Abstract
Pancreatic cancer is one of the most aggressive and intractable human malignant tumors and a leading cause of cancer-related deaths across the world, with incidence equaling mortality. Because of the extremely high malignance, this disease is usually diagnosed at its advanced stage and recurs even after surgical excision. Pancreatic adenocarcinoma is generally thought to arise from pathological changes of pancreatic duct, and the pancreatic ductal adenocarcinoma (PDA) accounts for more than 90% of malignant neoplasms of the pancreas. To date, scientists have revealed several risk factors for pancreatic cancer, including smoking, family history, and ageing. However, the underlying molecular mechanism remains unclear. Meanwhile, more mutations of DNA damage response factors have been identified in familial pancreatic cancers, implying a potential link between DNA damage and pancreatic cancer. DNA damage is a recurring phenomenon in our bodies which could be induced by exogenous agents and endogenous metabolism. Accumulated DNA lesions cause genomic instability which eventually results in tumorigenesis. In this study, we showed obvious DNA damages existed in human pancreatic cancer, which activated DNA damage response and the DNA repair pathway including ATM, DNA-PK, CHK1 and CHK2. The persistent DNA damage in pancreatic tissue may be the source for its tumorigenesis.
Keywords: DNA damage response, pancreatic cancer, checkpoint
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in America and twelfth worldwide. With a five-year survival rate of less than 3% and an average survival of less than six months, this disease ranks the highest rate of death of any form of cancer, surpassed only by lung cancer (Bardeesy and DePinho 2002; Rustgi 2014).Because of the absence of specific symptoms and limitations in diagnostic methods, pancreatic cancer often eludes detection during its formative stages (Eckel et al. 2006; Mohammed et al. 2012).
There are two broad classifications of pancreatic cancer, dependent on whether or not the cancer affects the exocrine or endocrine functions of the pancreas. The most common form of pancreatic cancer is that affects exocrine functions of the pancreas (Bardeesy and DePinho 2002; Garcea et al. 2005). Of these, around 95% are classified as adenocarcinoma or pancreatic ductal adenocarcinoma (PDA), which typically arises in ducts of the pancreas (Warshaw and Fernandezdel Castillo 1992).PDA is a highly aggressive malignancy showing significant resistance to chemotherapies (Bardeesy and DePinho 2002).Annually, the incidence of PDA closely matches its mortality, highlighting the inefficacy of existing treatment options (Jemal et al. 2006), and this malignancy will be the second leading cause of cancer-related mortality in the next decade (Ma and Jemal 2013).Many efforts were made to reveal the cellular events and specific biochemical reactions of pancreatic cancer, especially the PDA. However, at this time the exact pathology remains unclear.
It is a well-known fact that DNA damage is a recurring phenomenon in biology and is a general precipitating factor of cancer. Our genome encounters a large amount of DNA damages per day, which can be induced by exogenous physical agents, spontaneous chemical reactions, and products of endogenous metabolism (Ciccia and Elledge 2010). To cope with these threats, cells employ a DNA damage response system to detect DNA damage, activate the cell cycle checkpoint, and initiate DNA repair process. Errors in DNA damage response lead to accumulated DNA lesions and induce genomic instability which eventually results in tumorigenesis (Jackson and Bartek 2009; Lord and Ashworth 2012). In particular, germline mutations in some of the DNA repair genes have been discovered in pancreatic cancer (Rustgi 2014), implying that unrepaired DNA lesions may be a reason for inducing pancreatic cancers. Notably, around 10% of sporadic PDAs were shown to harbor mutations of BRCA2 (Ozcelik et al. 1997), a tumor suppressor which functions for DNA break repair during homologous recombination (HR)(Zhang et al. 2009; Patel et al. 1998).As a functional partner of BRCA2, PALB2 also plays a crucial role in HR dependent DNA damage repair (Zhang et al. 2009). In a study of nearly 100 families with hereditary pancreatic cancer, four families were found to bear protein-truncating mutations in PALB2 (Jones et al. 2009). Moreover, germline heterozygous ATM mutations were found in the families with hereditary pancreatic cancer (Roberts et al. 2012), which further links DNA damages to pancreatic cancers since ATM is a core kinase in DNA damage response network and impairment of ATM could result in the failure of DNA repair (Matsuoka et al. 2007; Morrison et al. 2000). In this study, by using the pancreas samples from human, we observed obvious DNA damages in PDA pancreas. Meanwhile, DNA damage response and different repair pathways were also found to be activated in these cancer samples. Our results showed a general elevated DNA damage response in human pancreatic cancer, and the accumulated DNA lesions could be an original source of pancreatic tumorigenesis.
