Abstract
Dietary and waterborne exposure to CuO and ZnO nanoparticles (NPs) was conducted using a simplified model of an aquatic food chain consisting of zooplankton (Artemia salina) and goldfish (Carassius auratus) to determine bioaccumulation, toxic effects and particle transport through trophic levels. Artemia contaminated with NPs were used as food in dietary exposure. Fish were exposed to suspensions of the NPs in waterborne exposure. ICP-MS analysis showed that accumulation primarily occurred in the intestine, followed by the gills and liver. Dietary uptake was lower, but was found to be a potential pathway for transport of NPs to higher organisms. Waterborne exposure resulted in about a tenfold higher accumulation in the intestine. The heart, brain and muscle tissue had no significant Cu or Zn. However, concentrations in muscle increased with NP concentration, which was ascribed to bioaccumulation of Cu and Zn released from NPs. Free Cu concentration in the medium was always higher than that of Zn, indicating CuO NPs dissolved more readily. ZnO NPs were relatively benign, even in waterborne exposure (p≥0.05). In contrast, CuO NPs were toxic. Malondialdehyde levels in the liver and gills increased substantially (p<0.05). Despite lower Cu accumulation, the liver exhibited significant oxidative stress, which could be from chronic exposure to Cu ions.
Keywords: CuO nanoparticle, ZnO nanoparticle, Waterborne exposure, Dietary exposure, Bioaccumulation, Toxicity, Goldfish
Introduction
Nanomaterials are used in a range of appliances and household products, in the manufacture of textiles and electronics, as well as for medical products and in bioremediation technology. Metal oxide nanoparticles (NPs) are commonly used due to their exceptional physicochemical properties, which make them highly suitable and desirable for many consumer products and industrial technologies (Montes et al., 2012). Large-scale production and consumption will result in discharge of the products containing nanomaterials to aquatic ecosystems and agricultural lands (Chatterjee, 2008; Fabrega et al., 2011). Though the history of nanomaterials is relatively new, the release of nanomaterials from commercial products into the aquatic environment has already been reported (Benn and Westerhoff, 2008). Not only does this result in long-term contamination to natural resources, but also could accelerate transport, bioaccumulation and bio–magnification of hazardous substances and by–products of nanomaterials along the trophic food chain (Fisk et al., 2001). Therefore, the increasing concerns about the risks of nanotechnology need to be balanced against their undoubted benefits to society (Crane and Handy, 2007; Owen and Handy, 2007).
Metal oxide NPs are among the most used nanomaterials and have received considerable attentions over their potential ecological effects (Klaine et al., 2008). Nanoscale zinc oxide (ZnO) and copper oxide (CuO) are manufactured in large scale for both industrial and household use since they possess greater promise for future applications, such as fillers, opacifiers, catalysts, semiconductors, cosmetics, and microelectronics (Nel et al., 2006; Reijnders, 2006). ZnO NPs are additives in personal care products, including toothpaste, beauty products and sunscreens, as well as in textiles (Serpone et al., 2007; Becheri et al., 2008), wastewater treatment, and chemotherapy (Paynton et al., 2011). CuO NPs also have potential wide industrial applications, such as gas sensors, photovoltaic cells, catalyst, and heat transfer nano-fluids (Klaine et al., 2008). Thus, ZnO and CuO NPs are among the most probable contaminants to environment.
NPs have been shown to accumulate in cells, such as macrophages and hepatocytes (Johnston et al., 2010). Moreover, they are taken up by aquatic organisms, such as mollusks, crustaceans, fish, bacteria, protozoa, algae, and zooplankton (Kashiwada, 2006; Lee et al., 2007; Gallego et al., 2007; Heinlaan et al., 2008; Tao et al., 2009; Ward and Kach, 2009; Aruoja et al., 2009; Ates et al., 2013a; Ates et al., 2013b). Therefore, both aquatic and terrestrial habitats are likely to be affected adversely from discharges of nano-size of ZnO and CuO. It has been found that both aquatic and dietary metals could also invoke toxic effects on aqueous organisms (Taylor et al., 1998; Sofyan et al., 2007). Zinc (Zn) and copper (Cu) which are essential trace elements for living organisms, but could cause cellular damage as they are released at high concentrations from discharges of NPs (Goyer and Clarkson 2001; Gottschalk et al., 2009).
Toxicological studies of metal–based NPs have focused on the aqueous phase exposure of test organisms (Lovern and Klaper, 2006; Federici et al., 2007). Often studies have failed to include toxic effects associated with dietary intake of NPs. Currently, little is known about the rate of transfer and bio–magnification of nanomaterials through the trophic food chain (Bouldin et al., 2008). Fish are good sentinels of poor environmental quality, because there appears to be a relationship between environmental quality and fish health (NOAA, 1988). The health of fish could provide important information about the health of lower trophic levels; therefore, the use of fish has become prevalent to address ecotoxicological issues in recent years (Matranga and Corsi, 2012). As such, the chemical fate of some metal oxide–based NPs in the aquatic environment has been examined through exposure of fish under various exposure conditions (Zhu et al., 2010). However, bioavailability and toxic effects through dietary uptake remain unclear.
The zooplankton (e.g., Artemia salina) is major source of food for fish. Furthermore, they accumulate pollutants associated with suspended particles and sediments and hence are prime candidates for uptake of NPs from environmental discharges. Within this context, feeding on contaminated zooplankton could provide the capability to understand the influences of dietary uptake of NPs, and their transport pathways and rate from lower trophic levels to fish (Galloway et al., 2002; Livingstone, 2001). In this study, we conducted exposure studies on goldfish (Carassius auratus) and investigated the effects of waterborne and dietary exposure to CuO and ZnO NPs. Fish were exposed to the NPs either in aqueous suspensions of NPs or by feeding with artemia contaminated with the NPs to elucidate NP uptake, transport between trophic levels and sub-chronic toxicity towards particle-ingesting aquatic species.
Materials and Methods
Reagents and Chemicals
The powders of uncoated ZnO and CuO nanoparticles were purchased from Skyspring Nanomaterials Inc., Houston, TX, USA. All nanopowders were stored at room temperature in the laboratory until the implementation of the experimental studies. Deionized water with resistivity 18.0 MΩ cm was used for preparation of the exposure medium and experimental solutions. Trace metal grade nitric acid (HNO3, Fisher Scientific, NJ, USA) were used for digestion of the goldfish tissues collected after the exposure to determine the total uptake levels of NPs. Standard stock solutions (1000 μg mL−1) of copper (Cu), and zinc (Zn) were purchased from SpexCertiprep (Metuchen, NJ). Calibration standards for inductively coupled plasma mass spectrometry (ICP–MS) determinations were prepared within a range from 0 to 500 μg L−1 (ppb) from the stock solutions in 5% HNO3. Carbon coated Cu TEM grids (300 mesh) were purchased from Electron Microscopy Sciences (EMS), Hatfield, PA.
