Abstract
Androstenedione is a common precursor of sex steroids produced and secreted in the human adrenal gland and produced by 3β-hydroxysteroid dehydrogenase (3βHSD), 17α-hydroxylase/17-20 lyase (CYP17) and cytochrome b5 (CYB5A). 3βHSD is expressed in the zona glomerulosa (ZG) and fasciculata (ZF), CYP17 in the ZF and zona reticularis (ZR) and CYB5A in the ZR, respectively. We previously demonstrated the presence of cortical parenchymal cells co-expressing 3βHSD and CYB5A with hybrid features of both ZF and ZR in human adrenal cortex and hypothesized that these cells may play an important role in androstenedione production in human adrenal gland. Age-related morphologic development of these hybrid cells has, however, not been studied. Therefore, in this study, 48 human adrenal specimens from various age groups were retrieved. Double-immunohistochemical analyses were used in order to study the correlation between this hybrid cell type and age. In both male and female adrenal cortex, the mean of total adrenocortical area, the area of CYB5A positive cells and the mean of its ratio reached highest peak in the 21–40 year-old (y.o.). The greatest overlap between 3βHSD and CYB5A in both total and relative area was present in the 13–20 y.o. group. For all of the markers above, statistically significant differences were detected among the different age groups examined (P<0.05). These findings all indicated that both area and ratio of 3βHSD and CYB5A double positive cells, which could represent the hybrid cells of ZF and ZR, are correlated with human adrenal development and could subsequently influence age-related serum androstenedione levels.
Keywords: 3β-hydroxysteroid dehydrogenase (3βHSD), cytochrome b5 (CYB5A), human adrenal gland, development, double-immunohistochemistry
INTRODUCTION
The mechanisms causing the rise and decrease of adrenal androgen production during the course of adrenarche and aging have remained to be clarified These changes are, however, clearly considered to be associated with a series of intra-adrenal changes in the expression of steroidogenic enzymes, as well as morphological change of the adrenal zona reticularis (ZR) [1].
Androstenedione is a common precursor of sex steroids, and known to be produced in human adrenal cortex [2, 3]. Cytochrome b5 (CYB5A) is also known as an important regulator of androstenedione production by acting as an allosteric effector that interacts primarily with the 17α-hydroxylase/17-20 lyase (CYP17)/oxidoreductase complex to stimulate 17,20-lyase activity, while [4]. 3β-hydroxysteroid dehydrogenase (3βHSD) is considered a key enzyme converting DHEA to androstenedione [4–11]. Results of previous in vitro studies all demonstrated that an inhibition of both 3βHSD and CYB5A resulted in marked repression of androstenedione production, suggesting that efficient androstenedione production may require the presence of both enzymes in the one cell [12].
In human adrenals, 3βHSD is distinctively expressed in both zonae glomerulosa (ZG) and fasciculata (ZF), whereas ZR expresses very little 3βHSD but specifically expresses CYB5A [8]. In addition, CYP17 is expressed in both the ZF and ZR, including the cells expressing 3βHSD or CYB5A [8]. We previously demonstrated that CYP17 immunoreactivity was increased in both ZF and ZR after age 5 y.o. and reached a plateau level at age 13 y.o, while that of CYB5A became more pronounced in the ZR after age 5 y.o. and reached a plateau at 13 y.o. [13]. In addition, we also reported a marked decrease in the ZR after age 8 y.o. with little alterations in the adjacent ZG and ZF [13]. Results of our recent study also revealed for the first time the presence of adrenocortical parenchymal cells located between the ZF and ZR co-expressing both CYB5A and 3βHSD [12]. Therefore, it is also reasonably postulated that these cells are positive for CYP17 since they are located in the border between ZF and ZR. In addition, no other cell types of adrenal cortex co-expressed both of these enzymes and this hybrid zone that shares the characteristic of both ZF and ZR may be uniquely involved in the production of androstenedione. Serum androstenedione level has been reported to be associated with adrenarche and aging as well as DHEA and DHEAS [14–28]. The area of ZR has been generally postulated to correspond to age related changes in of DHEA and DHEAS [29, 30]. However, age-related morphologic development of these hybrid cells has not been examined so far.