Material and Methods
Chemicals and Antibodies
All chemicals and media were purchased from Sigma Chemical Company (St. Louis, MO) except for those specifically mentioned. Anti- pATM (4526), pCHK1 (2341), pCHK2 (2661), antibodies were purchased from Cell Signaling. Anti- ATM (ab2631), CHK1 (ab47574), CHK2 (ab8108), and pDNA-PK (ab18192) were purchased from Abcam. And anti- β-actin (A5441) antibody was from Sigma.
Samples
Nine samples of pancreatic tissues were collected from the Department of Pathology, Xuanwu Hospital, Capital Medical University, between January and August 2013. In details, six tissues from the patients were harvested following the criteria: (1) histologic diagnosis of pancreatic ductal adenocarcinoma (moderate differentiation); (2) pancreatic tumor was primary, and the tumor size ranged from 3 cm to 5 cm; (3) tumor samples derived from biopsy or surgical resection specimens were suitable for immunohistochemical assessment; (4) patients received radiotherapy or chemotherapy before biopsy or surgery were excluded. Three normal adult pancreatic tissues were obtained from organ donors who were free from any history of malignancy and died from acute myocardial infarction. All the human pancreatic tissues used in this study were harvested after obtaining approval from the ethics committees at Capital Medical University and University of Michigan and from the patients who gave written informed consent.
Hematoxylin and Eosin (H&E) Staining
Pancreatic tissues from both experimental and control samples were fixed overnight in 10% neutral buffered formalin, embedded in paraffin, and sectioned. Embedding and sectioning were performed by the University of Michigan Microscopy & Image Analysis Core. Sections were then subjected to hematoxylin and eosin staining.
Immunofluorescence Microscopy
Immunofluorescence was performed as described previously (Collins et al. 2012). For staining of γH2AX, pSQ/TQ, RAD51, or KU70, tissues were fixed in 4% paraformaldehyde in PBS (pH 7.4) for at least 3 hours at room temperature. After being permeabilized with 0.5% Triton X-100 at room temperature for 30 minutes, tissues were blocked in 1% BSA-supplemented PBS for 1 hour and incubated overnight at 4°C with the indicated antibodies, respectively. After three washes in PBS containing 0.1% Tween 20 and 0.01% Triton X-100 for 5 minutes each, the tissues were labeled with 1:500 FITC-conjugated IgG or Rho-conjugated IgG for 1 hour at room temperature. After washing in PBS containing 0.1% Tween 20 and 0.01% Triton X-100, the tissues were co-stained with Hoechst 33258 (10 mg/ml in PBS). Finally, the tissues were mounted on glass slides and examined with a fluorescent microscope (Olympus, Japan).
Western Blot
Protein samples from the tissues were extracted by using total protein extraction kit (Millipore, #2140). The proteins were separated by SDS-PAGE and then electrically transferred to polyvinylidene fluoride membranes. Following transfer, the membranes were blocked in TBST (TBS containing 0.1% Tween 20) containing 5% skimmed milk for 2 hours, followed by incubation overnight at 4°C with the indicated antibodies, respectively. After washing in TBST, the membranes were incubated for 1 hour at room temperature with 1:1000 horseradish peroxidase (HRP)-conjugated IgG. To detect total ATM, CHK1 or CHK2, the membranes were washed in the washing buffer (100 mM β-mercaptoethanol, 20% SDS, and 62.5 mM Tris, pH 6.7) for 30 minutes at 55°C, and then subjected to another round of incubation. Finally, the membranes were detected by the enhanced hemiluminescence detection system (Amersham, Piscataway, NJ).
Statistical analysis
All the experiments were performed at least three times. Results were analyzed using unpaired two-tailed Student’s t test and data expressed as mean ± s.d. p values less than 0.05 were considered statistically significant.