Instrumental Analysis
ICP-MS Analysis of Goldfish Organs and Exposure Medium
All elemental analysis of artemia, fish organs and exposure medium for Cu and Zn were made using a Varian 820MS inductively coupled plasma mass spectrometer (Varian, Australia). The instrument was equipped with a peltier–cooled double–pass glass spray chamber, a Teflon Ari–mist nebulizer (SCP Science, Champlain NY), quartz torch, Ni sampler and skimmer cones, and a Model AF250 all–digital detector (DDEM, ETP Australia). Samples were introduced manually. The instrument was optimized on daily basis for sensitivity, doubly charged ions (<1%) and oxides (<3%) with 5 μg L−1 138Ba, 25Mg, 115In, 140Ce, 208Pb solution. Data collection was achieved by ICP–MS Expert software package (version 2.2 b126) using 63Cu, 65Cu, 66Zn and 68Zn isotopes of Cu and Zn. Germanium (72Ge) was used as internal standard (IS) element to correct for possible instrumental drift and sensitivity changes. The internal standard solution (5.0 μg L−1 Ge in 1% HNO3) was mixed on–line with the sample solution. Two certified reference materials were used for quality assurance of the results. Dogfish liver (DOLT-3) and freshwater (SRM 1643e) reference materials were analyzed by ICP-MS during the analysis of fish tissue samples and water samples from exposure medium. SRM 1643e samples were directly analyzed without any further treatment. DOLT-3 samples were digested in HNO3. Details of sample preparation are given below in chemical analysis section.
Free Cu and Zn ions' levels in the exposure medium were determined by ultrafiltration which is conducted by centrifuging 2 mL of suspension from the medium at 12,000 rpm for 30 minutes. A portion of the supernatant solution was then passed through 3 kDa ultra filtration filters (VWR, Suwanee, GA, USA) to remove suspended NPs. This filter rejects particles greater than 1.3 nm; therefore, the filtrate is assumed to contain free Cu and Zn ions predominantly and all NPs and aggregates greater than 1.3 nm are retained on the filter. The final supernatant solution contained only Cu and Zn ions from the dissolution of CuO and ZnO NPs. The filtrate was then analyzed by ICP-MS for Cu and Zn in the solution.
Characterization of the Nanoparticles
Particle size characterization of the NPs was made by transmission electron microscopy (TEM) using a JEOL–1011 TEM instrument, providing a JEM–1011 resolution of 0.2 nm lattice with magnification of 50 to 1x106 under the accelerating voltage of 40 to 100 kV. For TEM measurements, a drop of the colloidal solution of NPs was placed on a 50 Å thick, carbon–coated copper grid (CF300 – Cu), and was allowed to dry to record TEM images. Particle size distribution was determined by ImageJ software package. To estimate the mean particle size, approximately 100 NPs were measured in random fields of view of three images. Zeta potential and hydrodynamic size distribution of the NPs were determined by dynamic light scattering (DLS) using a Nano ZS Zetasizer (Malvern Instruments). A portion of the suspension from stock and exposure medium was diluted to the appropriate range with water and briefly vortexed to homogenize the contents. Five DLS measurements were taken successively for each solution to estimate the hydrodynamic particle size distribution.
Preparation of Nanoparticle Suspensions
Stock solutions of the nanopowders (1.0 g L-1) were prepared by suspending an appropriate amount of the nanopowders in deionized water in polypropylene tubes. To achieve maximum dispersion, the suspensions were vortexed for two minutes at 2000 rpm and then exposed to ultrasounds for ten minutes in a sonicator bath. Appropriate volumes of the stock suspensions were immediately transferred into the exposure containers.
The distribution of free Cu and Zn levels in the exposure medium were determined by centrifuging 2 mL of suspension from the medium at 12,000 rpm for 30 minutes. A portion of the supernatant solution was then passed through 3 kDa centrifugal filters (VWR, Suwanee, GA, USA) to remove suspended NPs. The final supernatant solution contained only Cu and Zn ions from the dissolution of CuO and ZnO NPs.
Preparation of Artemia salina and Contamination with NPs for Dietary Exposure
Artemia salina cysts, the Great Salt Lake (GSL), Utah origin, were purchased from Artemia International LLC, Houston, and were kept at 4 °C in a refrigerator. The cysts were hatched in artificial seawater (3% w/v). The seawater was prepared by dissolving appropriate amount of Instant Ocean® salt mix (Aquatic Eco–Systems, Apopka, FL, USA) in deionized water. The seawater was stirred for 24 hours under aeration and then filtered through 30–μm millipore cellulose filters. For hatching, the method described in Persoone et al. (1989) was modified and followed as described in our previous studies (Ates et al., 2013a; Ates et al., 2013b). Briefly, encysted artemia were first cleaned by hydrating in distilled water at 4 °C for 12 hours. The floating cysts were removed by washing. Approximately 3 g of the cleaned cysts were incubated in 1.5 L seawater in a graduated conical plastic container at 30 ± 1 °C. The pH of the medium was adjusted to pH 8.5. A 1500 lux day–light was provided continuously by a fluorescent lamp. Aeration was maintained by a small line extending to the bottom of the hatching device from an aquarium air–pump. Under these conditions, artemia hatched within a period of approximately 24 hours.
Artemia salina larvae were then transferred to 0.5 L exposure tanks and exposed to 10 and 100 μg mL-1 suspensions of CuO and ZnO NPs for 24 hours as described elsewhere (Ates et al., 2013b). Prior to beginning the experiments, trials were conducted to estimate the mass of wet artemia required to achieve comparable dietary concentrations to those of waterborne exposure. After a 24 hour exposure, the samples of artemia were digested in 2 mL concentrated HNO3 and analyzed for Cu and Zn to estimate total CuO and ZnO accumulation. CuO levels were 210 ± 20 and 620 ± 55 μg g-1 for 10 and 100 μg mL-1 suspensions, respectively. Total ZnO levels were similar; 255 ± 35 and 705 ± 45 μg g-1 from exposure to 10 and 100 μg mL-1 suspensions of ZnO NP, respectively. Mass of wet artemia required for dietary exposure of fish (see Section Exposure of goldfish) was estimated accordingly using these concentrations.
Exposure of Goldfish
A group of goldfish (Carassius auratus) were purchased from a local pet shop. The initial average body weight and length of the fish were 4.1 ± 0.3 g and 5.5 ± 0.7 cm, respectively. All fish were kept in 50–L glass aquarium equipped with a static renewal flow–through system and dechlorinated tap water. They were acclimated for a period of 7 to 10 days and fed with commercial goldfish food (TetraFin goldfish flakes, Germany). Exposure of fish to NPs was performed in accordance with the Guide for the Care and Use of Laboratory Animals that was established by the United States National Institute of Health (NIH), and a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Jackson State University (Jackson, MS). Exposure was carried out in a 50-L glass aquaria. Volume for 40 L was marked and filled with dechlorinated freshwater.