Therefore, in this study, we hypothesized that a similar association may be detected between the age related changes in serum androstenedione and the CYB5A and 3βHSD positive layer of the adrenal between ZF and ZR. The age-related morphologic development of these hybrid cells has not been examined at all to the best of our knowledge. We thus performed double-immunohistochemical analyses in order to precisely identify these hybrid cells in order to obtain detailed information regarding the relationship between age related adrenal development and expression and localization of CYB5A and 3βHSD in adrenals of different age groups.
MATERIALS AND METHODS
Human tissue preparation
Human adrenal autopsy specimens were retrieved from autopsy files of Tohoku University Hospital from 1990 to 2007 (Sendai, Japan). Forty-eight specimens were subsequently selected for this study from the large group of archival tissue specimens following careful histological screening from the standpoints of the following four criteria: tissue collection in less than 3 h postmortem; no histories of administration of adrenocortical steroids or chronic illness prior to demise; no pathological abnormalities including adrenocortical nodules or neoplasms; full area of the adrenal extending from the capsule to medulla available in the specimens. From these paraffin-embedded specimens, 3 μm area tissue sections were prepared for immunostaining. The research protocol was approved by Institutional Review Board of Tohoku University Graduate School of Medicine.
Immunohistochemistry
We used double immunostaining with diaminobenzidine (DAB) for CYB5A using a polyclonal antibody kindly provided by Dr. Allen Conley (University of California, Davis CA) and vector-blue for 3βHSD using a polyclonal antibody kindly provided by Dr. J. Ian Mason (University of Edinburgh, Edinburgh, U.K.), respectively [13, 31]. A full description of the methods used for double immunostaining has been previously published [32].
Quantitative measurement of adrenal cortex
Quantitative analysis of adrenal sections was performed using an Olympus BX41 (®) microscope and an Olympus DP72 (®) camera with Olympus cellSens (®) software (Olympus, Tokyo, Japan). Digital images of each adrenal section designating five separate quadrangle-shaped areas that contained full-area cross sections of the cortex were prepared. Five values were measured for each section and the average value was determined for the final analysis. To avoid any bias, all measurements were conducted in a blinded fashion or without the knowledge of the age and sex of the donors. The data for analysis included the total cortical area, the area of CYB5A and 3βHSD/CYB5A (double) positivity, and the ratio of the area of CYB5A and double positivity to that of the total cortex expressed as a %. After gender stratification, the specimens were tentatively assigned into five age groups: group 4–6 years of age (y.o.) (n=4 in male; n=3 in female); group 7–12 years of age (y.o.) (n=5 in male; n=6 in female); group 13–20 y.o. (n=5 in male; n=3 in female); group 21–40 y.o. (n=4 in male; n=6 in female); group 41–80 y.o. (n=6 in male; n=6 in female) based on previous reports [13, 28, 33].
Statistical analysis
All the data were expressed as the mean ± S.D. We used one-way analysis of variance followed by the Tukey-Kramer test for comparisons between two different groups. P<0.05 was considered significant.
RESULTS
Immunohistochemical localization of 3βHSD and CYB5A in the human adrenal cortex
In the adrenal glands, 3βHSD immunoreactivity was predominantly detected in the cytoplasm of the ZF while CYB5A immunoreactivity was mainly detected in cytoplasm of the ZR of human adrenal glands (Figure 1A–E). The double positive cortical cells were detected in the morphologically identified border between the ZF and ZR (Figure 1F–J).
Figure 1.
