Abstract
Background
Prior studies reveal that bone mineral density (BMD) in congenital adrenal hyperplasia (CAH) is mostly in the osteopenic range and is associated with lifetime glucocorticoid dose. The forearm, a measure of cortical bone density, has not been evaluated.
Objective
We aimed to evaluate BMD at various sites, including the forearm, and the factors associated with low BMD in CAH patients.
Methods
Eighty CAH adults (47 classic, 33 nonclassic) underwent dual-energy-x-ray absorptiometry and laboratory and clinical evaluation. BMD Z-scores at the AP spine, total hip, femoral neck, forearm, and whole body were examined in relation to phenotype, body mass index, current glucocorticoid dose, average 5-year glucocorticoid dose, vitamin D, 17-hydroxyprogesterone, androstenedione, testosterone, dehydroepiandrosterone, and dehydroepiandrosterone sulfate (DHEAS).
Results
Reduced BMD (T-score < −1 at hip, spine, or forearm) was present in 52% and was more common in classic than nonclassic patients (P = .005), with the greatest difference observed at the forearm (P = .01). Patients with classic compared to nonclassic CAH, had higher 17-hydroxyprogesterone (P = .005), lower DHEAS (P = .0002), and higher non-traumatic fracture rate (P = .0005). In a multivariate analysis after adjusting for age, sex, height standard deviation, phenotype, and cumulative glucocorticoid exposure, higher DHEAS was independently associated with higher BMD at the spine, radius, and whole body.
Conclusion
Classic CAH patients have lower BMD than nonclassic patients, with the most affected area being the forearm. This first study of forearm BMD in CAH patients suggests that low DHEAS may be associated with weak cortical bone independent of glucocorticoid exposure.
Key terms: Congenital adrenal hyperplasia (CAH), osteopenia, dehydroepiandrosterone (DHEA)
Introduction
Congenital adrenal hyperplasia (CAH) is an autosomal recessive group of disorders characterized by impaired steroidogenesis and varying degrees of cortisol deficiency and androgen excess 1. The most common form of CAH is 21-hydroxylase (21OH) deficiency which presents in different clinical forms reflecting the severity of enzyme impairment1. Clinical phenotype is classified into classic [consisting of salt-wasting (SW) and simple-virilizing (SV) subtypes], and nonclassic CAH.
Management of classic CAH consists of life-long glucocorticoid (GC) replacement, and often presents a challenge because adequate androgen suppression may require GC over-replacement1. Nonclassic CAH patients may require GC therapy, but lifetime GC therapy is often not warranted 1. As such, patients with CAH are exposed to different hormonal derangements, which may affect bone mineral density (BMD) among various other metabolic and developmental factors.
Studies of BMD in adult CAH patients have reported conflicting findings, ranging from low BMD 2–4 to normal BMD 5, 6. Factors that have been associated with low BMD in CAH populations have included excess GC exposure and low adrenal androgens. In a study of 32 adult patients with CAH, Jaaskelainen et al found a significant negative correlation between GC exposure and lower BMD 7. Similarly, in a cohort of 13 women with CAH, Hagenfeldt et al found a strong negative association between BMD and calculated index of accumulated postmenarchal GC dose, but not between BMD and circulating androgen levels 8. King et al attributed low BMD to oversuppression of androgens (DHEA, DHEAS) in a cohort of 26 women with CAH treated with GC 9, with a trend of higher daily hydrocortisone equivalent dose in osteopenic (22 mg/m2) than in non-osteopenic CAH women (15 mg/m2) 9.
The aim of this study was to perform a comprehensive assessment of BMD status in adult patients with CAH, and evaluate risk factors associated with low BMD in a population of adult CAH patients. In addition to the typical sites evaluated in prior BMD studies in CAH patients (lumbar spine, hip, and WB areas), our study is the first to evaluate BMD of the forearm (distal radius), an assessment of cortical bone mass.
Subjects and Methods
Patients
Eighty adults (defined as age ≥ 20) enrolled in a Natural History Study of CAH at the National Institutes of Health Clinical Center in Bethesda, MD between 2006 and 2013 (www.ClinicalTrials.gov identifier no. NCT00250159) underwent a BMD DEXA scan 10. Subjects were recruited to be evaluated on a Natural History Study through listings on www.Clinicaltrials.gov, the national support group for CAH (Congenital Adrenal Hyperplasia Research Education & Support Foundation), or self-referral. Approximately 5% were physician referrals and around 15% patients were local to the Bethesda, Maryland area. The study was not designed or advertised to evaluate BMD. BMD was part of the comprehensive clinical evaluation performed. The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board and all patients provided written informed consent.