Results and Discussion
Occurrence of DNA damages in pancreas cancer
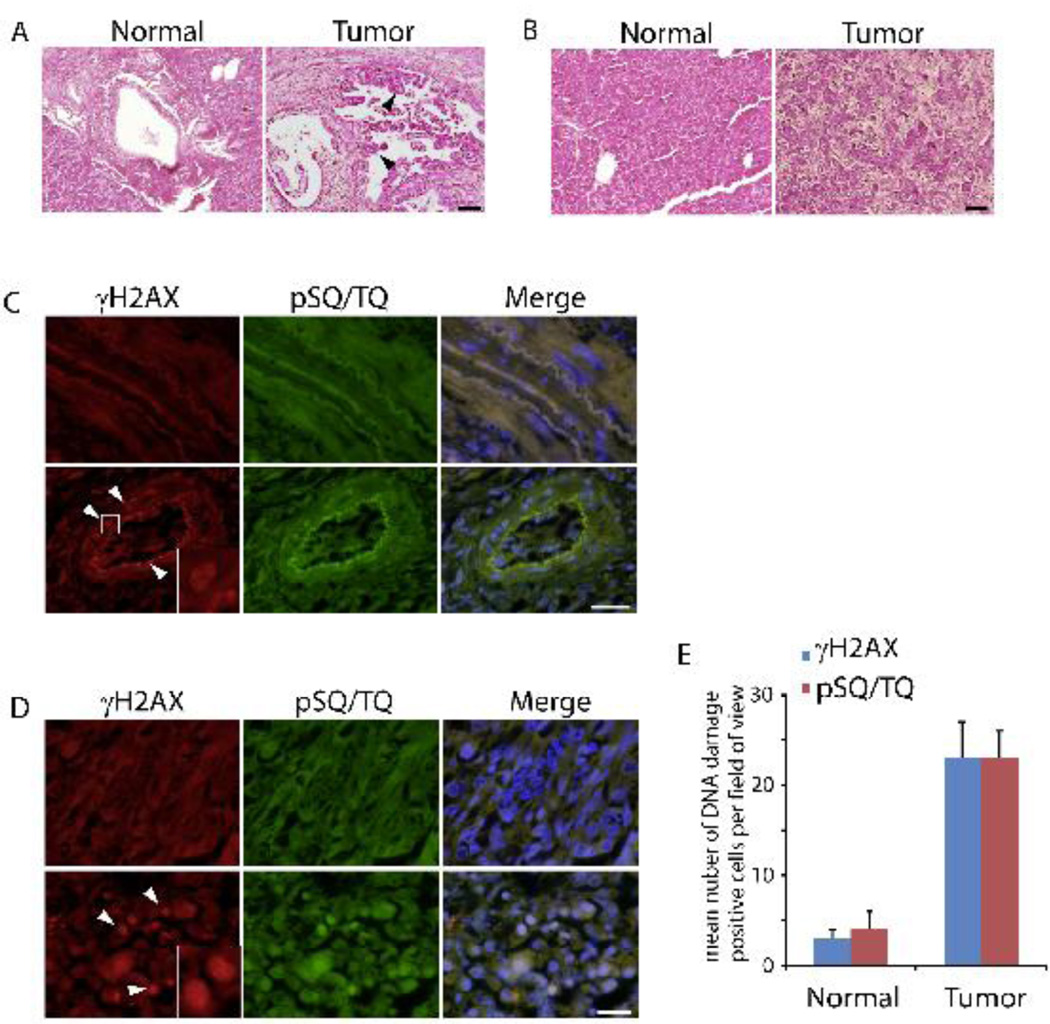
The six patients with pancreatic tumor (Table 1) included 4 male and 2 female patients with a mean age of 55.8 years (T1-T6), and all had clinical symptoms, such as jaundice and hepatomegaly. Three patients had lymph node metastasis and one had liver metastasis. The three control patients (Table 1) include 2 male and 1 female with a mean age of 53.7 years (C1-C3). Once the tissues received, each sample was divided into three groups for subsequent experiments. Figure 1A shows the representative morphologies of the normal pancreatic duct and the duct of pancreatic cancer with H&E Staining. The wall of the duct that originally neatly surrounded the normal sample became considerably thicker in the tumor sample. This change indicated the obvious adenocarcinoma of the duct, which may originate from pancreatic intraepithelial neoplasias (PanIN). For the body of the pancreas (non-duct part), obvious fibrosis was found in the PDA sample compared to the regular cells in the normal pancreas. Also, the cells were misaligned, jagged, and the entire structure of the outer lining of the pancreas seemed damaged (Figure 1B). These results indicate the severe cancerous cells in patient pancreatic tissues.
Table 1.
Characteristics of the patients and tumors
| No. | Sex | Age | Tumor size (cm) |
Differitiation | Lymph node metastasis |
Liver metastasis |
|---|---|---|---|---|---|---|
| T1 | M | 54 | 4.2 | moderate | − | − |
| T2 | F | 52 | 3.7 | moderate | + | − |
| T3 | M | 58 | 3.2 | moderate | + | − |
| T4 | M | 53 | 4.4 | moderate | − | − |
| T5 | F | 58 | 4.7 | moderate | − | − |
| T6 | M | 60 | 3.6 | moderate | + | + |
| C1 | M | 53 | - | - | − | − |
| C2 | M | 51 | - | - | − | − |
| C3 | F | 57 | - | - | − | − |
Figure 1. DNA damage in human pancreatic cancers.
