Abstract
Tunneling nanotubes (TnTs) represent a novel mechanism by which intercellular components such as proteins, Golgi vesicles, and mitochondria can be transferred from cell to cell in the complex tumor microenvironment. Here, we report data showing that microRNAs (miRNAs) are transferred through TnTs in osteosarcoma and ovarian cancer as in vitro model systems. miRNA array analysis demonstrated significant upregulation of miR-19a in osteosarcoma tumors resected from human patients, and differential expression of miR-199a in ovarian cancer cell lines resistant or sensitive to platinum chemotherapy. K7M2 murine osteosarcoma cells were transfected with miR-19a and cultured with non-transfected K7M2 cells in low-serum, hyperglycemic medium for up to 72 hours to induce TnT formation. miRNA transfer via TnTs was detected by time-lapse microscopic imaging. miR-19 was also transported via TnTs connecting transfected K7M2 cells and non-transfected stromal MC3T3 murine osteoblast cells. Similar findings were observed in studies of TnT-mediated transport of miR-199a among SKOV3 ovarian cancer cells and non-malignant IOSE human ovarian epithelial cells. To quantify TnT-mediated transport of miRNAs, we used modified Boyden chambers to separate miR19a-transfected K7M2 cells (top chamber) and DiI-labeled MC3TC cells (bottom chamber) as compared to open culture of these cells. FACS analysis of cells collected after 48-hours of culture indicated that miR19a-positive MC3TC cells was 3-fold higher in open culture; this finding suggest that miR-19a occurred via TnTs, exclusive of other forms of cell-cell communication. These studies demonstrate that TnTs mediate direct transfer of genetic material between tumor and stromal cells.
Introduction
Intercellular communication among distant and proximal cells in the heterogeneous tumor microenvironment has emerged as an important paradigm for understanding tumor growth and invasion. Gap junctions, chemical messengers (eg, cytokines), and exosomes are well established forms of intercellular communication[1, 2]; however, tunneling nanotubes (TnTs), represent a novel mechanism by which intercellular components can be transferred from cell to cell in the complex tumor microenvironment[3, 4]. TnTs are long, thin, actin-based, cytoplasmic extensions that form de novo and can serve as conduits for intercellular shuttling of cargo such as proteins, Golgi vesicles, and mitochondria. We identified TnTs in solid tumors resected from patients with mesothelioma and lung adenocarcinomas[4]; this observation provided the first evidence of the potential in vivo relevance of TnTs in human solid tumor malignancies.
Intercellular exchange of genetic materials has the potential for inducing malignant transformation and for affecting gene regulation in recipient cells. Small noncoding RNAs in particular have gained strong interest for their critical role in tumor cell regulation, specifically by posttranscriptional modification of key regulator gene products. We hypothesized that TnTs were capable of transporting microRNAs (miRNAs) as a novel mechanism of tumor-tumor and tumor-stromal cross-talk among neighboring and distant cells. Using osteosarcoma (OS) and ovarian cancer as model systems, we examined whether miRNAs are transferred via TnTs in invasive cancers of high metastatic potential.
Materials and Methods
For additional pertinent details, please also see Supplemental Information section. This paper conforms to the relevant ethical guidelines for human and animal research.
Culture conditions for TnT formation
TnT formation was assessed in standard and hyperglycemic culture conditions. Standard conditions consisted of 10% FBS, 25 mM glucose RPMI-1640. Cells were also cultured in a low-serum, high-glucose environment to assess the growth of TnTs as previously described[4]. Specifically, culture conditions we used to stimulate TnT formation consisted of RPMI-1640 medium with 2.5% FBS, 50mM glucose, 1% P-S, 2% L-Glutamine, 10 nM ammonium lactate, and pH 6.6.