The study included 47 classic and 33 nonclassic patients with a mean age of 34.7 years (range: 20.1–69.8 years). Diagnoses were confirmed through biochemical and genetic testing. Phenotypic classification was determined based on clinical and hormonal criteria and has been previously reported 11.
Assays
Fasting blood was drawn in the morning before taking medication. Hormones were analyzed at the National Institutes of Health Clinical Center (Bethesda, MD) unless noted otherwise. Testosterone, androstenedione, 17-hydroxyprogesterone (17-OHP) and dehydroepiandrosterone (DHEA) were measured by liquid chromatography-tandem mass spectrometry (Mayo Medical Labs, Rochester, MN); dehydroepiandrosterone sulfate (DHEAS) by automated chemiluminescent immunometric assay (Mayo Medical Laboratories) until 2011 and by chemiluminescence immunoassay on Siemens Immulite 2500 analyzer (NIH) after 2011; parathyroid hormone (PTH) by electrochemiluminescence immunoassay on Roche Cobas e601 analyzer; and 25-hydroxyvitamin D by electrochemiluminescence binding assay on Roche Cobas.
Clinical Data and Definitions
Demographic, hormonal, and medication data was obtained from the review of medical records. Height standard deviation (SD) was obtained using data at 20 years of age from the National Health and Nutrition Examination Survey (NHANES). Body Mass Index (BMI) (kg/m2) was calculated for all patients. GC equivalencies were calculated based on prior studies in CAH: prednisone doses were multiplied by five and dexamethasone doses by 8011. Average daily GC dose over the past 5 years was calculated and corrected for body surface area. Androstenedione, testosterone, DHEA, and DHEAS were transformed into percentile of the corresponding normal range in order to age- and sex-adjust. Vitamin D deficiency was defined as 25-OH-vitamin D < 50 nmol/L12. Fracture data, reported as history of any lifetime fracture was obtained and further classified as traumatic or non-traumatic.
BMD was obtained using a dual-energy x-ray absorptiometry scan with the Hologic QDR4500A Instrument through 3/2011 or the Hologic Discovery instrument after 3/2011 (Hologic, Inc, Bedford, MA). The sites analyzed included AP spine, femoral neck, total hip, non-dominant distal 1/3rd radius, and whole body (WB). BMD measurements (g/cm2) at the AP spine and 1/3rd radius were compared to normal adults at peak bone mass (T-score) and sex- and age-matched adults (Z-score) using the Hologic reference database13. The T- and Z- scores for the femoral neck were calculated using the NHANES III reference data 14. The WB T- and Z- scores were calculated using sex and race (Black, White or Hispanic) specific reference data from NHANES 2008 15. Reduced BMD, based on the World Health Organization (WHO) definition of T-score <-1 at the hip, spine or forearm, 16 was used in order to compare results to prior studies of CAH adults2–4, 9, 17.
Statistical Analyses
Descriptive statistics were reported as percentage or as mean ± SD. Hormonal data was categorized for descriptive statistics only. Androstenedione, testosterone, and DHEAS were categorized as suppressed (below normal range), normal, or elevated (above normal range).
Demographic information and BMD Z-scores were compared between classic and nonclassic patients using t-test. Low BMD was defined as T-score < −1 at either the hip area (femoral neck or total hip), AP spine, or forearm, according to WHO definition 16. Continuous Z-score measures at each site were used for correlation analyses, since our cohort consistent mostly (88%) of young adults (6 postmenopausal women and 5 men age 50 or older), in concordance with the 2013 ISCD Official Position18. Independent variables evaluated included age, sex, clinical phenotype, BMI, height SD, 5- year GC exposure, vitamin D status, and hormonal levels including 17OHP, androstenedione, testosterone, DHEA, and DHEAS. 17OHP was log-transformed for inclusion in analysis because of lack of normality. Univariate analysis was performed to assess the association between fracture rate and the different exposure variables. Traumatic and finger fractures were excluded from the analyses.
Univariate analysis was performed to assess the association between BMD Z-scores and each independent variable for each of the 5 sites. Linear regression analysis was used to assess the association between BMD Z-scores and continuous independent variables; one-way ANOVA was used to determine the association between BMD Z-scores and categorical independent variables. Variables that were significantly associated with BMD were subsequently included in the multivariate regression model for each site. Variables tested included age, sex, height SD, BMI, current and 5-year average GC dose, hormonal status (17-OHP, androstenedione, DHEA, DHEAS, testosterone) and vitamin D status. All models were adjusted for age and sex. Additionally, adjustment for GC exposure was performed in the spine BMD model. Backward stepwise regression was performed to obtain variables that were significantly and independently associated with BMD (at each site) in the final model. P-value less 0.05 was considered statistically significant. Analysis was performed using JMP Statistical Software 10.0.2 (2012, SAS Institute).