(A) H&E staining of duct histomorphology was performed from normal (C1-C3) and tumor (T1-T4, and T6) pancreatic tissues. Representative images from C2 and T4 are shown. Arrowheads indicate the carcinoma area in duct. (B) H&E staining of pancreatic body histomorphology was performed from normal (C1-C3) and tumor (T1-T6) pancreatic tissues. Representative images from C1 and T3 are shown. (C) Immunostaining of phosphorylated H2AX (γH2AX) and SQ/TQ motif (pSQ/TQ) of duct was performed from normal (C1-C3) and tumor (T2, T4, and T5) pancreatic tissues. Representative results from C3 and T3 are shown. (D) Immunostaining of phosphorylated H2AX (γH2AX) and SQ/TQ (pSQ/TQ) of pancreatic body was performed from normal (C2 and C3) and tumor (T1, T2, and T6) pancreatic tissues. Representative results from C2 and T2 are shown. Enlarged box denotes γH2AX foci. Scale bar: 20 µm. (E) Numbers of positive γH2AX and pSQ/TQ cells around the duct in normal pancreas (C1-C3) and pancreatic tumor (T2, T4, and T5) were counted. Three independent experiments were averaged. The error bars represent the SD.
To investigate the link between DNA damage and pancreatic cancers, we tested the existence of DNA damages in these pancreas tissues. It is well realized that H2AX, a variant of canonical histone H2A, plays an important role in spreading the signal of the damage, and phosphorylated H2AX (γH2AX) is required for the stabilization of numerous of DNA damage response factors at DNA lesions (Paull et al. 2000; Srivastava et al. 2009). Therefore, γH2AX is usually used for the surrogate marker of DNA damagepresence (Li et al. 2013; Li and Yu 2013). As shown in Figure 1C, no staining of γH2AX was detected in the normal samples of pancreas. While the signal of γH2AX was highly positive in both duct and the tissue around duct in the tumor sample. Also, we found evident foci within the cells from these samples (enlarged box in Figure 1C). To further confirm the occurrence of DNA damages, we tested the status of serine or threonine residues that precede glutamine residues, called SQ/TQ motifs, which are a large number of phosphorylation substrates and phosphorylated by kinases upon DNA damages (Cortez et al. 1999; Traven and Heierhorst 2005). As expected, phosphorylated SQ/TQ was also detected in the tumor sample but not the normal sample, and the positive signal of pSQ/TQ was co-localized with γH2AX, indicating the obvious existence of DNA damages in human pancreatic cancer. Similarly, the signals of γH2AX and pSQ/TQ were also positive in the body of pancreas from the tumor samples (Figure 1D), and they were exactly co-localized as that in Figure 1C. Both the numbers of γH2AX and pSQ/TQ positive cells around duct in the normal and tumor tissue were summarized in Figure 1E.
Checkpoint pathway in pancreas cancer
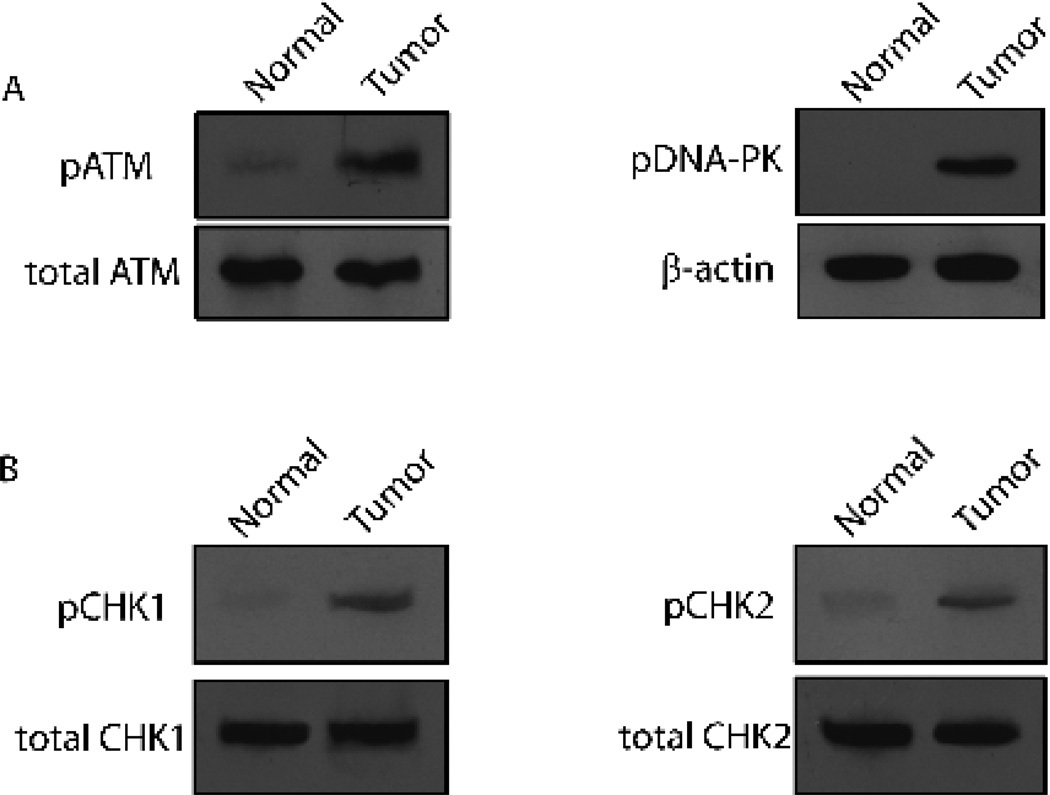