Transfection and co-culture of cells and time-lapse imaging
For osteosarcoma (OS) studies: K7M2 cells were reverse-transfected with Alexa-488 labeled miR-19a (250 ng of RNA per 1 × 106 cells). Transfection efficiency was assessed by visual inspection by fluorescent microscopy (Olympus IX70), 15 hrs after transfection. Cells were collected by trypsinization 16 hrs post-transfection, and subjected to co-culture experiments as needed. DiI and DiO are commercially available lipophilic dyes that fluoresce in the red and green channels, respectively (Life Technologies, Carlsbad, CA) and whose use for TnT studies we have described previously[4]. Equal proportions of miR-19a transfected-K7M2 cells were co-cultured with DiO stained MC3T3 cells in 1:1 mixture of 10% FBS and antibiotic-containing alpha MEM and DMEM under standard conditions. The cells were allowed to adhere at standard cell culture conditions for 16 hrs before imaging.
For ovarian cancer studies: SKOV3 cells were reverse transfected with Alexa-488 labeled miR-199a. Equal proportion of miR-199a transfected-SKOV3 cells were co-cultured with DiO-stained IOSE cells in 1:1 mixture of 10% FBS and antibiotic-containing alpha MEM and DMEM under standard conditions.
Live imaging of miR-19a transfer from K7M2 to MC3T3cells
Alexa-488 labeled miR-19a transfected K7M2 cells were mixed with DiI stained MC3T3 cells in a 1:1 ratio (10 × 105 cells each) and plated in 35mm petri dishes with glass bottom (MatTek corporation, Ashland, MA. Catl no: P35G-1.5-20-C) in a 1:1 mixture of alpha MEM and DMEM with 10 % FBS and antibiotics. The cells were allowed to adhere at standard cell culture conditions for overnight before imaging. Cells were imaged by live cell microscopy (Vivaview, Olympus Corporation) at 20 X objective lens every 5 minutes for 48 hrs at phase, green and red fluorescence channels and analyzed using Metamorph software.
Live imaging of miR-19a transfer from K7M2 to K7M2 cells
miR-19a transfected K7M2 cells (4 × 105 cells) were plated on to the 4 chambered 35mm petri dishes with glass bottom (InvitroSci, Sunnyvale, CA; Catl no: D35C4-20-1-N) and allowed to adhere at standard cell culture conditions for 16 hrs and imaged by live cell microscopy every 5 minutes for 48 hrs (BiostatinIMQ, Nikon Instruments Inc) at phase, green and phase channels using 20X objective lens. Images were analyzed using NIS elements AR (Nikon, version 4.00.07).
Live imaging of miR-199a transfer among SKOV3 and IOSE cells
Cultures were prepared as described above. Time-lapse imaging was performed using Olympus, Zeiss, Nikon, and Incucyte microscopes. All environmental chambers were maintained at 37°C and 5% CO2. Brightfield images were taken every 15 minutes for 24–48 hours. DIC (differential interference contrast) and fluorescent imaging was performed concurrently.
Results
To provide supportive evidence of potential in vivo relevance of nanotubes in osteosarcoma (OS) and ovarian cancers, and also in both human and animal tumors in general, we performed microscopic imaging of ex vivo tumor explants from both of these invasive cancers. Samples were obtained by surgical resection of tumors from an orthotopic syngeneic mouse model of osteosarcoma and from human patients with ovarian adenocarcinoma. We detected nanotube conduits in murine orthotopic osteosarcoma (Figure 1A, 1B; a low-magnification image is also provided in Supplemental Figure S1) and human ovarian tumors (Figure 1C). We also evaluated 6 tumors from ovarian cancer patients. These tumors were more dense than OS. Nonetheless, we did visualize nanotube-like structures following tissue staining with MitoTracker dye (Figure 1C). These data demonstrate that TnTs were present in both human and murine tumor explants, and further support their existence and potential in vivo relevance. We next investigated whether our in vitro models of osteosarcoma and ovarian cancer formed TnTs. We cultured murine osteosarcoma and osteoblast cells, and separately human ovarian adenocarcinoma and normal ovarian epithelial cells, using previously published protocols. These in vitro data confirmed that TnTs form tumor-tumor and tumor-stromal cell connections in both osteosarcoma (Figure 2) and ovarian cancer (Figure 3) (see Figure Legend for details).
Figure 1. Identification of nanotube structures in resected tumors.