Results
The cohort consisted of 80 adult patients (age > 20) with CAH. The majority (59 %) had classic CAH (Table 1). On average, classic compared to nonclassic patients, had a significantly lower height SD (P = .0003), similar BMI, and had been exposed to significantly higher doses of GC (P < .0001), especially the classic females. All classic patients were currently on GC medication and were being treated with a variety of regimens. Nonclassic patients who were receiving GC treatment were on regimens similar to the classic patients; however 36 % of nonclassic patients were not receiving GC treatment. One woman had taken a non-steroidal anti-androgen (bicalutamide) in the past (data not presented) and one woman was on bicalutamide at the time of the study. Five premenopausal women (2 nonclassic, 3 classic) were receiving spironolactone. Twelve women were on some form of estrogen, and 2 men were on testosterone replacement. Six women were postmenopausal. Out of 50 premenopausal women, 30 (60%) had regular menses, 15 had irregular menses, and 5 had unknown menstruation status. Five patients (4 classic and 1 nonclassic) had taken bisphosphonates for some length of time before their DEXA scan and prior to being seen at our center. Twenty-three patients (29%) were receiving vitamin D and/or calcium supplementation.
Table 1.
Clinical characteristics of adult patients with CAH by phenotype
| Variablei | Classic (N=47) | Nonclassic (N=33) | P-value |
|---|---|---|---|
| Age (years) | 32.4 (13) | 38.1 (12.4) | 0.05 |
| Female N (%) | 27 (58%) | 29 (88%) | 0.004* |
| Height SD | −1.20 (1.2) | −0.19 (1) | 0.0003* |
| BMI (Kg/m2) | 28.8 (6.9) | 27.3 (5.3) | 0.31 |
| Current GC | |||
| Hydrocortisone (mg/m2/day) | 17.7 (4.5) | 13.1 (10.6) | 0.17 |
| Dexamethasone (mcg/day) | 467 (207) | 464 (94.5) | 0.97 |
| Prednisone (mg/day) | 6.5 (3) | 6.3 (2.6) | 0.90 |
| Current GCii dose (mg/m2/day) | 18.3 (9.2) | 10.4 (10) | 0.0005** |
| Avg 5-year GC iii (mg/m2/day) | 17.9 (8.1) | 8.2 (8.6) | <0.0001*** |
| Anti-androgen (% females) | 4 (15%) | 3 (10%) | 0.61 |
| Vitamin D deficiency iv N (%) | 9 (27%) | 3 (12%) | 0.17 |
| Lifetime Fractures (non-traumatic) | 17 (36%) | 1 (3%) | 0.0005** |
Data presented as mean (SD) unless indicated otherwise.
Current glucocorticoid dose in hydrocortisone equivalents. Patients not receiving glucocorticoid excluded.
Average glucocorticoid dose in hydrocortisone equivalents over the past 5 years.
25-hydroxyvitamin D < 50 nmol/L.
P <.05.
P < .001.
P < .0001.
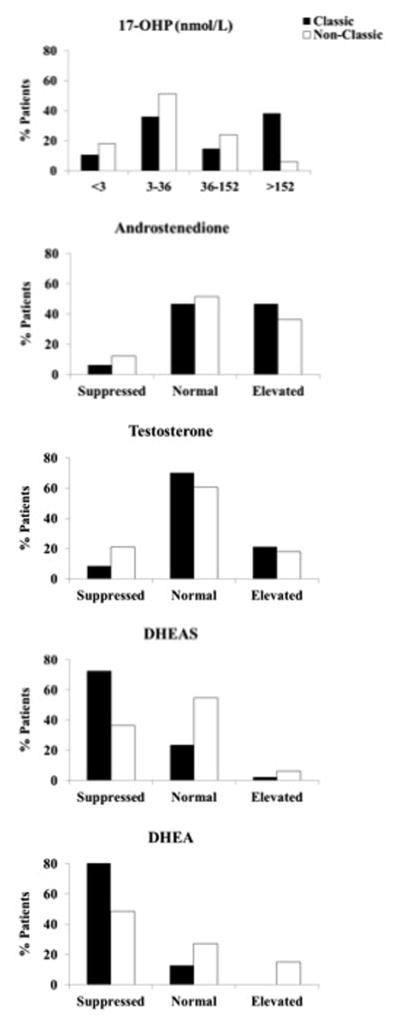
Classic patients compared to nonclassic patients had significantly higher 17-hydroxyprogesterone (P = .005), and significantly lower DHEA (P < .0001) and DHEAS (P = .0002) levels. Approximately half of classic and nonclassic patients had androstenedione within the normal range and the majority had normal testosterone levels (Figure 2B–C). Interestingly, despite the majority of patients having normal or elevated 17-hydroxyprogesterone, androstenedione and testosterone, the majority of classic patients had DHEA and DHEAS (72.3% and 89.4%, respectively) suppressed (Figure 2D–E). Of patients who had vitamin D levels measured, 20% (12/59) had vitamin D deficiency (defined as total 25-hydroxyvitamin D < 50 nmol/L) 12 however mean vitamin D or PTH were not statistically different among the 2 groups. All patients had normal PTH levels. The rate of non-traumatic fractures was significantly higher in classic as compared to nonclassic patients (36% vs. 3% respectively, P = .0005), with fractures involving different body parts with no obvious pattern. Apart from CAH phenotype, fracture rate was not significantly associated with any other variable on univariate analysis. Lower BMD at the radius (but not other sites) was significantly associated with non-traumatic fractures (P = .008) (data not shown).
