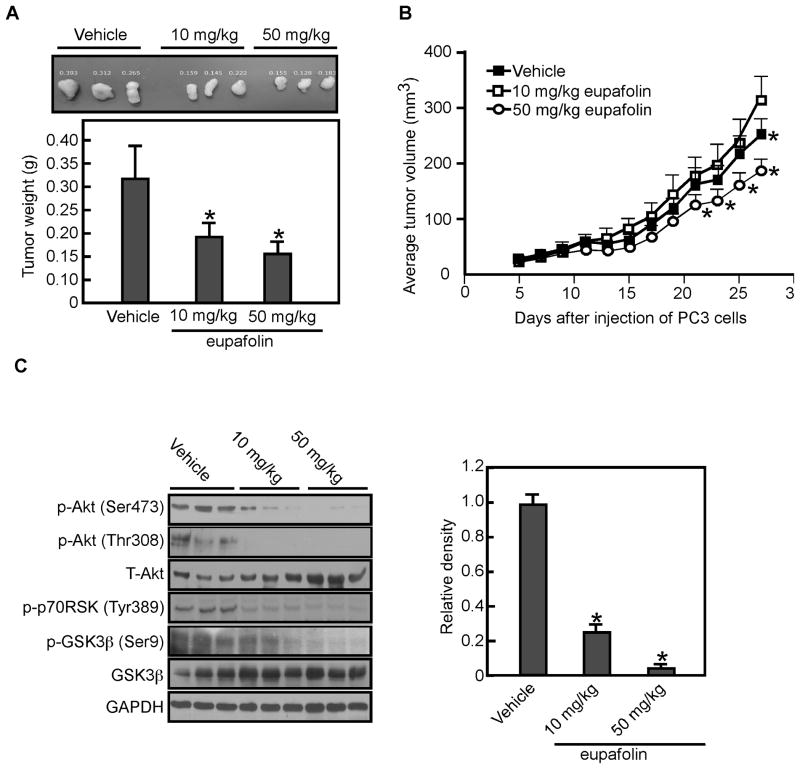
Eupafolin suppresses xenograft tumor growth by inhibiting PI3-K/Akt activation
Based on our in vitro and ex vivo results, we next determined whether eupafolin could suppress tumor growth in vivo. Results revealed that the mean tumor weight was decreased in eupafolin-treated groups (Figure 6A; p < 0.05) and the mean tumor volume in the vehicle-treated group increased faster than that in the eupafolin–treated groups (Figure 6B; p < 0.05). The body weights of the vehicle- and eupafolin-treated (50 mg/kg) mice were not different (32.9 ± 0.65 g vs 32.5 ± 0.48 g, p > 0.05). Tumor extracts from vehicle-treated and eupafolin-treated mice (i.e., euthanized on the same day of the experiment) were prepared and subjected to Western blot analysis to measure the phosphorylation of Akt. Results indicated that the eupafolin-treated tumor extracts exhibited substantially decreased Akt phosphorylation at Ser473 compared with vehicle-treated tumors (Figure 6C).
Fig. 6.
Eupafolin suppresses tumor growth in vivo by inhibiting the PI3-K-related signaling pathway. A, the total average tumor weight in the eupafolin-treated groups is significantly less (*, p < 0.05) than that of the vehicle-treated group. Tumors were extracted and weighed after mice were sacrificed. Data are shown as means ± S.D. and significant differences were determined by one-way ANOVA. B, total average tumor volume in the eupafolin-treated group was significantly (*, p < 0.05) less than that of the vehicle-treated group. Tumor volume was measured and recorded 3 times a week throughout the study. Data are shown as means ± S.D. C, the protein levels of total and phosphorylated Akt, p70RSK, and GSK3β were assessed by Western blot analysis in vehicle- and eupafolin-treated tumor tissues. The relative density was compared with actin (lower panel). Data are shown as means ± S.E.
Discussion
Accumulating evidence demonstrates that activation of signaling pathways by gene alterations plays a vital role at the hormone-independent stage of prostate cancer and tumor metastasis [1,33,34]. PTEN mutation or loss is frequently found in prostate cancer [35,36]. PTEN mutation/loss activates the PI3-K/Akt signaling pathway, which contributes to the tumorigenesis of prostate cells [37,38]. Emerging evidence indicates that the PTEN/PI3-K/Akt signaling pathway is a promising target for cancer treatment and prevention [39,40]. Therefore, small molecules that can suppress the PI3K/Akt signaling pathway are potential effective candidates for the prevention and/or treatment of prostate cancer. Eupafolin is a flavonoid from Eupatorium perfoliatum L. Previous research studies indicated that eupafolin affected mitochondrial energetic metabolism and caused cell apoptosis at high concentrations (25–200 μM) [23,41]. Our studies demonstrated that eupafolin could inhibit prostate cancer cell proliferation and anchorage-independent cell growth (Figure 1). Its activity was associated with G1 cell cycle arrest and inhibition of cyclin D1 transcription activity (Figure 3). These results indicated eupafolin has cellular targets other than mitochondrial targets.