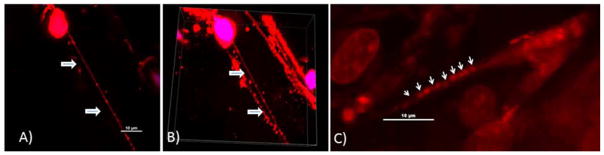
A) Visualization of a long nanotube in a murine orthotopic model of osteosarcoma. Tumors were sectioned using a Vybratome and stained using MitoTracker Red dye to visualize mitochondria. The samples we imaged represent only a very small portion of the entire tumor volume. Nonetheless, for surgically excised murine OS tumors, using confocal microscopy and 3-dimensional reconstruction with Imaris software analysis, we readily identified and visualized nanotubes in 2 of 3 tumors excised from 3 mice. We measured nanotube width in order to differentiate these protrusions from other cellular extensions, and confirmed these nanotubes were well under 1.0 μm in width. These intratumoral nanotubes measured ~80 μm in length, and were consistent with our in vitro findings of OS and other forms of cancer cells culture in vitro (both cell lines and primary).
B) Snapshot of 3-dimensional z-stacked imaging of several nanotubes within the matrix of a murine orthotopic osteosarcoma tumor.
C) Nanotube in a tumor surgically resected from a human patient with ovarian adenocarcinoma. The tumor was stained with fluorescing MitoTracker Orange dye and imaged using confocal microscopy with z-stacking of images.
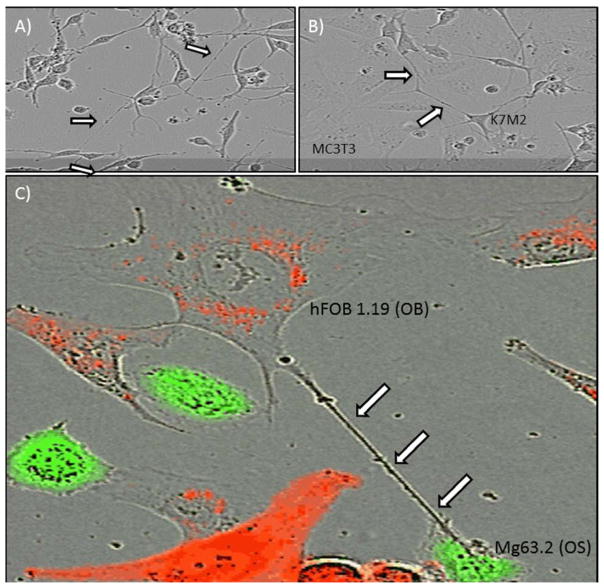
Figure 2. In vitro demonstration of intercellular tumor-tumor and tumor-stromal interaction via TnTs in osteosarcoma.
TnTs formed between (A) K7M2 murine osteosarcoma cells; (B) K7M2 and MC3T3 murine osteoblast cells; and (C) Mg63.2 human OS cells and hFOB1.19 human OB cells. Cells were labeled using the lipophilic dyes DiI (red) and DiO (green) per the manufacturer’s protocol and per our prior demonstration using these dyes to visualize TnTs with fluorescence microscopy[4]. In cases of co-culture, cells were cultured in a 1:1 ratio.
Figure 3. . In vitro demonstration of intercellular tumor-tumor and tumor-stromal interaction via TnTs in ovarian cancer.
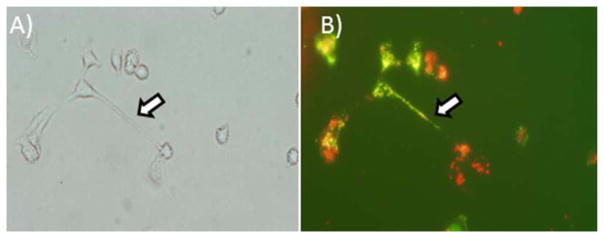
Ovarian cancer SKOV3 cells also formed TnTs to each other and to IOSE cells in co-culture. TnTs connecting DiO-labeled SKOV3 human ovarian cancer cells (green) with DiI-labeled IOSE human ovarian epithelial cells (red) are demonstrated in the following panels. (A) Brightfield image; and (B) same frame imaged using TRITC filter. Cells were labeled using the lipophilic dyes DiI (red) and DiO (green) per the manufacturer’s protocol and per our prior demonstration using these dyes to visualize TnTs with fluorescence microscopy[4]. In cases of co-culture, cells were cultured in a 1:1 ratio. These in vitro data confirmed that TnTs form tumor-tumor and tumor-stromal cell connections in both osteosarcoma and ovarian cancer.