Figure 2.
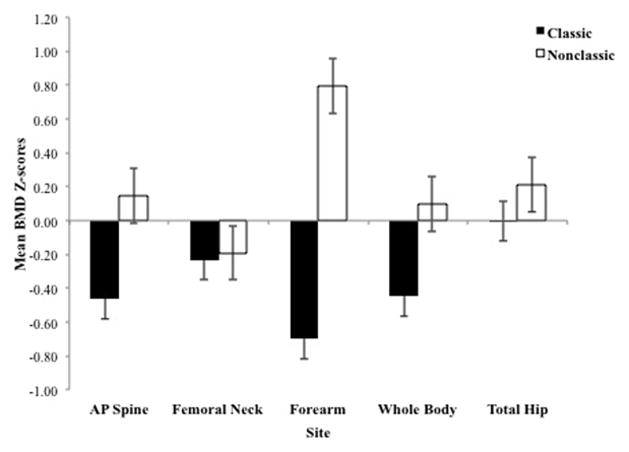
Low BMD at the spine, hip, or radius was more common in classic than nonclassic patients (65 vs. 33%; P = .005). Overall, patients with classic CAH tended to have lower BMD Z-scores than nonclassic patients at all sites; significant differences were observed at the AP spine (P = .015), forearm (P < .0001) and WB P = .048); with the greatest difference observed at the forearm (Figure 1). Similarly, low BMD, defined as T-score < −1, was more common in classic than nonclassic patients at the AP spine, (36% vs. 9% respectively, P = .006); forearm (37% vs. 12% respectively, P = .01), and WB (32% vs. 9% respectively, P = .02). Similar BMD was found at the other sites, namely total hip (13% vs. 21% respectively, P = .31) and femoral neck (23% vs. 27% respectively, P = .81). Women tended to have higher BMD Z-scores than men at all sites, however this was statistically different only at the spine (−0.01 ± 1.14 vs. −0.68 ± 0.9 respectively; P = .01) and forearm (0.24 ± 1.1 vs. −0.81 ± 2 respectively; P = .004).
Figure 1.
Correlates of BMD
Height SD was positively correlated with BMD at all sites (P < .05) whereas BMI was positively correlated with BMD only at the total hip and femoral neck (P < .05) (Table 2). Females had higher BMD than males, and age was positively related to BMD at the spine and radius even after adjusting for other variables in the multivariate analysis. DHEAS showed a significant positive correlation with BMD at the spine, radius, and WB in the multivariate analysis. DHEA was associated with total hip and femoral neck BMD however this association was no longer observed after adjustment for age, sex, height SD, and BMI. At sites where BMD differed between CAH subtypes (spine, radius, and WB), higher DHEAS was independently associated with higher BMD (Table 3). There was no correlation between height SD and DHEAS or DHEA. Due to the heterogeneous nature of the cohort, the data were re-analyzed excluding (6) post-menopausal women; followed by excluding (5) patients on bisphosphonates, and finally excluding both these groups in addition (2) men on testosterone, and all remaining women on estrogen replacement (final N =56). The final results including multivariable regression models were unchanged, with DHEAS remaining independently associated with BMD at the spine, radius, and WB.
Table 2.