The exposure regime summarized in Table 1 consists of four treatments and a control group. In each tank (40 L), 20 goldfish were exposed to NPs in duplicate. Sub-chronic (21 days) waterborne and dietary exposure was conducted according to OECD 203 testing guidelines (OECD, 1992). A control group fish was setup without exposure to NPs. In waterborne exposure, fish were exposed directly to the suspensions of CuO and ZnO NPs. The NPs were added from stock suspensions into the exposure tanks to produce 1.0 and 10 μg mL−1 NPs, respectively. For dietary exposure, contaminated artemia were fed to fish. Approximately, 0.2 to 0.25 g wet artemia contaminated with CuO NPs were added to 40 L tanks to accommodate CuO concentration of around 1.0 μg mL−1 in the medium. For 10 μg mL−1 CuO, 0.6 to 0.65 g artemia was added to the 40 L tanks. In case of ZnO NPs, 0.15 to 0.2 g and 0.55 to 0.6 g wet artemia were added to exposure tanks to yield around 1.0 and 10 μg mL−1 ZnO. Fish were fed with fresh (e.g., live) artemia once daily. Exposed artemia were collected from seawater, filtered through a plankton net, rinsed with freshwater and then immediately added to the dietary exposure tanks.
Table 1.
Expanded design for sub–chronic exposure of goldfish to CuO and ZnO NPs. Waterborne concentrations were adjusted by adding appropriate volume of 1.0 mg L-1 suspensions into 40 L exposure tanks. Dietary concentrations were provided by adding appropriate amounts of Artemia salina larvae contaminated through exposure to 10 and 100 μg mL−1 suspensions of CuO and ZnO NPs
| Exposure scheme | Groups | Concentration (μg mL−1) | Duration (day) | Number of fish | Replicates | |
|---|---|---|---|---|---|---|
|
| ||||||
| CuO NPs | ZnO NPs | |||||
| Control | n/a | n/a | 21 | 20 | 2 | |
|
| ||||||
| Dietary | Low dose | 1.1 - 1.3 | 1.0 - 1.3 | 21 | 20 | 2 |
| High dose | 9.3 - 10.1 | 9.7 – 10.6 | 21 | 20 | 2 | |
|
| ||||||
| Waterborne | Low dose | 1.0 | 1.0 | 21 | 20 | 2 |
| High dose | 10 | 10 | 21 | 20 | 2 | |
Preparation of Fish Tissue Samples for Chemical Analysis
At the end of the exposure, fish were removed from the tanks and anesthetized in an oxygenated solution of MS-222 (3–aminobenzoic acid ethyl ester (Aqua Life, Syndel Laboratories Ltd., Vancouver, BC, Canada) at a lethal dose (excess of 200 mg L−1). Fish were then dissected to collect the organs, including the intestine, liver, gills, heart, brain, muscle tissue for chemical and biochemical analysis. The organs were digested in HNO3. Approximately, 0.1 g of wet tissue was digested in teflon vessels in 2 mL concentrated HNO3 at 160 °C for two hours using a digestion block (DigiPrep MS, SCP Science) according to protocols described elsewhere (Arslan et al., 2011). Once completely dissolved, the contents were diluted to 10 mL with deionized water. All sample solutions were analyzed by ICP–MS in 10– or 100–fold diluted solutions. Calibration was performed with aqueous multi-element standards ranging from 5 to 200 μg L−1 (ppb) for Zn and Cu. For quality assurance of the results from the experimental fish samples, Dogfish Liver Certified Reference Material (DOLT-3) was analyzed for Cu and Zn. Preparation of the DOLT-3 samples were made as described above. About 0.1 g sub-samples (n=4) from the DOLT-3 was weighed and digested in HNO3 and then diluted to 10 mL with deionized water. For ICP-MS analysis 10-fold diluted solutions were analyzed to verify the instrumental results. The Cu and Zn concentrations for Cu and Zn were 30.6 ± 3.1 and 84.2 ± 4.1 μg g-1, respectively.
Biochemical Analysis
The decomposition of lipid hydro–peroxides leads to a variety of end products, one of which is malondialdehyde (MDA), a colorless by–product that is considered as a reliable marker of lipid peroxidation (Ohkawa and Ohishi, 1979). This assay is based upon the formation of a red adduct between thiobarbituric acid (TBA) and MDA, exhibiting an absorption maximum at 532 nm (Janero, 1990). Thiobarbituric acid–reactive substances (TBARS) were measured to determine the lipid peroxidation products as a measure of oxidative stress induced by NP exposure. The values were expressed as total malondialdehyde (MDA) concentration per gram of tissue.
The MDA concentration was measured in liver and gill tissues as described by Van Ye et al. (1993). The liver and gill were washed with ice cold water and immediately frozen in liquid nitrogen and then stored at −80°C until assayed (ca. 10 days). For assaying, each tissue was thawed and about 0.1 g sub-sample was assayed using MDA kit (Northwest Life Science, LLC, Vancouver, Canada). Samples were homogenized in a 2 mL phosphate buffer solution (pH 7.2) using an ultrasonic homogenizer and then centrifuged at 6,000 rpm for ten minutes. The resulting supernatant was used for biochemical assay immediately. Briefly, 10 μL butylated hydroxytoluene (BHT), 0.25 mL of sample supernatant, 0.25 mL of phosphoric acid (1.0 M), and 0.25 mL of TBA were added to a vial. A set of MDA standards were freshly prepared from tetramethoxypropane in a concentration range of 0 to 10 μM. All samples and standards were incubated at 90 °C for one hour, and then centrifuged at 12,000 rpm for 15 minutes to separate the suspending tissue. The absorbance of the supernatant was measured at 532 nm. Measurements were performed in triplicate (biological replicates) for all experimental groups.
Water Quality
Water quality parameters, including pH, dissolved oxygen, and temperature were monitored during the course of the exposure by using a multimeter (Model HI 9828, Hanna instruments, Michigan, USA). The values were 6.8 ± 0.2 for pH, 6.0 ± 1.4 mg L-1 for dissolved oxygen and 23 ± 2.5 °C for temperature. Total hardness was measured as 130 mg L-1 CaCO3 using the titration method. Water was renewed daily leading to an exchange rate of 10 L/day/tank. Contaminated water was collected and treated with activated charcoal before discharging. The water quality in the sub-chronic toxicity experiment was not significantly different from that in the trophic transfer experiment. However, turbidity increased at 10 mg L-1 NP concentration as a result of the formation of visible CuO and ZnO NPs aggregates. No mortality was observed in any exposure regime.
Statistical Analysis
All experiments were repeated twice independently, and data were recorded as a mean value with standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey's multiple comparisons was used to detect significant differences among groups. A p-value of less than 0.05 was considered statistically significant.
Results and Discussion
Size Distribution of NPs by TEM and DLS
Stability of NPs in aqueous solution is influenced by their physicochemical properties as well as the chemistry of the medium. In general, NPs tend to aggregate in solution, and the degree of aggregation has been shown to alleviate toxicity in vitro. Therefore, successful preparation of the stable suspensions of NPs is one of the most difficult tasks for testing the actual toxic effects. In this study, we opted for exposing both suspensions of CuO and ZnO NPs to ultrasounds for two minutes to improve dispersion in water. In our previous study, we found that aeration provided effective means of mixing/agitation to maintain homogeneity of the suspensions in exposure tanks, yet aggregation of NPs occurred as observed for TiO2 and Zn NPs (Ates et al., 2013a; Ates et al., 2013b). These effects were due to the reduced surface charge as well as the surface hydration.