When DNA damages occur, cells must start the system of DNA damage response to activate the cell cycle checkpoint, initiate DNA repair process, or drive the cells to apoptosis (Ghosal and Chen 2013; Sengupta and Harris 2005). In the system of the DNA damage response, ataxia-telangiectasia mutated (ATM) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) are members of the phosphoinositide-3-kinase-related protein kinase (PIKK) family. Theyare rapidly activated in response to DNA damage and are believed to be the earliest sensor of DNA damages (Falck et al. 2005). To explore whether DNA response was activated in the tumor samples, we tested the status of ATM and DNA-PK. As shown in Figure 2A, both ATM and DNA-PK were activated in tumor pancreas rather than in normal pancreas, indicting the active response upon the DNA damages in the pancreases of the patients. As ATM and DNA-PK respond mainly to DNA double-strand breaks (DSBs)for promotinghomologous recombination (HR) and Non-homologous end joining (NHEJ), respectively (Falck et al. 2005), these data suggested that the DSBs may be the major DNA lesions in the cancerous tissue. DSBs are more deleterious than other types of DNA lesions since they do not leave an intact complementary strand to be used as a template for repair. If accumulated, they can ultimately cause chromosome translocations that result in tumorigenesis (Jackson and Bartek 2009; Polo and Jackson 2011). Thus, these results built a potential link between DNA damage to tumorigenesis of pancreas. The locally increased kinase (e.g. ATM and DNA-PKs) activation is believed to be important for efficient phosphorylation of their substrates, including the downstream effector kinases, checkpoint kinase 1 (CHK1) and checkpoint kinase 2 (CHK2)(Reinhardt and Yaffe 2009; Falck et al. 2005; Kastan and Bartek 2004).CHK1 and CHK2 regulate checkpoint network in responses to DSBs and transiently delay cell-cycle progression in G1, S or G2 phases, or even impose prolonged, durable cell-cycle arrests in either G1 or G2, before entry into the subsequent S phase or mitosis, respectively (Kastan and Bartek 2004). To further confirm our proposition, we examined the activation of CHK1 and CHK2. As expected, CHK1 and CHK2 were both phosphorylated in the samples from the pancreatic tumor but not in thenormal pancreas (Figure 2B). These results indicated an overall activation of the checkpoint pathway upon the DNA damages in pancreas cancer and the cell-cycle was arrested by these active kinases. Normally, the G2/M checkpoint only transiently exists, which allows the completion of quick DNA damage repair before entering into mitosis so that the DNA lesions would not be transmitted from mother cells to daughter cells (Chen et al. 2000; Abraham 2001). However, prolonged arresting at the G2/M boundary may cause the mitotic exit and genomic instability (Chiu et al. 2005; Yang et al. 2010; Hirose et al. 2001), which eventually leads to tumorigenesis.
Figure 2. Checkpoint activation in human pancreatic cancer.
(A) Phosphorylation status of ATM and DNA-PK was tested by Western Blot from normal (C1-C3) and tumor (T2-T5) pancreatic tissues. Representative results from C3 and T5 are shown. Total ATM and β-actin were used for the loading controls, respectively. (B) Phosphorylation status of CHK1 and CHK2 was tested by Western Blot from normal (C1-C3) and tumor (T2-T5) pancreatic tissues. Representative results from C1 and T1 are shown. Total CHK1 and CHK2 were used for the loading controls, respectively.