Site-specific Z-score univariate regression models
| Variable | AP Spine | Forearm | Whole Body | Femoral Neck | Total Hip |
|---|---|---|---|---|---|
| Age | 0.02 (0.048)* | 0.03 (0.02)* | NS | NS | NS |
| Sex (F/M) | 0.66 (0.014)* | 1.05 (0.004)* | NS | NS | NS |
| Height SD | 0.3 (0.002)* | 0.46 (0.0004)** | 0.32 (0.002)* | 0.23 (0.005)* | 0.16 (0.046)* |
| BMI (kg/m2) | NS | NS | NS | 0.04 (0.01)* | 0.05 (0.003)* |
| Phenotype (NC/C) | 0.61 (0.015)* | 1.49 (<.0001)*** | 0.54 (0.047)* | NS | NS |
| GCi (mg/m2/day) | NS | NS | −0.04 (0.01)* | NS | NS |
| 17-OHP | NS | NS | NS | NS | NS |
| Androstenedione | NS | NS | NS | NS | NS |
| Testosterone | NS | NS | NS | NS | NS |
| DHEA | 0.82 (0.02)* | 1.4 (0.005)* | 1.07 (0.005)* | 0.89 (0.002)* | 0.74 (0.01)* |
| DHEAS | 1.05 (0.003)* | 1.62 (0.0007)** | 1.12 (0.002)* | NS | NS |
| Vitamin D deficiencyii | NS | NS | NS | NS | NS |
Data expressed as Estimate (P-value).
Average glucocorticoid dose in hydrocortisone equivalents over the past 5 years.
25-hydroxyvitamin D < 50 nmol/L.
P < .05.
P < .001.
P < .0001.
NS = Non-significant.
Table 3.
Site-specific Z-score multivariate regression models
| Variable | AP Spine i | Forearmii | Whole Bodyiii | Femoral Neckiv | Total Hipv |
|---|---|---|---|---|---|
| Age | 0.02 (0.02)* | 0.03 (0.006)* | NS | NS | NS |
| Sex (F/M) | 0.58 (0.02)* | 0.9 (0.005)* | NS | NS | NS |
| Height SD | 0.25 (0.01)* | 0.39 (0.002)* | 0.27 (0.008)* | 0.29 (0.0002)** | 0.22 (0.005)* |
| BMI (kg/m2) | NS | NS | 0.05 (0.001)* | 0.05 (0.0005)** | |
| Phenotype (NC/C) | NS | NS | NS | ||
| GCvi (mg/m2/day) | NS | NS | |||
| DHEA | NS | NS | |||
| DHEAS | 0.72 (0.04)* | 1.08 (0.01)* | 0.98 (0.006)* |
Data expressed as Estimate (P-value).
Final model R2 value = 0.29, R2 adjusted = 0.25, P < .0001, N= 78.
Final model R2 value = 0.39, R2 adjusted = 0.36, P < .0001, N= 76.
Final model R2 value = 0.27, R2 adjusted = 0.23, P = .0005, N= 66.
Final model R2 value = 0.24, R2 adjusted = 0.19, P = .0007, N= 78.
Final model R2 value = 0.22, R2 adjusted = 0.18, P = .001, N= 78.
Average glucocorticoid dose in hydrocortisone equivalents over the past 5 years.
Discussion
We performed evaluation of BMD at various sites in a well-established cohort of patients with CAH due to 21-hydroxylase deficiency. In this first study of forearm BMD in patients with CAH, we found that the greatest difference in BMD among classic and nonclassic CAH patients involves cortical BMD. In our comprehensive analyses of factors that may influence BMD, only DHEAS was independently (positively) associated with BMD at sites that displayed significant differences between classic and nonclassic CAH patients, suggesting that lifetime DHEAS exposure may play a role in determining bone mass. These findings provide insight into hormonal influences on bone mineral density and the long-term implications of the multiple hormonal imbalances characteristic of CAH.
Using the WHO definition of reduced BMD 16, the prevalence of reduced BMD in previous CAH studies ranges between 45–60% 3, 4, 17 as compared to 52% in our cohort. We found lower BMD in classic compared to nonclassic patients and this has been reported 4, 17, 19. Unique to our study was the evaluation of BMD at the forearm, an assessment of cortical bone. We did not find a correlation between GC exposure and BMD, similar to some prior studies 19, 20. However, because of the well-established adverse effects of GC on trabecular bone, we controlled for GC in our final model for spine BMD nevertheless.
A major finding of our study was the correlation between DHEAS and BMD, particularly at the radius. A limited number of studies have evaluated DHEA or DHEAS in relation to trabecular or mixed trabecular/cortical BMD in CAH patients with mixed results. No relationship between DHEA and BMD was found in 11 patients with CAH 5, and 17 pediatric patients with classic CAH 21; although CAH patients in these two studies had normal BMD. A third study of 28 Romanian children and young adults with CAH evaluated BMD only at the spine 22 and also did not find a correlation between adrenal metabolites, including DHEA, and BMD. Small cohort size and the young age of these patient populations may have contributed to the findings. Conversely, one study of 26 adult women with classic CAH, including 12 postmenopausal women, found that oversuppression of adrenal androgens was associated with increased risk for bone loss 9. Those with osteopenia (45% of SW and 13% of SV) had significantly lower DHEAS and were taking higher GC dose as compared to those with normal BMD, suggesting that oversuppression of adrenal androgens was due to excessive GC exposure. Interestingly, we found that low DHEAS had an independent adverse effect on bone (spine, radius, and WB) after controlling for GC exposure in our final multivariate models.