We next performed in vitro studies to assess whether TnTs can directly transport miRNAs between connected cancer cells. For this study, we not only examined tumor cell-tumor cell interactions but also tumor-stromal transport of miRNAs via TnTs. We first sought to identify specific miRNAs relevant to the biology of osteosarcoma and ovarian cancer. miRNA array analysis demonstrated significant upregulation of miR-19a in osteosarcoma tumors resected from human patients (Figure 4A) consistent with our previous results[5], and differential expression of miR-199a in ovarian cancer cell lines resistant or sensitive to platinum chemotherapy (Figure 4B).
Figure 4. Identification of differential miRNA expression.
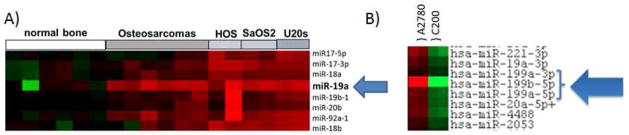
A) miRNA overexpression of miR-19a in human osteosarcoma compared with normal bone.
B) miRNA expression pattern of the miR-199 family in cisplatin-sensitive (A2780, on left) and resistant (C200, on right) ovarian cancer cell lines.
To determine whether TnTs mediate the transfer of miR-19a in osteosarcoma and miR-199 in ovarian cancer, we used time-lapse fluorescent microscopic imaging to visualize miRNAs and document their intercellular movement. We examined TnT formation and intercellular transfer of miRNA. miR-19a-transfected K7M2 cells were cultured in a 1:1 ratio with non-transfected K7M2 cells in low-serum, hyperglycemic medium for up to 72 hours to induce TnT formation. miRNA transfer via TnTs was detected by time-lapse microscopic imaging (Figure 5A; also Supplemental Movie 1). The approximate average rate of intercellular transport via TnTs was 1.6 μm/min. To further study potential tumor-stromal interactions in sarcoma, we performed a similar experiment using miR-19-transfected K7M2 cells with MC3T3 cells and miR-19-transfected MC3T3 cells with non-transfected K7M2 cells. Interestingly, miR-19 was in fact transported effectively and in a direct cell-to-cell manner via TnTs connecting transfected malignant K7M2 cells and non-transfected stromal MC3T3 cells (Figure 5B; also Supplemental Movie 2).
Figure 5. Intercellular transfer of miRNA via TnTs, captured using time-lapse imaging.
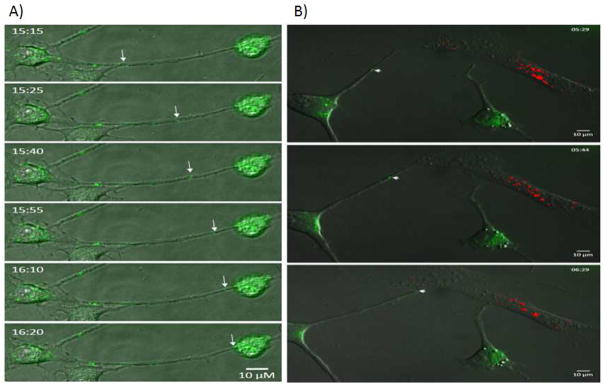
We performed in vitro transcription of miR-19a and -199a with the fluorophore, Alexa-488. Alexa-488 is a standard fluorophore that can be visualized reliably using fluorescent microscopy without interfering with transcription and movement of miRNA. Successful transfection of miRNAs into the appropriate cell lines was confirmed by direct visualization using fluorescent microscopy.
A) Images taken from time-lapse microscopy demonstrating intercellular transfer of Alexa-Fluor-488-tagged miR-19a between two K7M2 murine OS cells connected via a long TnT.