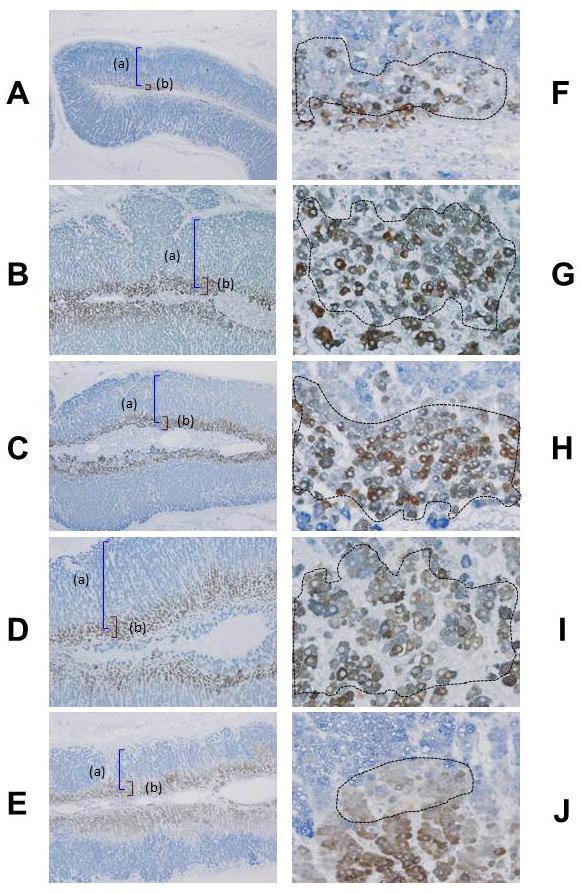
Immunohistochemical localization of 3β-hydroxysteroid dehydrogenase (3βHSD) (a) and cytochrome b5 (CYB5A) (b) in the human adult adrenal glands following age groups: 4–6 years of age (y.o.) (4 y.o., male) (A, F), 7–12 y.o. (10 y.o. male) (B, G), 13–20 y.o. (16 y.o. female) (C, H), 21–40 y.o. (38 y.o. female) (D, I) and 41–80 y.o. (51 y.o. female) (E, J). Immunopositive cells for CYB5A appear brown as a result of diaminobenzidine colorimetric reaction. Immunopositive cells for 3βHSD appear blue as a result of Vector-blue colorimetric reaction. Double-immunopositive cells are confirmed (circles) (F–J).
Quantitative analyses of age-related morphologic changes and immunoreativity of 3βHSD and CYB5A in human adrenal cortex
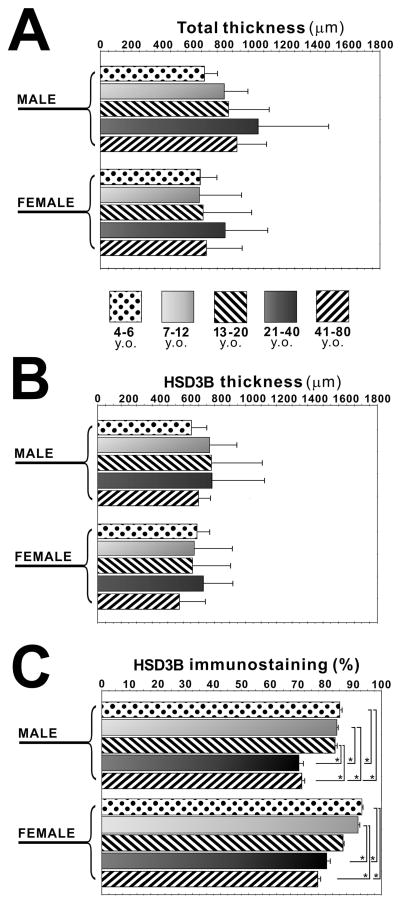
There were no significant differences of the total adrenal cortex area between different genders or among different age groups (Figure 2A). There were no significant differences of the thickness of the area positive for 3βHSD between different genders or among different age groups (Figure 2B). However, in both male and female adrenal cortex, there were significant differences of its ratio among the age groups (4–6 y.o., 7–12 y.o. and 13–20 y.o. group v.s. 21–40 y.o. and 41–80 y.o. group in male adrenal glands; 4–6 y.o. and 7–12 y.o. group v.s. 21–40 y.o. and 41–80 y.o. group in female adrenal glands (P<0.05) (Figure 2C).
Figure 2.