DHEAS and other adrenal androgens affect bone homeostasis throughout life and particularly during adrenarche, with a main effect on cortical bone. DHEA exerts is effect on bone by binding to the androgen receptor (AR) and stimulating osteoblast growth and differentiation 23; and a direct effect on osteoblasts has been suggested by a DHEA-specific receptor in mouse studies 24. The potential effect of DHEA on bone accrual was initially noted in girls with premature adrenarche, who were found to have higher bone mineral mass and density as compared to controls 25. Subsequent studies of healthy children confirmed that adrenal androgens play a role in periosteal bone deposition 26. In a cohort of 205 children, Remer et al examined cortical bone quality (including periosteal circumference, cortical density and area, and BMC) using peripheral quantitative CT (qCT) alongside measurement of 24-hour urinary adrenal metabolites. DHEA and its metabolites proved to be significant independent determinants of cortical density, particularly before the activation of pubertal sex hormones 26. In a cross-sectional and longitudinal study of 116 healthy males and females, DHEAS has been found to be negatively correlate with bone turnover before peak mass, which could further account for its role in skeletal bone maturation27.
Adrenarche is characterized by the onset of DHEA and DHEAS secretion by the zona reticularis that precedes pubarche in healthy children of both sexes. An important step in conversion of 17-hydoxypregnenolone to DHEAS is the activation of the enzyme 17, 20 lyase in addition to inhibition of 3βHSD in the zona reticularis 28. Factors that trigger adrenarche are not fully understood, but cortisol has been shown to inhibit 3βHSD in cultured cells, and a rise in intra-adrenal cortisol during childhood has been implicated in the regulation of adrenal DHEA secretion and may contribute to the initiation of adrenarche 29. Lack of intra-adrenal cortisol secretion has been shown to result in aberrant adrenal development with lack of zonation of the adrenal cortex in patients with classic salt-wasting CAH 30. This does not occur in patients with nonclassic CAH 31; patients with the most severe salt-wasting form of CAH have the most developmental impairment of the adrenal glands.
Interestingly, children with classic CAH fail to have a physiological rise in DHEAS levels during childhood, effectively accounting for absence of a typical adrenarche 32, 33. This is likely due to a development defect in the adrenal gland 30. This is further supported by the fact that patients with classic CAH have low DHEAS levels in the first year of life, prior to and unrelated to treatment control, as evidenced by untreated patients with classic CAH who have low DHEAS levels despite high 17-OHP and T levels 33. As such, low DHEAS levels in our cohort were not solely a reflection of GC control, but a well-known phenomenon of blunted DHEA secretion in patients with classic CAH. In one study of 22 women with classic CAH, DHEAS levels were found to be suppressed in a subset of 5 under-treated patients with elevated 17-OHP 34, supporting the notion that low DHEAS found in classic CAH patients is not solely a GC treatment effect. Patients with nonclassic CAH on the other hand may have high DHEAS levels upon diagnosis (as compared to controls) that subsequently decline upon treatment; however over time remain higher than patients with classic CAH but lower than unaffected subjects 35. As DHEA peaks higher in men, blunted adrenarche could affect BMD in men more profoundly than women; this might contribute to the lower BMD in CAH-affected men than women, as was seen in our cohort and others 17.
Our findings of low BMD in CAH patients is in agreement with other studies, however it is unknown whether this would translate to poor bone quality or higher fracture risk. Our study found that the lifetime, non-traumatic fracture rate was significantly higher in classic as compared to nonclassic patients, however, the nature, time, and severity of these fractures was unknown, and therefore any association with hormonal status or findings cannot be inferred. To date, only one study has found an increased fracture risk in adult women with CAH as compared to healthy controls, fractures particularly involved the wrist and vertebrae were found.4 Similarly, we found that CAH patients had the lowest Z-scores at the forearm (wrist) and spine. These same sites, and along with WB, also showed a significant correlation with DHEAS levels. Whether this would result in a higher fracture risk of these areas in our population is unknown, but the forearm is one of the most common sites for osteoporotic fractures in the middle aged and elderly women 36. Lower BMD at the radius was associated with non-traumatic fracture (nonspecific) in our study, however future studies evaluating BMD in patients with CAH should involve measures of the distal areas, and evaluate the prevalence of wrist in addition to other bone fractures.