DNA repair pathway in pancreatic cancer
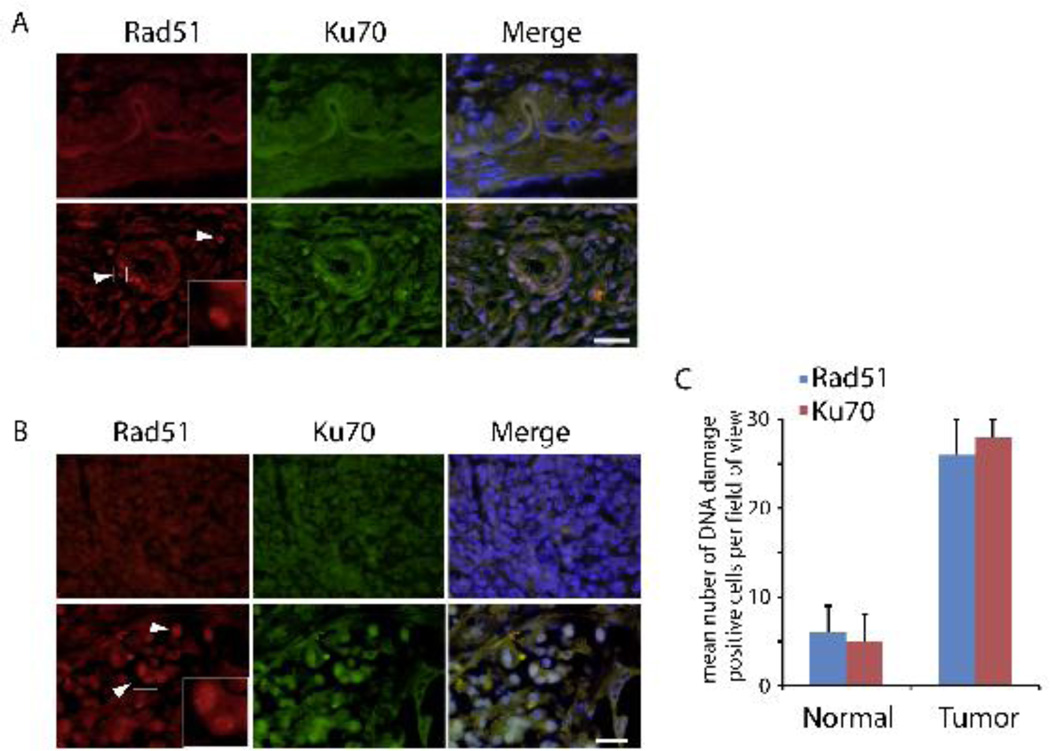
Accumulation of DSBs is one of the major reasons for tumorigenesis. The persistent DNA damages will keep the DNA repair pathway active in vivo until all the damages repaired (Sancar et al. 2004). It is well known that HR and NHEJ are the two major repair pathways for DSB repair (Bristow and Hill 2008). The NHEJ repair pathway can be activated in any phase of the cell cycle for error-prone repair. While the HR pathway is preferentially activated in the S and G2 phases of the cell cycle when a sister chromatid is available as the template for error-free repair of the DNA DSBs (Sandhu et al. 2000). Since RAD51 and KU70/80 play the central roles in the HR and NHEJ pathways respectively, we tested the signals of these two proteins by immunostaining. As seen in Figure 3A, the signals of RAD51 and KU70 were negative in normal pancreas (upper), but were notably stained in and around the duct of the patient pancreas, and these signals were well co-localized (lower). Similarly, this pattern was also found in the body of the patient pancreas (Figure 3B). Both the numbers of Rad51 and Ku70 positive cells around duct in the normal and tumor tissue were summarized in Figure 3C. These results indicated that both HR and NHEJ pathways were active in the tumor pancreas tissue, implying the persistent DSBs in this area. It is worth noting that persistent expressions of RAD51 and KU70/80 have been observed in many cancer cells (Nagathihalli and Nagaraju 2011; Komuro et al. 2005), further suggesting the correlation between DNA damages and pancreatic cancer.
Figure 3. Activation of DNA repair pathways in human pancreatic cancer.
(A) Immunostaining of RAD51 and KU70 of duct was performed from normal (C1-C3) and tumor (T1, T2, and T4) pancreatic tissues. Representative images from C2 and T1 are shown. (B) Immunostaining of RAD51 and KU70 of pancreatic body was performed from normal (C1-C3) and tumor (T1-T4) pancreatic tissues. Representative images from C2 and T3 are shown. Enlarged box denotes RAD51 foci. Scale bar: 20 µm. (C) Numbers of positive Rad51 and Ku70 cells around the duct in normal pancreas (C1-C3) and pancreatic tumor (T1, T2, and T4) were counted. Three independent experiments were averaged. The error bars represent the SD.