Quantitative analyses of age-related morphologic changes and immunoreativity of 3β-hydroxysteroid dehydrogenase (3βHSD) in human adrenal cortex. Total adrenocortical area (total thickness) in four age categories in both male and female adrenals (A). The area positive for 3βHSD (HSD3B thickness) in four age categories in both male and female adrenals (B). The ratio of area positive for 3βHSD to that of total cortex (HSD3B immunostaining (%)) in four age categories in both male and female adrenals (C) (*, P<0.05).
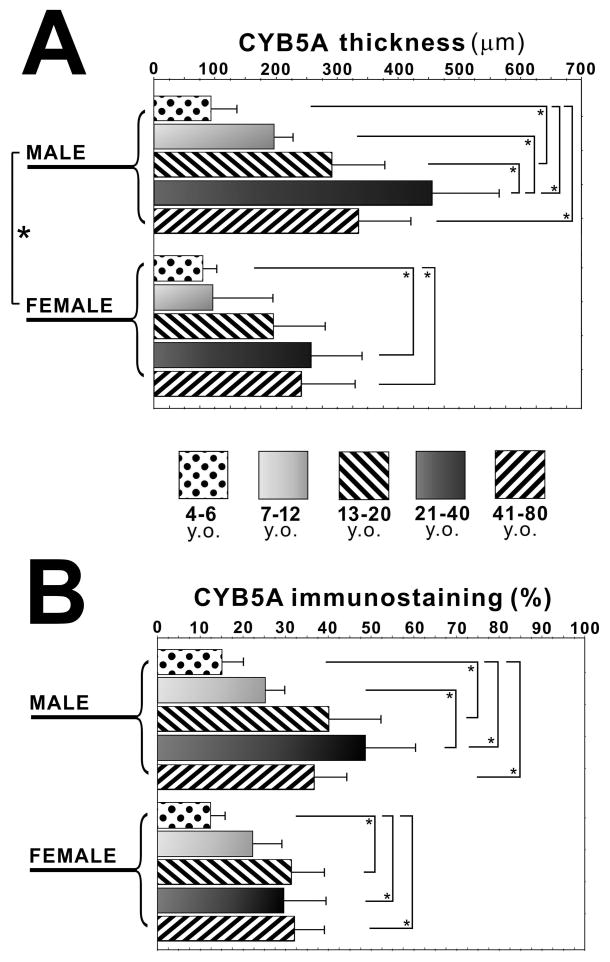
The area of CYB5A positive zone was significantly higher in the male adrenal group than that in the female adrenal group examined in this study (P<0.05) (Figure 3A). In the male adrenal cortex, the area positive for CYB5A was significantly smaller in 4–6 y.o. group than in 13–20 y.o., 21–40 y.o. and 41–80 y.o. group, and was significantly larger in 21–40 y.o. group than in 4–6 y.o. and 7–12 y.o. group (P<0.05) (Figure 3A). The ratio of CYB5A positive area was significantly smaller in 4–6 y.o. group than in 13–20 y.o., 21–40 y.o. and 41–80 y.o. group, and was significantly larger in 21–40 y.o. group than in 7–12 y.o. group (P<0.05) (Figure 3B). In the female adrenal cortex, the area positive for CYB5A was significantly smaller in 4–6 y.o. group than in 21–40 y.o. and 41–80 y.o. group (P<0.05), and its ratio was significantly lower in 4–6 y.o. group than in 13–20 y.o., 21–40 y.o. and 41–80 y.o. group (P<0.05) (Figure 3B).
Figure 3.
Quantitative analyses of immunoreativity of cytochrome b5 (CYB5A) in human adrenal cortex. The area positive for CYB5A (CYB5A thickness) in four age categories in both male and female adrenals (A). The ratio of area positive for CYB5A to that of total cortex (CYB5A immunostaining (%)) in four age categories in both male and female adrenals (B) (*, P<0.05).