Our study has several limitations. It is a cross-sectional study with limited historical data, and involves a heterogeneous sample. As the study was not designed to evaluate BMD per se, data on exercise or family history of osteoporosis was not consistently available. Data on lifetime exposure to GC was not available, so assumptions were made based on recent 5-year exposure as representative of long-term GC exposure. Glucocorticoid effects on bone are likely to be slow and it is possible that 5-year exposure data was not an accurate reflection of long-term glucocorticoid effect. We did not have bone turnover markers to further evaluate bone dynamics. Finally, BMD measures may not necessarily be reflective of bone quality, or risk of fractures.
In summary, we found that patients with classic CAH had lower BMD than patients with nonclassic CAH, with the greatest difference residing at the forearm. This is the first study to evaluate forearm BMD in CAH, which in turn reflects cortical bone. DHEAS was the only independent significant predictor of BMD at the forearm, WB and AP spine. Although this association does not imply causality, the finding of low cortical bone in association with low DHEAS in classic patients may be partially explained by disease-related DHEAS deficiency. Future studies of BMD in patients with CAH should include measurement on BMD at the forearm, with the goal of understanding the underlying etiology of cortical bone differences in CAH subgroups. Further studies to evaluate bone quality at the forearm using quantitative CT measures should be done to evaluate fracture risk. Our findings provide insight into the multiple hormonal determinants of BMD and thus may help guide future osteoporosis preventative strategies.
Acknowledgments
This work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child health and Human Development (NICHD) and the National Institutes of Health Clinical Center. DPM is a Commissioned Officer in the United States Public Health Service.
Footnotes
Disclosure Statement: D.P.M., D.E., S.C., M.P., and J.C.R. have nothing to disclose.
References
- 1.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, Stimson RH, Walker BR, Connell JM, Ross RJ. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–5121. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, Samara D, Nihoul-Fekete C, Kuttenn F, Polak M, Touraine P. Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2007;67:268–276. doi: 10.1159/000098017. [DOI] [PubMed] [Google Scholar]
- 4.Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjold A, Hagenfeldt K, Thoren M. Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:4643–4649. doi: 10.1210/jc.2007-0744. [DOI] [PubMed] [Google Scholar]
- 5.Guo CY, Weetman AP, Eastell R. Bone turnover and bone mineral density in patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 1996;45:535–541. doi: 10.1046/j.1365-2265.1996.00851.x. [DOI] [PubMed] [Google Scholar]
- 6.Gussinye M, Carrascosa A, Potau N, Enrubia M, Vicens-Calvet E, Ibanez L, Yeste D. Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics. 1997;100:671–674. doi: 10.1542/peds.100.4.671. [DOI] [PubMed] [Google Scholar]
- 7.Jaaskelainen J, Voutilainen R. Bone mineral density in relation to glucocorticoid substitution therapy in adult patients with 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 1996;45:707–713. doi: 10.1046/j.1365-2265.1996.8620871.x. [DOI] [PubMed] [Google Scholar]
- 8.Hagenfeldt K, Martin Ritzen E, Ringertz H, Helleday J, Carlstrom K. Bone mass and body composition of adult women with congenital virilizing 21-hydroxylase deficiency after glucocorticoid treatment since infancy. Eur J Endocrinol. 2000;143:667–671. doi: 10.1530/eje.0.1430667. [DOI] [PubMed] [Google Scholar]
- 9.King JA, Wisniewski AB, Bankowski BJ, Carson KA, Zacur HA, Migeon CJ. Long-term corticosteroid replacement and bone mineral density in adult women with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91:865–869. doi: 10.1210/jc.2005-0745. [DOI] [PubMed] [Google Scholar]
- 10.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96:E161–172. doi: 10.1210/jc.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 13.Kelly TL. Bone mineral reference databases for American men and women. J Bone Min Res. 1990;5:249. [Google Scholar]
- 14.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 15.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42:467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Chakhtoura Z, Bachelot A, Samara-Boustani D, Ruiz JC, Donadille B, Dulon J, Christin-Maitre S, Bouvattier C, Raux-Demay MC, Bouchard P, Carel JC, Leger J, Kuttenn F, Polak M, Touraine P. Impact of total cumulative glucocorticoid dose on bone mineral density in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2008;158:879–887. doi: 10.1530/EJE-07-0887. [DOI] [PubMed] [Google Scholar]