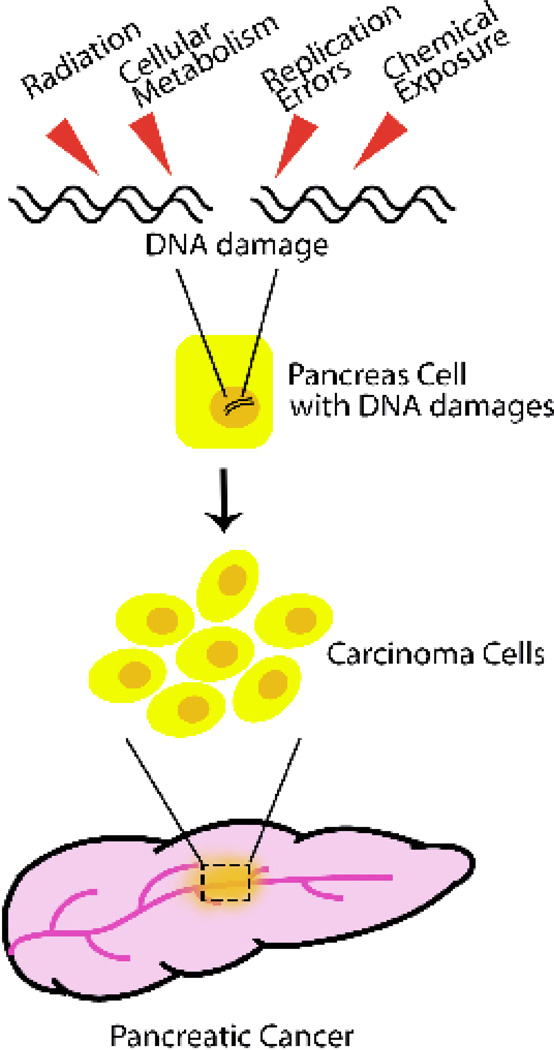
In the past decades, many advances have been made to understand DNA damage and human cancers. Research studies in pancreatic cancer have involved detection of DNA damage derived from carcinogen exposure and endogenous metabolic processes (Li et al. 2004). For instance, smoking-induced aromatic DNA adducts and other types of DNA damage have been detected in human pancreas (Wang et al. 1998; Li et al. 2002; Thompson et al. 1999; Kadlubar et al. 1998).In particular, an increasing number of mutations of DNA response factors, including ATM, BRCA2, PALB2, FANCC and FANCG, have been identified in chronic pancreatitis and pancreatic cancer (Rustgi 2014; van der Heijden et al. 2003), and genomic instability is a hallmark feature of sporadic PDA (Campbell et al. 2010).These studies and our current findings suggest that the human pancreas is susceptible to carcinogen exposure which causes DNA damages, and the accumulated DNA damages might contribute to genetic mutation, and in turn to tumorigenesis (Figure 4).
Figure 4. Model of DNA damage induced pancreatic cancer.
Pancreas is susceptive to DNA damages induced by radiation, cellular metabolism, replication error, and chemical exposure. If not repaired timely, massive DNA damages accumulates in pancreatic cells, which causes cellular genomic instability, mutations and chromosome translocations. These cells loss of control and eventually become carcinoma cells which results in pancreatic cancer.
Acknowledgments
This study was supported by National Institute of Health (CA132755 and CA130899 to X.Y.), Ovarian Cancer Research Fund (292728 to M. L.), and National Natural Science Foundation of China (81272756 to Fei Li).
References
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15(17):2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Trujillo K, Sung P, Tomkinson AE. Interactions of the DNA ligase IV-XRCC4 complex with DNA ends and the DNA-dependent protein kinase. J Biol Chem. 2000;275(34):26196–26205. doi: 10.1074/jbc.M000491200. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Li CH, Ung MW, Fuh TS, Chen WL, Fang K. Etoposide (VP-16) elicits apoptosis following prolonged G2-M cell arrest in p53-mutated human non-small cell lung cancer cells. Cancer Lett. 2005;223(2):249–258. doi: 10.1016/j.canlet.2004.10.049. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122(2):639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286(5442):1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- Eckel F, Schneider G, Schmid RM. Pancreatic cancer: a review of recent advances. Expert Opin Investig Drugs. 2006;15(11):1395–1410. doi: 10.1517/13543784.15.11.1395. [DOI] [PubMed] [Google Scholar]
- Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434(7033):605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- Garcea G, Neal CP, Pattenden CJ, Steward WP, Berry DP. Molecular prognostic markers in pancreatic cancer: a systematic review. Eur J Cancer. 2005;41(15):2213–2236. doi: 10.1016/j.ejca.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Ghosal G, Chen J. DNA damage tolerance: a double-edged sword guarding the genome. Transl Cancer Res. 2013;2(3):107–129. doi: 10.3978/j.issn.2218-676X.2013.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose Y, Berger MS, Pieper RO. p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001;61(5):1957–1963. [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324(5924):217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlubar FF, Anderson KE, Haussermann S, Lang NP, Barone GW, Thompson PA, MacLeod SL, Chou MW, Mikhailova M, Plastaras J, Marnett LJ, Nair J, Velic I, Bartsch H. Comparison of DNA adduct levels associated with oxidative stress in human pancreas. Mutat Res. 1998;405(2):125–133. doi: 10.1016/s0027-5107(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Komuro Y, Watanabe T, Hosoi Y, Matsumoto Y, Nakagawa K, Suzuki N, Nagawa H. Prognostic significance of Ku70 protein expression in patients with advanced colorectal cancer. Hepatogastroenterology. 2005;52(64):995–998. [PubMed] [Google Scholar]