Quantitative analyses of double immunoreactivty of CYB5A and 3βHSD5 and its relation to age in the human adrenal cortex
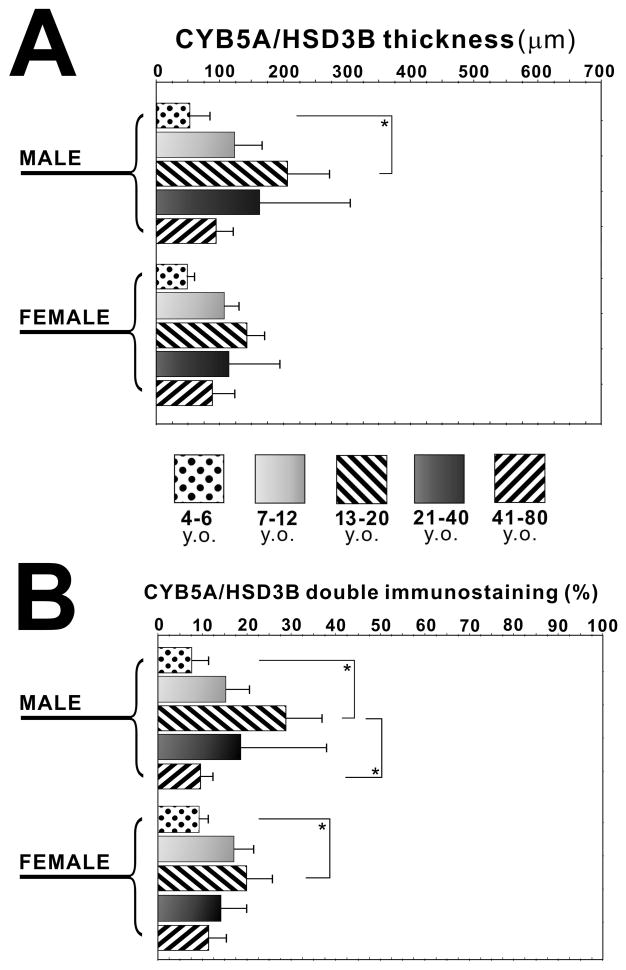
In the male adrenal cortex, the area double positive for both 3βHSD and CYB5A was significantly larger in 13–20 y.o. group than in 4–6 y.o. group, and its ratio was significantly higher than in 4–6 y.o. and 41–80 y.o. group (P<0.05) (Figure 4A, B). In the female adrenal cortex, its ratio was significantly higher in 13–20 y.o. group than in 4–6 y.o. group (P<0.05) (Figure 4A, B). There was no significant difference of these values between male and female subjects examined (Figure 4A, B).
Figure 4.
Quantitative analyses of age-related immunoreativity of 3β-hydroxysteroid dehydrogenase (3βHSD) and cytochrome b5 (CYB5A) in human adrenal cortex. The area positive for both 3βHSD and CYB5A (CYB5A/HSD3B thickness) in four age categories in both male and female adrenals (A). The ratio of area positive for both 3βHSD and CYB5A to that of total cortex (CYB5A/HSD3B double immunostaining (%)) in four age categories in both male and female adrenals (B) (*, P<0.05).
DISCUSSION
We previously identified the presence of adrenocortical cells co-expressing 3βHSD and CYB5A which is postulated to represent hybrid features of both ZF and ZR in human adrenals. In this study, we further demonstrated that the area and ratio of these double positive cortical cells were indeed associated with age indicating possible involvement in adrenal development/aging and changes of age-related serum androstenedione levels.