The activation of Akt requires phosphorylation at both Thr308 by PDK1 and at Ser473 by mTORC2 [25,39]. PI3-K inhibition suppresses Akt phosphorylation at both sites. Although Akt phosphorylation at Thr308 downstream of PI3-K is known to occur through PDK1, the means by which PI3-K affects Akt phosphorylation at Ser473 remains elusive. Our data indicate that eupafolin inhibits Akt phosphorylation both at Ser473 and Thr308. Accordingly, the phosphorylation of GSK3β and p70RSK, downstream kinases of Akt, was decreased in a dose-dependent manner by eupafolin treatment. ERKs and its downstream signaling were not affected by eupafolin treatment. These data suggested that eupafolin could suppress cancer cell growth mediated through the PI3-K/Akt pathway.
Compounds can inhibit kinase activities through different mechanisms. We found that eupafolin could bind to PI3-K ex vivo and in vitro. The ATP competition assay and computational docking showed that eupafolin competes with ATP for binding to PI3-K, suggesting that eupafolin might also bind to the ATP pocket of PI3-K (Figure 4). Results of an in vitro PI3-K kinase assay using phosphatidylinositol sodium salt as a substrate indicated that eupafolin could inhibit PI3-K activity in a dose-dependent manner (Figure 5). All these data indicated that eupafolin inhibits PI3-K activity by competing with ATP.
In verifying that eupafolin has antitumor effects in vivo, we found that eupafolin inhibited growth of prostate cancer xenografts in mice without any apparent signs of toxicity. Consistent with the findings in cells, eupafolin treatment reduced the phosphorylation of Akt at Ser473 and its downstream targets p70S6K (Thr398) and GSK3β (Ser9). Based on these observations, eupafolin most probably exerts its potential cancer preventive/therapeutic effects directly through the PI-3K/Akt signaling pathway. Our data demonstrating that eupafolin down-regulates constitutive Akt kinase activity provide a rationale to develop eupafolin as a chemotherapeutic agent or in combination therapy to overcome the chemoresistance associated with prostate cancer (Figure 6A, B).
In conclusion, eupafolin inhibits prostate cancer cell proliferation and anchorage-independent cell growth. We also provide clear evidence showing that eupafolin can bind to PI3-K and inhibit its enzyme activity. Importantly, eupafolin effectively suppresses in vivo tumor growth in nude mice bearing PC3 cancer cells by inhibiting Akt kinase activity. The anticancer effect of eupafolin occurs through its direct targeting of PI3-K, consequently suppressing the activation of the PI3-K/Akt downstream signaling pathway.
Supplementary Material
Acknowledgments
This work was supported by The Hormel Foundation and National Institutes of Health grants CA120388, CA166011, CA172457, R37 CA081064, and ES016548., the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2010-0029233); Leap Research Program (No. 2010- 0029233); WCI: World Class Institute Program founded by the Korea Research Foundation, Ministry of Education, Science and Technology; or WCI 2009-002 National Natural Science Foundation of China No. 81372269, Science Foundation of Henan Education department (No. 13HASTIT022)
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Shen MM, Abate-Shen C. Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res. 2007;67(14):6535–6538. doi: 10.1158/0008-5472.CAN-07-1271. [DOI] [PubMed] [Google Scholar]
- 2.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121(7):1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 3.Warde N. Prostate cancer: loss of PTEN promotes progression of prostate cancer in an androgen-independent manner. Nat Rev Urol. 2011;8(8):412. doi: 10.1038/nrurol.2011.109. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Kheir WG, Hynes PG, Martin PL, Pierce R, Kelly K. Characterizing the contribution of stem/progenitor cells to tumorigenesis in the Pten-/-TP53-/- prostate cancer model. Stem Cells. 2010;28(12):2129–2140. doi: 10.1002/stem.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulholland DJ, Kobayashi N, Ruscetti M, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72(7):1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29(35):4947–4958. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7(6):591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 8.Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19(6):792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2006;103(39):14477–14482. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carver BS, Tran J, Gopalan A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41(5):619–624. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69(7):2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 12.Squire JA. TMPRSS2-ERG and PTEN loss in prostate cancer. Nat Genet. 2009;41(5):509–510. doi: 10.1038/ng0509-509. [DOI] [PubMed] [Google Scholar]
- 13.Sun X, Huang J, Homma T, et al. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer Res. 2009;29(5):1739–1743. [PubMed] [Google Scholar]