- 18.ISCD Official Positions - Adult. The International Society for Clinical Densitometry; Tampa, FL: 2013. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/ [Google Scholar]
- 19.Paganini C, Radetti G, Livieri C, Braga V, Migliavacca D, Adami S. Height, bone mineral density and bone markers in congenital adrenal hyperplasia. Horm Res. 2000;54:164–168. doi: 10.1159/000053253. [DOI] [PubMed] [Google Scholar]
- 20.Sciannamblo M, Russo G, Cuccato D, Chiumello G, Mora S. Reduced bone mineral density and increased bone metabolism rate in young adult patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91:4453–4458. doi: 10.1210/jc.2005-2823. [DOI] [PubMed] [Google Scholar]
- 21.Fleischman A, Ringelheim J, Feldman HA, Gordon CM. Bone mineral status in children with congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2007;20:227–235. doi: 10.1515/jpem.2007.20.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann A, Sido PG, Schulze E, Al Khzouz C, Lazea C, Coldea C, Weber MM. Bone mineral density and bone turnover in Romanian children and young adults with classical 21-hydroxylase deficiency are influenced by glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 2009;71:477–484. doi: 10.1111/j.1365-2265.2008.03518.x. [DOI] [PubMed] [Google Scholar]
- 23.Kasperk CH, Wakley GK, Hierl T, Ziegler R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J Bone Miner Res. 1997;12:464–471. doi: 10.1359/jbmr.1997.12.3.464. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Wang YD, Wang WJ, Zhu Y, Li DJ. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J Mol Endocrinol. 2007;38:467–479. doi: 10.1677/jme.1.02173. [DOI] [PubMed] [Google Scholar]
- 25.Sopher AB, Thornton JC, Silfen ME, Manibo A, Oberfield SE, Wang J, Pierson RN, Jr, Levine LS, Horlick M. Prepubertal girls with premature adrenarche have greater bone mineral content and density than controls. J Clin Endocrinol Metab. 2001;86:5269–5272. doi: 10.1210/jcem.86.11.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remer T, Boye KR, Hartmann M, Neu CM, Schoenau E, Manz F, Wudy SA. Adrenarche and bone modeling and remodeling at the proximal radius: weak androgens make stronger cortical bone in healthy children. J Bone Miner Res. 2003;18:1539–1546. doi: 10.1359/jbmr.2003.18.8.1539. [DOI] [PubMed] [Google Scholar]
- 27.Walsh JS, Henry YM, Fatayerji D, Eastell R. Hormonal determinants of bone turnover before and after attainment of peak bone mass. Clin Endocrinol (Oxf) 2010;72:320–327. doi: 10.1111/j.1365-2265.2009.03606.x. [DOI] [PubMed] [Google Scholar]
- 28.Dardis A, Saraco N, Rivarola MA, Belgorosky A. Decrease in the expression of the 3beta-hydroxysteroid dehydrogenase gene in human adrenal tissue during prepuberty and early puberty: implications for the mechanism of adrenarche. Pediatr Res. 1999;45:384–388. doi: 10.1203/00006450-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Topor LS, Asai M, Dunn J, Majzoub JA. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J Clin Endocrinol Metab. 2011;96:E31–39. doi: 10.1210/jc.2010-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, Van Wyk JJ, Bornstein SR. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343:1362–1368. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, Green-Golan L, VanRyzin C, Drinkard B, Mehta SP, Weise M, Eisenhofer G, Merke DP. Adrenomedullary function in patients with nonclassic congenital adrenal hyperplasia. Horm Metab Res. 2010;42:607–612. doi: 10.1055/s-0030-1253385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sellers EP, MacGillivray MH. Blunted adrenarche in patients with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Res. 1995;21:537–544. doi: 10.1080/07435809509030471. [DOI] [PubMed] [Google Scholar]
- 33.Volkl TM, Ohl L, Rauh M, Schofl C, Dorr HG. Adrenarche and puberty in children with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2011;76:400–410. doi: 10.1159/000333696. [DOI] [PubMed] [Google Scholar]
- 34.Helleday J, Siwers B, Ritzen EM, Carlstrom K. Subnormal androgen and elevated progesterone levels in women treated for congenital virilizing 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1993;76:933–936. doi: 10.1210/jcem.76.4.8473408. [DOI] [PubMed] [Google Scholar]
- 35.Brunelli VL, Chiumello G, David M, Forest MG. Adrenarche does not occur in treated patients with congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 1995;42:461–466. doi: 10.1111/j.1365-2265.1995.tb02663.x. [DOI] [PubMed] [Google Scholar]
- 36.Dhainaut A, Hoff M, Syversen U, Haugeberg G. Cortical hand bone porosity and its association with distal radius fracture in middle aged and elderly women. PLoS One. 2013;8:e68405. doi: 10.1371/journal.pone.0068405. [DOI] [PMC free article] [PubMed] [Google Scholar]