The rise in the serum concentrations of DHEA and DHEAS occurs in association with puberty, and circulating DHEAS concentrations continue to rise and peak during the second decade of life and remain elevated into the 30s [13, 19, 25, 26]. In addition, Elmlinger et al. reported that, with the onset of puberty around the age of 10 y.o., serum androstenedione levels markedly increased, reaching a maximum at about 17 y.o. (10-fold in boys, 5-fold in girls) followed by a decline in both sexes [20]. The expression patterns reported in our present study could also account for the peak production of DHEA and androstenedione mentioned above. In the adrenal, CYB5A and 3βHSD as well as CYP17 control production of DHEA and androstenedione where CYB5A together with CYP17 also catalyzes the reaction. Spatial compartmentalization of 3βHSD and CYB5A above usually starts to develop in the adrenal from around 8 y.o. with 3βHSD located in the ZF and CYB5A in the ZR, while CYP17 is stably expressed in both the ZF and ZR after age 5 y.o. [13]. Results of our previous studies also demonstrated that the area of CYB5A and its ratio reached the peak during 21–40 y.o. in both male and female adrenal glands, corresponding to the age bracket in which peak production of serum DHEA and DHEAS levels are detected [13, 19, 25, 26]. It is entirely true that this finding regarding DHEA is confirmatory but the true novelty of our present study came from its finding on the age related alterations in the hybrid zone expressing both 3βHSD and CYB5A. The peak area of this particular zone in both male and female subjects was detected in the 13–20 y.o. age group which corresponds to peak production of androstenedione. In our study, it is therefore possible to regard the total area of adrenal cortex as the area positive for CYP17 (ZF and ZR) since the thickness of ZG is in general very thin. On the other hand, there were no significant differences of the total adrenal cortex area among different age groups. We therefore suggest that the peak production of adrenal androstenedione may be caused by the alteration of total ZR area and the transitional zone of hybrid cells of ZF and ZR expressing both 3βHSD and CYB5A in the same cortical cells but not the alteration of the total adrenal cortex.
Results of our present study suggest that the hybrid zone may represent an area of uncertain differentiation between ZF and ZR in adrenal cortex. We previously reported that the total area of the ZR in adrenal cortex of pediatric age groups was significantly greater in older age groups, with the ratio of ZR area of the female adrenal surpassing 50% of the total cortex at around 16–17 y.o., suggesting that the early stages of adrenarche were related to intra-adrenal remodeling [32]. Together with the results of our present study, continued adrenal remodeling throughout teens and twenties, in particular, remodeling of the ZR/ZF zone into a specialized ZR zone could explain the age related changes of these steroid hormones in circulation. However, the significance of association with steroid peaks and underlying mechanisms for the hybrid ZR/ZG zone still remains to be clarified in the future.
In conclusion, results of our present study of double immunohistochemistry firstly demonstrated that the area and the ratio of cortical zone co-expressing 3βHSD and CYB5A were indeed correlated with human adrenal development and that this likely contributed to the ability of the adrenal cortex to produce androstenedione.
Acknowledgments
This work was supported by National Institutes of Health grant, DK069950 (YN, WER, and HS). This work was also partly supported by the Takeda Science Foundation. We thank Ms. Kaori Suzuki (Department of Pathology, Tohoku University Graduate School of Medicine, Sendai, Japan) for the skillful technical assistance.
Footnotes
Declaration of Interest
None of the authors has conflict of interest.
References
- 1.Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord. 2009;10:19–26. doi: 10.1007/s11154-008-9092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidlingmaier F, Dorr HG, Eisenmenger W, Kuhnle U, Knorr D. Contribution of the adrenal gland to the production of androstenedione and testosterone during the first two years of life. J Clin Endocrinol Metab. 1986;62:331–335. doi: 10.1210/jcem-62-2-331. [DOI] [PubMed] [Google Scholar]
- 3.de Ronde W, Hofman A, Pols HA, de Jong FH. A direct approach to the estimation of the origin of oestrogens and androgens in elderly men by comparison with hormone levels in postmenopausal women. Eur J Endocrinol. 2005;152:261–268. doi: 10.1530/eje.1.01830. [DOI] [PubMed] [Google Scholar]
- 4.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar MK, Kelly SL, Kaderbhai MA. Cytochrome b(5) modulation of 17{alpha} hydroxylase and 17-20 lyase (CYP17) activities in steroidogenesis. J Endocrinol. 2005;187:267–274. doi: 10.1677/joe.1.06375. [DOI] [PubMed] [Google Scholar]