Aggregation of ZnO NPs was also observed for ZnO in distilled water and was considered to be an inherent property of unmodified oxide NPs (Franklin et al., 2007). The TEM images gathered from the dried NPs suspensions are illustrated in Fig. 1 along with detailed data in Table 2. Aggregation was minimal for the freshly prepared stock suspensions (Fig. 1 A and B) in that nanopowders were suspended in distilled water. Sizes of NPs ranged from 20 to 75 nm for CuO NPs and from 16 to 50 nm for ZnO NPs (see Fig. 1 A, B and Table 2). These values were within the values reported by the manufacturer (e.g., 40 nm for CuO and 10–30 nm for ZnO). However, the DLS data of the same NP suspensions showed a large distribution in size of the particles, ranging from 280 to 1172 nm for CuO and from 423 to 1275 nm for ZnO. The estimated mean sizes were 490 and 658 nm for the suspensions of CuO and ZnO NPs, respectively. These results indicate that the size of NPs increased by a factor of 20 to 100 in water due to the aggregation. The TEM image illustrated in Fig. 1C and D were gathered after 12 hours from the start of the exposure. Aggregation was more significant in salt water compared with the freshly prepared solutions because of the increasing counter ions and positively charged cations that reduced the surface stabilization.
Fig. 1.
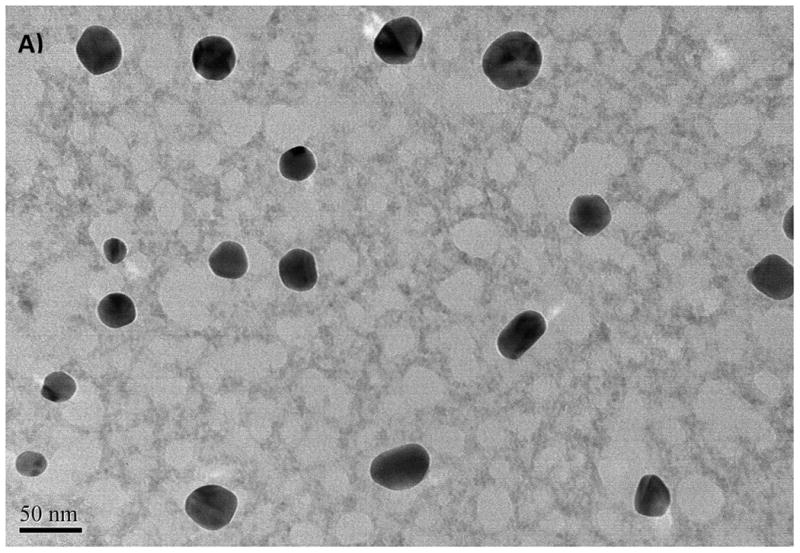
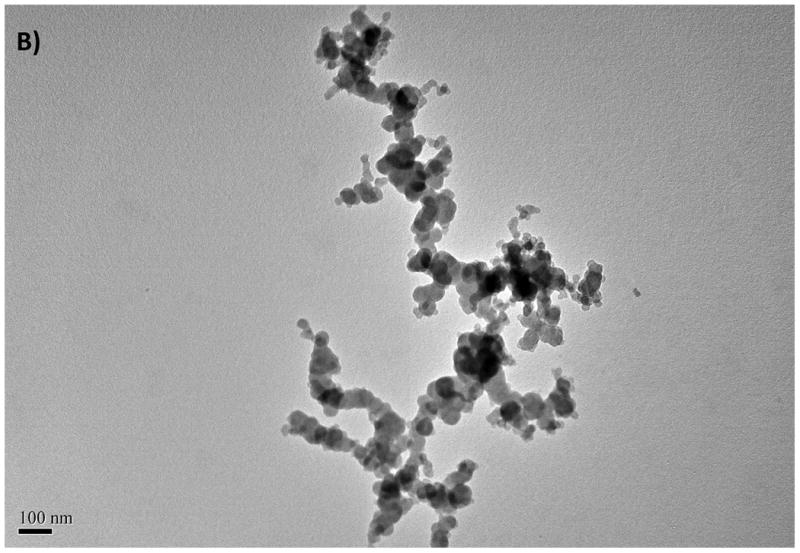
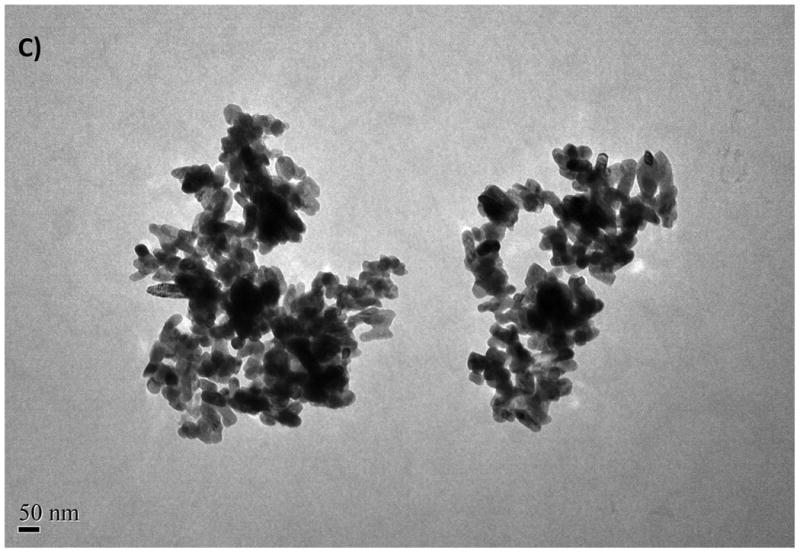
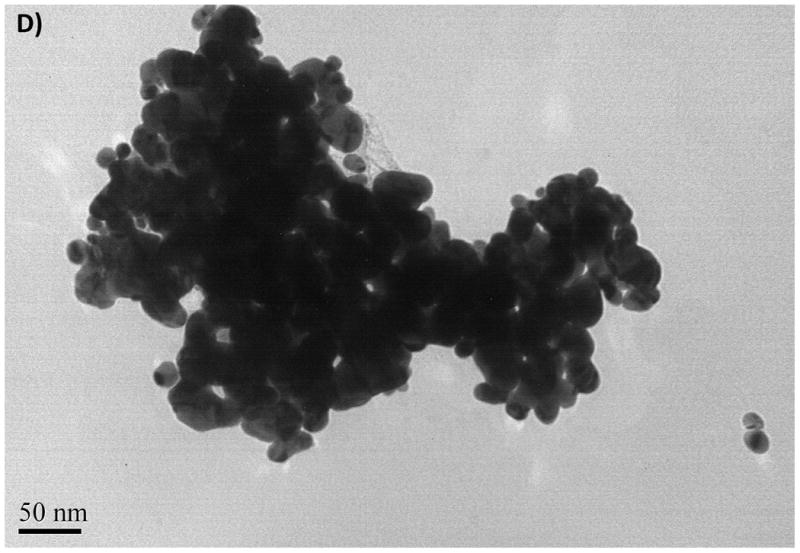
The TEM images of CuO NPs from stock solution (A), from exposure medium (C), TEM images of ZnO NPs from stock solution (B), from exposure medium (D)
Table 2. Descriptive physical properties and estimated static (TEM) and hydrodynamic (DLS) size distribution of CuO and ZnO NPs.
| Size (D50) a (nm) | Surface areaa (m2 g−1) | Dry sizeb (TEM, nm) | Hydrodynamic sizeb (DLS, nm) | Zeta potentialb | Appearance | |
|---|---|---|---|---|---|---|
| CuO (99%) | 40 | 20 | 20–75 | 280–1172 | -1.64 ± 0.32 | Black |
| ZnO (99.8%) | 10–30 | 30–50 | 16–50 | 423–1275 | -29.1 ± 4.1 | Pale yellow |
Values provided by the manufacturer.