- Li D, Firozi PF, Zhang W, Shen J, DiGiovanni J, Lau S, Evans D, Friess H, Hassan M, Abbruzzese JL. DNA adducts, genetic polymorphisms, and K-ras mutation in human pancreatic cancer. Mutat Res. 2002;513(1–2):37–48. doi: 10.1016/s1383-5718(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- Li M, Lu LY, Yang CY, Wang S, Yu X. The FHA and BRCT domains recognize ADP-ribosylation during DNA damage response. Genes Dev. 2013;27(16):1752–1768. doi: 10.1101/gad.226357.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Yu X. Function of BRCA1 in the DNA damage response is mediated by ADPribosylation. Cancer Cell. 2013;23(5):693–704. doi: 10.1016/j.ccr.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–294. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- Ma J, Jemal A. The rise and fall of cancer mortality in the USA: why does pancreatic cancer not follow the trend? Future Oncol. 2013;9(7):917–919. doi: 10.2217/fon.13.76. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- Mohammed A, Janakiram NB, Lightfoot S, Gali H, Vibhudutta A, Rao CV. Early detection and prevention of pancreatic cancer: use of genetically engineered mouse models and advanced imaging technologies. Curr Med Chem. 2012;19(22):3701–3713. doi: 10.2174/092986712801661095. [DOI] [PubMed] [Google Scholar]
- Morrison C, Sonoda E, Takao N, Shinohara A, Yamamoto K, Takeda S. The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J. 2000;19(3):463–471. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagathihalli NS, Nagaraju G. RAD51 as a potential biomarker and therapeutic target for pancreatic cancer. Biochim Biophys Acta. 2011;1816(2):209–218. doi: 10.1016/j.bbcan.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Ozcelik H, Schmocker B, Di Nicola N, Shi XH, Langer B, Moore M, Taylor BR, Narod SA, Darlington G, Andrulis IL, Gallinger S, Redston M. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat Genet. 1997;16(1):17–18. doi: 10.1038/ng0597-17. [DOI] [PubMed] [Google Scholar]
- Patel KJ, Yu VP, Lee H, Corcoran A, Thistlethwaite FC, Evans MJ, Colledge WH, Friedman LS, Ponder BA, Venkitaraman AR. Involvement of Brca2 in DNA repair. Mol Cell. 1998;1(3):347–357. doi: 10.1016/s1097-2765(00)80035-0. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10(15):886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25(5):409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol. 2009;21(2):245–255. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, Gallinger S, Schwartz AG, Syngal S, Cote ML, Axilbund J, Schulick R, Ali SZ, Eshleman JR, Velculescu VE, Goggins M, Vogelstein B, Papadopoulos N, Hruban RH, Kinzler KW, Klein AP. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;2(1):41–46. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustgi AK. Familial pancreatic cancer: genetic advances. Genes Dev. 2014;28(1):1–7. doi: 10.1101/gad.228452.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Sandhu JK, Haqqani AS, Birnboim HC. Effect of dietary vitamin E on spontaneous or nitric oxide donor-induced mutations in a mouse tumor model. J Natl Cancer Inst. 2000;92(17):1429–1433. doi: 10.1093/jnci/92.17.1429. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6(1):44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Gochhait S, de Boer P, Bamezai RN. Role of H2AX in DNA damage response and human cancers. Mutat Res. 2009;681(2–3):180–188. doi: 10.1016/j.mrrev.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Thompson PA, Seyedi F, Lang NP, MacLeod SL, Wogan GN, Anderson KE, Tang YM, Coles B, Kadlubar FF. Comparison of DNA adduct levels associated with exogenous and endogenous exposures in human pancreas in relation to metabolic genotype. Mutat Res. 1999;424(1–2):263–274. doi: 10.1016/s0027-5107(99)00024-x. [DOI] [PubMed] [Google Scholar]
- Traven A, Heierhorst J. SQ/TQ cluster domains: concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. Bioessays. 2005;27(4):397–407. doi: 10.1002/bies.20204. [DOI] [PubMed] [Google Scholar]
- van der Heijden MS, Yeo CJ, Hruban RH, Kern SE. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003;63(10):2585–2588. [PubMed] [Google Scholar]
- Wang M, Abbruzzese JL, Friess H, Hittelman WN, Evans DB, Abbruzzese MC, Chiao P, Li D. DNA adducts in human pancreatic tissues and their potential role in carcinogenesis. Cancer Res. 1998;58(1):38–41. [PubMed] [Google Scholar]
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326(7):455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543. doi: 10.1038/nm.2144. 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Ma J, Wu J, Ye L, Cai H, Xia B, Yu X. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol. 2009;19(6):524–529. doi: 10.1016/j.cub.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]