- 6.Fluck CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J Clin Endocrinol Metab. 2003;88:3762–3766. doi: 10.1210/jc.2003-030143. [DOI] [PubMed] [Google Scholar]
- 7.Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, et al. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 8.Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simard J, de Launoit Y, Labrie F. Characterization of the structure-activity relationships of rat types I and II 3 beta-hydroxysteroid dehydrogenase/delta 5 -delta 4 isomerase by site-directed mutagenesis and expression in HeLa cells. J Biol Chem. 1991;266:14842–14845. [PubMed] [Google Scholar]
- 10.Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF. C21 steroid side chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17 alpha-hydroxylase/C17,20-lyase cytochrome P-450. J Biol Chem. 1984;259:3971–3976. [PubMed] [Google Scholar]
- 11.Dharia S, Slane A, Jian M, Conner M, Conley AJ, et al. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71:83–88. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Xing Y, Hui XG, Kurotaki Y, Ono K, et al. Human adrenal cells that express both 3beta-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) contribute to adrenal androstenedione production. J Steroid Biochem Mol Biol. 2011;123:122–126. doi: 10.1016/j.jsbmb.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 14.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 15.Brody S, Carlstrom K, Lagrelius A, Lunell NO, Rosenborg L. Serum levels of 4-androstene-3,17-dione in menstruating and postmenopausal women. Evaluation of a radioimmunoassay and correlation with bone mineral content and endometrial pathology. Acta Obstet Gynecol Scand. 1983;62:531–534. doi: 10.3109/00016348309154231. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan JR, Hospodar P, Myers C, Leuenberger P, Demers LM. Effect of excess endogenous androgens on bone density in young women. J Clin Endocrinol Metab. 1988;67:937–943. doi: 10.1210/jcem-67-5-937. [DOI] [PubMed] [Google Scholar]
- 17.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 18.Dickerman Z, Grant DR, Faiman C, Winter JS. Intraadrenal steroid concentrations in man: zonal differences and developmental changes. J Clin Endocrinol Metab. 1984;59:1031–1036. doi: 10.1210/jcem-59-6-1031. [DOI] [PubMed] [Google Scholar]
- 19.Ducharme JR, Forest MG, De Peretti E, Sempe M, Collu R, et al. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42:468–476. doi: 10.1210/jcem-42-3-468. [DOI] [PubMed] [Google Scholar]
- 20.Elmlinger MW, Kuhnel W, Wormstall H, Doller PC. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab. 2005;51:625–632. [PubMed] [Google Scholar]
- 21.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 22.Jensen J, Riis BJ, Hummer L, Christiansen C. The effects of age and body composition on circulating serum oestrogens and androstenedione after the menopause. British Journal of Obstetrics and Gynaecology. 1985;92:260–265. doi: 10.1111/j.1471-0528.1985.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 23.Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 24.Likitmaskul S, Cowell CT, Donaghue K, Kreutzmann DJ, Howard NJ, et al. ‘Exaggerated adrenarche’ in children presenting with premature adrenarche. Clin Endocrinol (Oxf) 1995;42:265–272. doi: 10.1111/j.1365-2265.1995.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 25.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 26.Smith MR, Rudd BT, Shirley A, Rayner PH, Williams JW, et al. A radioimmunoassay for the estimation of serum dehydroepiandrosterone sulphate in normal and pathological sera. Clin Chim Acta. 1975;65:5–13. doi: 10.1016/0009-8981(75)90328-9. [DOI] [PubMed] [Google Scholar]
- 27.Tung YC, Lee JS, Tsai WY, Hsiao PH. Physiological changes of adrenal androgens in childhood. J Formos Med Assoc. 2004;103:921–924. [PubMed] [Google Scholar]
- 28.Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22:337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- 29.Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche) Beitr Pathol. 1973;150:357–377. doi: 10.1016/s0005-8165(73)80086-1. [DOI] [PubMed] [Google Scholar]
- 30.Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr. 1977;90:766–770. doi: 10.1016/s0022-3476(77)81244-4. [DOI] [PubMed] [Google Scholar]
- 31.Mapes S, Tarantal AF, Parker CR, Moran FM, Bahr JM, et al. Adrenocortical cytochrome b5 expression during fetal development of the rhesus macaque. Endocrinology. 2002;143:1451–1458. doi: 10.1210/endo.143.4.8718. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura Y, Miki Y, Suzuki T, Nakata T, Darnel AD, et al. Steroid sulfatase and estrogen sulfotransferase in the atherosclerotic human aorta. Am J Pathol. 2003;163:1329–1339. doi: 10.1016/S0002-9440(10)63492-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, et al. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203:241–252. doi: 10.1677/JOE-09-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]