Experimental values measured in this study from 10 μg mL-1 suspensions.
Elemental Analysis of Goldfish Organs for NP Accumulation
The gills and intestine are known to be the major organs of fish that toxicants predominantly accumulate (Farkas et al., 2010). Gills serve for gas exchange, osmoregulation, acid–base regulation, nitrogenous waste excretion, and endocrine regulation (Olson, 1998; Evans et al., 2005). Therefore, cultures of fish gill cells have been used as model systems for toxicological measurements (Lilius et al., 1995). In this study, intestine, liver, gill, heart, brain, and muscle of the exposed fish were analyzed for total Cu and Zn by ICP–MS as a measure of CuO and ZnO accumulation (Fig. 2). The results showed that accumulation of the NPs primarily took place in the intestine followed by the gills, and liver (see Fig. 2). The concentrations of Cu and Zn in the heart and brain were very low and were not significantly different from those of the controls (p≥0.05). Including the controls, Cu levels in the heart ranged from 19 to 23 μg kg-1 (ppb) and that for Zn were between 29 and 35 μg kg-1. The brain contained about 19-22 μg kg-1 Cu and 24-27 μg kg-1 Zn. The levels in the muscle tissue were also very low ranging from 13 to 28 μg kg-1 for Cu and 11 to 27 μg kg-1 for Zn. However, the levels in the muscle tissue exhibited an increasing trend with NP concentration and were statistically higher than those of the controls (p<0.05).
Fig. 2.
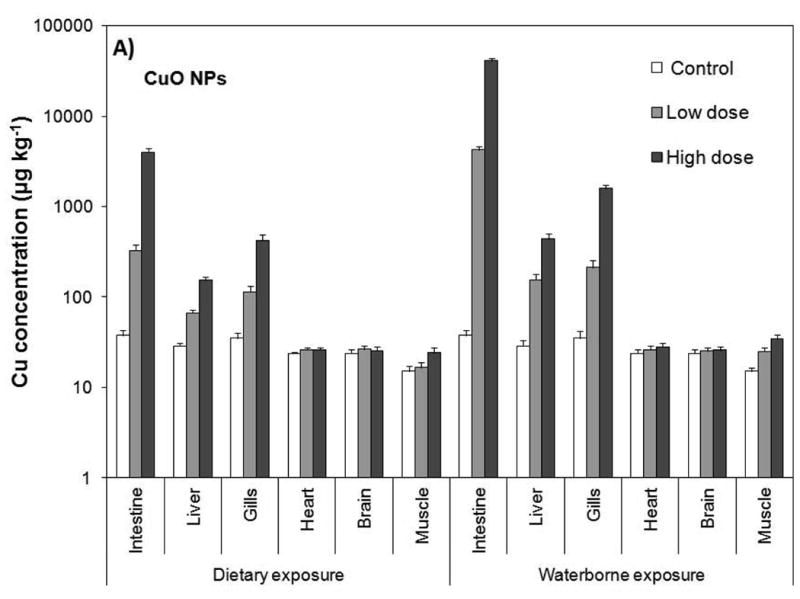
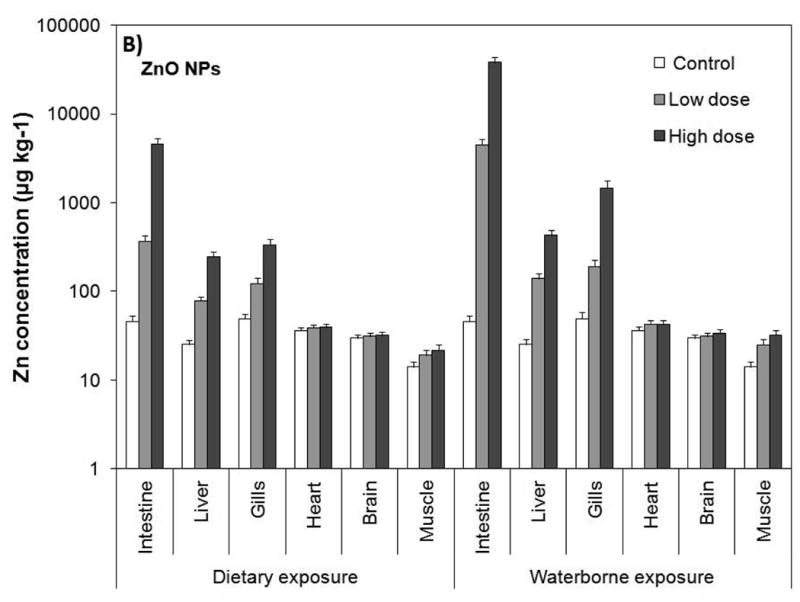
Tissue/organ Cu and Zn levels resulting from waterborne and dietary exposure to CuO (A) and ZnO (B) NPs. Values are given as average±standard deviation of triplicate exposures. The significant level is p=0.05.
The liver, gills and intestine possessed significantly higher concentrations of Cu and Zn than those of the controls (p<0.05). Cu concentration in the liver ranged from 53 to 153 μg kg-1 for dietary exposure and 123 to 350 μg kg-1 for waterborne exposure, while Zn levels ranged from 63 to 199 μg kg-1 for dietary exposure and 113 to 350 μg kg-1 for waterborne exposure. The gills possessed between 91 and 337 μg kg-1 Cu for dietary exposure, and between 170 and 1,274 μg kg-1 Cu for waterborne exposure. Zn levels in the gills varied from 99 to 269 μg kg-1 for dietary exposure and from 153 to 1,174 μg kg-1 for waterborne exposure. The levels in the intestine were the highest ranging from 261 to 3,183 μg kg-1 Cu for dietary exposure and 3,400 to 32,583 μg kg-1 Cu for waterborne exposure. Zn concentrations were similar, ranging from 295 to 3,710 μg kg-1 for dietary exposure and 3,594 to 30,591 μg kg-1 for waterborne exposure.
Effect Route of Exposure and NP Dissolution on Accumulation
It is important to note that waterborne exposure resulted in significantly higher accumulation compared with the dietary exposure (Fig. 2, p<0.05). Both Cu and Zn levels were approximately 2-, 4- and 10-fold higher in the liver, gills and intestine, respectively. Within the same exposure regime, on the other hand, the differences between total Cu and Zn were marginal. For instance, total Cu and Zn concentration in the intestines of fish were 261 and 292 μg kg−1, respectively, from dietary exposure to low dose of CuO and ZnO NPs. Likewise, intestinal Cu and Zn levels increased to 3,183 and 3,710 μg kg−1, respectively, as a result of dietary exposure to high doses of CuO and ZnO NPs. Waterborne exposure showed a similar profile, but the intestinal elemental concentrations were 10-fold higher; 3400 μg kg−1 Cu and 3,594 μg kg−1 Zn from low dose exposure, and 32,571 μg kg−1 Cu and 30,583 μg kg−1 Zn from high dose exposure to suspensions of CuO and ZnO NPs. The dietary and waterborne exposure to low and high doses of NPs affected total Cu and Zn similarly in the liver and gills. These results point to the fact that CuO and ZnO NPs' concentrations in the organs were dependent on the ambient concentrations in water and food.