- 14.de Muga S, Hernandez S, Agell L, et al. Molecular alterations of EGFR and PTEN in prostate cancer: association with high-grade and advanced-stage carcinomas. Mod Pathol. 2010;23(5):703–712. doi: 10.1038/modpathol.2010.45. [DOI] [PubMed] [Google Scholar]
- 15.Agell L, Hernandez S, Salido M, et al. PI3K signaling pathway is activated by PIK3CA mRNA overexpression and copy gain in prostate tumors, but PIK3CA, BRAF, KRAS and AKT1 mutations are infrequent events. Mod Pathol. 2010 doi: 10.1038/modpathol.2010.208. [DOI] [PubMed] [Google Scholar]
- 16.de Souza PL, Russell PJ, Kearsley J. Role of the Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2009;9(2):163–175. doi: 10.2174/156800909787581006. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Bailey CG, Ng C, et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 2011;71(24):7525–7536. doi: 10.1158/0008-5472.CAN-11-1821. [DOI] [PubMed] [Google Scholar]
- 18.Kladney RD, Cardiff RD, Kwiatkowski DJ, et al. Tuberous sclerosis complex 1: an epithelial tumor suppressor essential to prevent spontaneous prostate cancer in aged mice. Cancer Res. 2010;70(21):8937–8947. doi: 10.1158/0008-5472.CAN-10-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–1545. [PubMed] [Google Scholar]
- 20.Hensel A, Maas M, Sendker J, et al. Eupatorium perfoliatum L: phytochemistry, traditional use and current applications. J Ethnopharmacol. 2011;138(3):641–651. doi: 10.1016/j.jep.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Miura K, Kikuzaki H, Nakatani N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L) measured by the oil stability index method. J Agric Food Chem. 2002;50(7):1845–1851. doi: 10.1021/jf011314o. [DOI] [PubMed] [Google Scholar]
- 22.Maas M, Deters AM, Hensel A. Anti-inflammatory activity of Eupatorium perfoliatum L. extracts, eupafolin, and dimeric guaianolide via iNOS inhibitory activity and modulation of inflammation-related cytokines and chemokines. J Ethnopharmacol. 2011;137(1):371–381. doi: 10.1016/j.jep.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 23.Chung KS, Choi JH, Back NI, et al. Eupafolin, a flavonoid isolated from Artemisia princeps, induced apoptosis in human cervical adenocarcinoma HeLa cells. Mol Nutr Food Res. 2010;54(9):1318–1328. doi: 10.1002/mnfr.200900305. [DOI] [PubMed] [Google Scholar]
- 24.Lee DE, Lee KW, Song NR, et al. 7,3′,4′-Trihydroxyisoflavone inhibits epidermal growth factor-induced proliferation and transformation of JB6 P+ mouse epidermal cells by suppressing cyclin-dependent kinases and phosphatidylinositol 3-kinase. J Biol Chem. 2010;285(28):21458–21466. doi: 10.1074/jbc.M109.094797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 26.Colburn NH, Wendel EJ, Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrödinger. Schrödinger Suite 2011. New York: Schrödinger, LLC; 2011. [Google Scholar]
- 28.Zhang X, Vadas O, Perisic O, et al. Structure of lipid kinase p110beta/p85beta elucidates an unusual SH2-domain-mediated inhibitory mechanism. Mol Cell. 2011;41(5):567–578. doi: 10.1016/j.molcel.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman HM, Westbrook J, Feng Z, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albanese C, Johnson J, Watanabe G, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270(40):23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 31.Shimura T. Acquired radioresistance of cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. J Radiat Res. 2011;52(5):539–544. doi: 10.1269/jrr.11098. [DOI] [PubMed] [Google Scholar]
- 32.Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 34.Jiao J, Wang S, Qiao R, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67(13):6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 35.Bar-Shira A, Matarasso N, Rosner S, Bercovich D, Matzkin H, Orr-Urtreger A. Mutation screening and association study of the candidate prostate cancer susceptibility genes MSR1, PTEN, and KLF6. Prostate. 2006;66(10):1052–1060. doi: 10.1002/pros.20425. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Yoshimoto M, Trpkov K, et al. Detection of ERG gene rearrangements and PTEN deletions in unsuspected prostate cancer of the transition zone. Cancer Biol Ther. 2011;11(6):562–566. doi: 10.4161/cbt.11.6.14376. [DOI] [PubMed] [Google Scholar]
- 37.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142(11):4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Ittmann MM, Ayala G, et al. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005;8(2):108–118. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 39.Hill KM, Kalifa S, Das JR, et al. The role of PI 3-kinase p110beta in AKT signally, cell survival, and proliferation in human prostate cancer cells. Prostate. 2010;70(7):755–764. doi: 10.1002/pros.21108. [DOI] [PubMed] [Google Scholar]
- 40.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118(9):3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrerias T, de Oliveira BH, Gomes MA, et al. Eupafolin: Effect on mitochondrial energetic metabolism. Bioorg Med Chem. 2008;16(2):854–861. doi: 10.1016/j.bmc.2007.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.