Fig. 3 illustrates the distribution of free Cu and Zn levels in the exposure medium. Average Cu concentration in the supernatant ranged from 0.04 to 0.6 μg mL-1 for dietary exposure and from 0.98 to 1.75 μg mL-1 for waterborne exposure to low and high doses of CuO NPs, respectively. The values for Zn were lower ranging from 0.02 to 0.21 μg mL-1 for dietary exposure and 0.55 to 1.05 μg mL-1 for waterborne exposure to low and high doses ZnO NPs, respectively. These results clearly show that CuO NPs dissolved more readily than ZnO NPs. Additionally, the free Cu and Zn ions' concentrations in the exposure medium increased with increasing NP concentration. The average Cu and Zn levels in muscle tissue of control (not exposed) studies are not significantly different than both exposure studies (p≥0.05). This suggests that neither waterborne nor dietary exposure resulted in any significant accumulation of NPs (see Fig. 2) in brain, heart and muscle tissues.
Fig. 3.
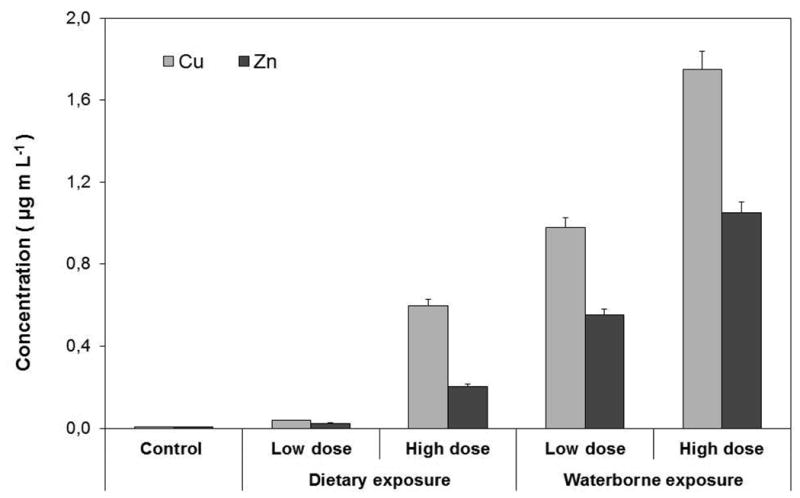
Free Cu and Zn ion concentrations released from dissolution of CuO and ZnO NPs. Values are given as average±standard deviation of triplicate exposures. The significant level is p=0.05.
Transfer of NPs between Trophic Levels
Aquatic species, specifically fish, are an important component of the human diet. In relation to this, concerns about the adverse effects of NPs on the food chain have grown rapidly due to the fact that ingested NPs at the lower trophic levels could be mobilized to higher organisms. Uptake of nanomaterials by fish and other aquatic species has been reported previously (Kashiwada, 2006; Moore, 2006). Absorption via the gills is one of the potential routes of uptake in fish resulting in accumulation of particles in the intestine (Handy et al., 2008). Ferry et al., (2008) reported that NPs could pass from the water column to the aquatic food web. It was also found that NPs, such as carboxylated and biotinylated quantum dots (QDs) could be transferred to higher trophic organisms (rotifers) through dietary intake of ciliated protozoan (Holbrook et al., 2008). Similarly, transfer of QDs was found in a simple food chain from algae (Pseudokirchneriella subcapitata) to zooplankton (Ceriodaphnia dubia) (Bouldin et al., 2008).
The results in this study indicated that despite significant accumulation of CuO and ZnO NPs in the intestine, liver and gills, neither dietary uptake nor waterborne exposure could lead to bioaccumulation in the muscle, heart and brain. However, ionic Cu and Zn could bioaccumulate in the tissues under long-term exposure since CuO and ZnO NPs are known to decompose in time. These results are consistent with those observed in zebrafish and rainbow trout (NOAA, 1988; Handy et al., 2008). Similarly, dietary uptake of NPs by zebrafish were found to lead to deposition in the intestine, but mobilized to other organs in the fish by diffusion and blood circulation (NOAA, 1988). Rainbow trout (Oncorhynchus mykiss) were exposed to TiO2 NPs (34 nm) and bulk (>100 nm) TiO2 via waterborne (0.5 and 5.0 μg mL−1), dietary (0.1 and 1 mg g−1 food) and intravenous injection (1.3 mg kg−1 body weight). Titanium dioxide (TiO2) levels in the tissues of trout after waterborne and dietary exposure was found to be very low (Handy et al., 2008).
Oxidative Stress Associated with CuO and ZnO NP Exposure
MDA is an end product of lipid peroxidation and hence is a robust index of oxidative stress. The MDA levels measured from the liver and gills of goldfish are summarized in Table 3. The results for the gills indicate that ZnO NPs did not increase the MDA levels via dietary exposure nor in waterborne exposure; the MDA levels measured from treatments were not statistically different from that of controls (p≥0.05). Dietary exposure to low dose of CuO NPs did not exhibit any toxicity (p≥0.05), but MDA levels in the gills increased significantly during dietary exposure to high doses of CuO NPs (p<0.05). Waterborne exposure to suspensions of CuO NPs induced oxidative stress such that MDA levels were higher than that of controls at any NP concentration (p<0.05). Dietary and waterborne exposure to ZnO NPs resulted in marginal increase in liver MDA levels in comparison with the controls (p=0.041). In contrast, oxidative stress levels from exposure CuO NPs were significant for both dietary and waterborne exposure (p<0.05). Apparently, the liver was more sensitive to the effects of ZnO and CuO NPs despite the fact that liver possessed lower concentrations of Cu and Zn as a result of exposure.
Table 3. Malondialdehyde levels (nmol g−1) measured in liver and gills of goldfish.
| Exposure route | Groups | CuO NPs | ZnO NPs | ||
|---|---|---|---|---|---|
|
| |||||
| Liver | Gill | Liver | Gill | ||
| Control | 30.0 ± 1.9 | 14.6 ± 2.5 | 31.5 ± 2.1 | 12.0 ± 2.4 | |
| Dietary | Low dose | 59.0 ± 4.0 | 16.3 ± 3.4 | 37.5 ± 4.9 | 13.4 ± 1.6 |
| High dose | 71.6 ± 3.5 | 25.6 ± 4.9 | 42.8 ± 3.5 | 13.1 ± 2.8 | |
| Waterborne | Low dose | 71.0 ± 6.3 | 35.0 ± 1.7 | 40.0 ± 6.1 | 13.8 ± 2.2 |
| High dose | 83.2± 2.2 | 41.5 ± 3.7 | 45.0 ± 4.0 | 14.1 ± 4.2 | |
ZnO NPs have been found to be toxic to the microalgae Pseudokirchneriella subcapitata (LC50 = 0.04 mg L−1), to crustaceans Daphnia magna (LC50 = 3.2 mg L−1) and Thamnocephalus paltyurus (LC50 = 0.18 mg L−1), and to the bacteria Vibrio fischeri (LC50 = 1.9 mg L−1) (Heinlaan et al., 2008; Aruoja et al., 2009). These studies also reported that ZnO NPs exhibited nearly identical toxicities with bulk ZnO and Zn ions (ZnSO4 or ZnCl2). This has brought the conclusion that toxic effects on the test species were due to the production of ionic Zn (Heinlaan et al., 2008; Aruoja et al., 2009). Accordingly, toxic effects from ZnO NPs were marginal in this study even in waterborne exposure, which could be attributed to the fact that free Zn ion concentrations were not high enough to induce oxidative stress on goldfish (Ates et al., 2013b). Although the effects of CuO NPs vary among species, they were consistently more toxic then the bulk CuO. For instance, the LC50 levels on the crustaceans Daphnia magna and Thamnocephalus paltyurus were 3.2 and 2.1 mg L−1, respectively. The LC50 values for the bulk CuO, on the other hand, were 165 mg L−1 for D. magna and 95 mg L−1 for T. paltyurus (Heinlaan et al., 2010). Micro–organisms showed similar patterns of vulnerability as the aquatic crustaceans. The LC50 level for bacteria Vibrio fischeri was 79 mg L−1 for the CuO NPs compared with 3811 mg L−1 for the bulk CuO, and to that for the algae Pseudokirchneriella subcapitata was 0.71 mg L−1 for CuO NP compared to 11.5 mg L−1 for the bulk CuO (Ward and Kach, 2009; Aruoja et al., 2009). The elevated toxicity of the CuO NPs over the bulk CuO could be affected by the increase in the surface area of the NPs since aqueous suspension of the CuO NPs possessed 39–fold larger surface area than the bulk CuO.
The results here clearly show that suspensions of CuO NPs were more toxic than ZnO NPs regardless of the route of exposure. These results are consistent with those reported by Heinlaan et al. (2010) who found that CuO NPs were more toxic than bulk CuO. While bulk CuO is insoluble, CuO NPs are more soluble in water. This assumption is supported with experimental results that Cu ion concentration was significantly higher in suspensions of CuO NPs (Fig. 3) than that of Zn in suspensions of ZnO NPs. Therefore, it could be suggested that elevated concentration of Cu ions might also contribute to the higher toxicity of CuO NPs.
Conclusion
In this study, bioaccumulation profiles and toxicological effects of ZnO and CuO NPs were examined by dietary and waterborne exposure of goldfish. It was found that NPs accumulated mainly in the intestine, gills and liver (up to 32,583, 1,274 and 350 μg kg-1 respectively for high concentration of CuO NP waterborne exposure) of goldfish. The intestine was the major biological repository followed by the gills and the liver. The gills accumulated significant level of NPs in waterborne exposure through uptake, but was not significant in dietary exposure. The heart, brain and muscle tissue did not exhibit any significant NP accumulation under any exposure condition. However, the results imply that ionic Cu and Zn could bioaccumulate in the muscle tissue as the NPs undergo dissolution.
Uptake of NPs was lower in dietary exposure in comparison to waterborne exposure. Nevertheless, the results demonstrated that the ingestion of contaminated food is a pathway for the transfer of the NPs from lower trophic levels to higher organisms in the food chain. The results also suggest that toxic effects of NPs could vary with route of exposure and chemical properties of NPs. ZnO NPs did not increase MDA level significantly through dietary exposure. The effects under sub–chronic waterborne exposure were also marginal, which implied that the suspensions of the ZnO NPs were not acutely toxic to goldfish. The suspensions of the CuO NPs, on the other hand, were more toxic in both dietary and waterborne exposure. This study shed light onto the short-term effects of exposure to ZnO and CuO NPs. The effects of possible exposure could be different under long–term conditions considering the bioavailability and decomposition of NPs in natural systems, which need to be studied to better understand toxicological impact and fate of NPs along the food chain.
Acknowledgments
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (grant no: G12RR013459) and the U.S. Department of Defense (DOD) through the Engineer, Research and Development Center (Vicksburg, MS) (contract #W912HZ-10-2-0045). The views expressed herein are those of the authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies. The authors thank Jackson State University, Biostatistical Support Unit for assistance in statistical analysis.
References
- Arslan Z, Ates M, McDuffy W, Agachan MS, Farah IO, Yu WW, Bednar AJ. Probing metabolic stability of CdSe nanoparticles: alkaline extraction of free cadmium from liver and kidney samples of rats exposed to CdSe nanoparticles. J Hazard Mater. 2011;192:192–199. doi: 10.1016/j.jhazmat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoja V, Dubourguier HC, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ. 2009;407:1461–1468. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Ates M, Daniels J, Arslan Z, Farah IO. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environ Monit Assess. 2013a;185:339–3348. doi: 10.1007/s10661-012-2794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M, Daniels J, Arslan Z, Farah IO, Félix-Rivera H. Comparative evaluation of impact of Zn and ZnO nanoparticles on brine shrimp (Artemia salina) larvae: effects of particle size and solubility on toxicity. Environ Sci: Processes Impacts. 2013b;15:225–233. doi: 10.1039/c2em30540b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becheri A, Dürr M, Nostro PL, Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV–absorbers. J Nanopart Res. 2008;10:679–689. [Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- Bouldin JL, Ingle TM, Sengupta A, Alexander R, Hannigan RE, Buchanan RA. Aqueous toxicity and food chain transfer of quantum dots™ in freshwater algae and Ceriodaphnia dubia. Environ Toxicol Chem. 2008;27(9):1958–1963. doi: 10.1897/07-637.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin JL, Ingle TM, Sengupta A, Alexander R, Hannigan RE, Buchanan RA. Aqueous toxicity and food chain transfer of Quantum DOTs in freshwater algae and Ceriodaphnia dubia. Environ Toxicol Chem. 2008;27:1958–1963. doi: 10.1897/07-637.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R. The challenge of regulating nanomaterials. Environ Sci Technol. 2008;42:339–343. [PubMed] [Google Scholar]
- Crane M, Handy RD. An assessment of regulatory testing strategies and methods for characterizing the ecotoxicological hazards of nanomaterials. Defra; London, UK: 2007. [Google Scholar]
- Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: Dominant site of gas exchange, Osmoregulation, Acid–Base regulation, and excreation of nitrogenous waste. Physiol Rev. 2005;85:97–177. doi: 10.1152/physrev.00050.2003. [DOI] [PubMed] [Google Scholar]
- Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR. Silver nanoparticles: Behavior and effects in the aquatic environment. Environ Int. 2011;37:517–531. doi: 10.1016/j.envint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Farkas J, Christian P, Urrea JA, Roos N, Hassellöv M, Tollefsen KE, Thomas KV. Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol. 2010;96:44–52. doi: 10.1016/j.aquatox.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007;84:415–430. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Ferry JL, Craig P, Hexel C, Sisco P, Frey R, Pennington PL, Fulton MH, Scott IG, Decho AW, Kashiwada S, Murphy CJ, Shaw TJ. Transfer of gold nanoparticles from the water column to the estuarine food web. Nat Nanotechnol. 2009;4:441–444. doi: 10.1038/nnano.2009.157. [DOI] [PubMed] [Google Scholar]
- Fisk AT, Hobson KA, Norstrom RJ. Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the northwater polynya marine food web. Environ Sci Technol. 2001;35:732–738. doi: 10.1021/es001459w. [DOI] [PubMed] [Google Scholar]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalge (Pseudokirchneriella subcapituta): the importance of particle solubility. Environ Sci Technol. 2007;41:8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- Gallego A, Martín–González A, Ortega R, Gutiérrez JC. Flow cytometry assessment of cytotoxicity and reactive oxygen species generation by single and binary mixtures of cadmium, zinc and copper on populations of the ciliated protozoan Tetrahymena thermophila. Chemosphere. 2007;68:647–661. doi: 10.1016/j.chemosphere.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Galloway TS, Sanger RC, Smith KL, Fillmann G, Readman JW, Ford TE. Rapid assessment of marine pollution using multiple biomarkers and chemical immunoassays. Environ Sci Technol. 2002;36:2219–2226. doi: 10.1021/es010300w. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- Goyer RA, Clarkson TW. In: Toxic effects of metals, in Casarett and Doull's Toxicology: The Basic Science of Poisons. Klaassen CD, editor. McGraw–Hill; Columbus, OH: 2001. pp. 811–867. [Google Scholar]
- Handy R, Ramsden C, Smith T, Shaw B. Toxicology of dietary titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol A: Mol Integr Physiol. 2008;150:56–63. [Google Scholar]
- Heinlaan H, Ivask A, Blinova I, Dubourguier HC, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus paltyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Heinlaan M, Kahru A, Kasemets K, Arbeille B, Prensier G, Dubourguier HC. Changes in the Daphnia magna midgut upon ingestion of copper oxide nanoparticles: A transmission electron microscopy study. Water Res. 2010;45:179–190. doi: 10.1016/j.watres.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Holbrook RD, Murphy KE, Morrow JB, Cole KD. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat Nanotechnol. 2008;3:352–355. doi: 10.1038/nnano.2008.110. [DOI] [PubMed] [Google Scholar]
- Janero DR. Malondialdehyde and thiobarbituric acid reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Johnston HJ, Semmler-Behnke M, Brown DM, Kreyling W, Tran L, Stone V. Evaluating the uptake and intracellular fate of polystyrene nanoparticles by primary and hepatocyte cell lines in vitro. Toxicol Appl Pahrm. 2010;242:66–78. doi: 10.1016/j.taap.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Kashiwada S. Distribution of nanoparticles in the see–through Medaka (Oryzias latipes) Environ Health Perspect. 2006;114:1697–1702. doi: 10.1289/ehp.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR. Nanomaterials in the environment: behavior, fate, bioavailability and effects. Environ Toxicol Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XN. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius H, Sandbacka M, Isomaa B. The use of freshly isolated gill epithelial cells in toxicity testing. Toxicol in Vitro. 1995;9:299–305. doi: 10.1016/0887-2333(95)00010-6. [DOI] [PubMed] [Google Scholar]
- Livingstone DR. Contaminant–stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull. 2001;42:656–666. doi: 10.1016/s0025-326x(01)00060-1. [DOI] [PubMed] [Google Scholar]
- Lovern SB, Klaper R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem. 2006;25:1132–1137. doi: 10.1897/05-278r.1. [DOI] [PubMed] [Google Scholar]
- Matranga V, Corsi I. Toxic effects of engineered nanoparticles in the marine environment: Model organisms and molecular approaches. Mar Environ Res. 2012;76:32–40. doi: 10.1016/j.marenvres.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Montes MO, Hanna SK, Lenihan HS, Keller AA. Uptake, accumulation and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. J Haz Mater. 2012:225–226. 139–145. doi: 10.1016/j.jhazmat.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- NOAA. Fish as Sentinels of Environmental Health. US National Marine Fisheries Service, Office of the Press Department of Commerce; Springfield, VA, USA: 1988. [Google Scholar]
- OECD. Organization for Economic Co-operation and Development, Guideline for the Testing of Chemicals: Part 203 1992 [Google Scholar]
- Ohkawa H, Ohishi N. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Olson KR. Hormone metabolism by the fish gill. Comp Biochem Physiol Part A: Mol Integr Physiol. 1998;119:55–65. doi: 10.1016/s1095-6433(97)00406-6. [DOI] [PubMed] [Google Scholar]
- Owen R, Handy RD. Formulating the problems for environmental risk assessment of nanomaterials. Environ Sci Technol. 2007;41:5582–5588. doi: 10.1021/es072598h. [DOI] [PubMed] [Google Scholar]
- Paynton HC, Lazorchak JM, Impellitteri CA, Smith ME, Rogers K, Patra M, Hammer KA, Allen HJ, Vulpe CD. Differential gene expression in Daphnia magna suggests distinct models of action and bioavailability for ZnO nanoparticles and Zn ions. Environ Sci Technol. 2011;45:762–768. doi: 10.1021/es102501z. [DOI] [PubMed] [Google Scholar]
- Persoone G, Van de Vell A, Van Steertegem M, Nayer B. Predictive value for laboratory tests with aquatic invertebrates: influence of experimental conditions. Aquat Toxicol. 1989;14:149–166. [Google Scholar]
- Reijnders L. Cleaner nanotechnology and hazard reduction of manufactured nanoparticles. J Cleaner Prod. 2006;14:124–133. [Google Scholar]
- Serpone N, Dondi D, Albini A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg Chim Acta. 2007;360:794–802. [Google Scholar]
- Sofyan A, Rosita G, Price DJ, Birge WJ. Cadmium uptake by Ceriodaphnia dubia from different exposures: Relevance to body burden and toxicity. Environ Toxicol Chem. 2007;26:470–477. doi: 10.1897/06-232r.1. [DOI] [PubMed] [Google Scholar]
- Tao X, Fortner JD, Zhang B, He Y, Chen Y, Huges JB. Effects of aqueous stable fullerene nanocrystals (nC60) on Daphnia magna: Evaluation of sub–lethal reproductive responses and accumulation. Chemosphere. 2009;77:1482–1487. doi: 10.1016/j.chemosphere.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Taylor G, Baird DJ, Soares AMVM. Surface binding of contaminants by algae: Consequences for lethal toxicity and feeding to Daphnia magna straus. Environ Toxicol Chem. 1998;17:412–419. [Google Scholar]
- Van Ye TM, Roza AM, Pieper GM. Inhibition of intestine lipid peroxidation does not minimize morphologic damage. J Surg Res. 1993;55:553–558. doi: 10.1006/jsre.1993.1183. [DOI] [PubMed] [Google Scholar]
- Ward JE, Kach DJ. Marine aggregates facilitate ingestion of nanoparticles by suspension–feeding bivalves. Mar Environ Res. 2009;68:137–142. doi: 10.1016/j.marenvres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang J, Zhang X, Chang Y, Chen Y. Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere. 2010;79:928–933. doi: 10.1016/j.chemosphere.2010.03.022. [DOI] [PubMed] [Google Scholar]