B) Time-lapse images demonstrating a miR-19a-expressing K7M2 cell (green) extending a long TnT that connects with an MC3T3 OB cell marked by fluorescent DiI (red). The K7M2 forms a TnT connection which then allows transfer of the miRNA cargo to the OB cell.
Similar findings were observed for TnT-mediated tumor cell interactions and tumor-stroma interactions in ovarian cancer. We used malignant SKOV3 and non-malignant IOSE cells to assess the transfer of miR-199a. This miRNA family is differentially expressed in ovarian tumor cells that display platinum chemotherapy resistance, a major clinical problem in ovarian cancer for which the underlying biologic mechanisms are not yet well understood. miR-199a was transported via TnTs connecting SKOV3 cells as seen on time-lapse microscopic imaging (Figure 6A). Additionally, co-cultures of SKOV3 and IOSE cells exhibited TnT-mediated transfer of miR-199a from SKOV3 to IOSE (Figure 6B; please also see Supplemental Movie 3 and Supplemental Figure S2). The approximate length of time of miRNA transport was 1.15 μm/min between connected ovarian cancer cells (comparable to the in vitro transport rate for osteosarcoma). Take together, these time-lapse fluorescence microscopic imaging data provide visual evidence of direct cell-to-cell transfer of miRNAs via TnTs in in vitro models of osteosarcoma and ovarian cancer.
Figure 6. Demonstration of miR-199a in multiple TnTs connecting SKOV3 human ovarian adenocarcinoma cells in vitro.
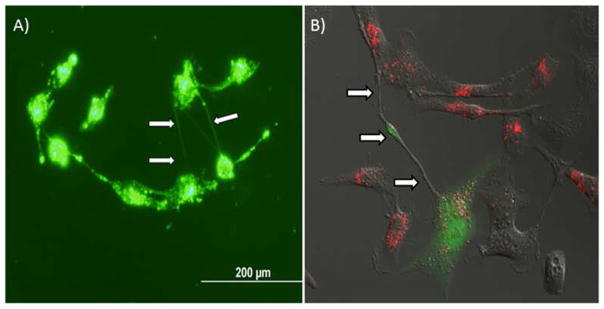
SKOV3 cells transfected with Alexa-Fluor-88-tagged miR-199a were cultured in a low-serum, hyperglycemic culture medium.
A) Multiple TnTs connecting SKOV3 cells expressing fluorescing miR-199a.
B) Demonstration of a long TnT connecting a miR-199a-transfected SKOV3 cell (green) to a DiI-marked IOSE (red) ovarian epithelial cell, with intercellular transport of miR-199a in progress.
We further adapted invasion assays using modified Boyden chambers to quantitate transport of miRNAs specifically taking place via TnTs. The rationale for this approach is that filters used for standard available invasion assays would create a physical barrier that could significantly reduce intercellular exchange of diffusible signals including cytokines and exosomes. In addition, they might exclude formation and maintenance of gap junctions by preventing immediate contact formation by adjacent cells, while yet permitting potential TnT formation between separated cells. A recent study suggested that use of transwell assays may cut down on contact-independent forms of intercellular transfer of miRNAs (including, for example, microvesicular or exosomal trafficking) by as much as 84%[6]. We performed several variations of co-culture, using miR19a-transfected K7M2 cells in the top chamber co-cultured with DiI labeled MC3TC cells in the bottom well. We performed FACS analysis on cells collected from a 48-hour culture of miR19a-transfected K7M2 cells openly co-cultured with DiI labeled MC3T3 cells without membrane barrier. FACS analysis demonstrated the proportion of double-positive population was 44.8% in open culture (Figure 7A), compared with only 16.4% of MC3T3 cells from the transwell culture (Figure 7B). Thus these data support our hypothesis that intercellular transfer of miR-19a can occur via TnTs, exclusive of other forms of cell-cell communication, in a quantifiable manner, and that this approach can be used in future studies examining the effects of TnT-mediated transfer of genetic materials between cells. Based on these data, we are developing and refining further techniques and methods to measure TnT-specific intercellular transfer of cargo, including miRNAs.
Figure 7. FACS analysis of cells grown in open culture vs. separated by membrane filter in modified invasion chambers.
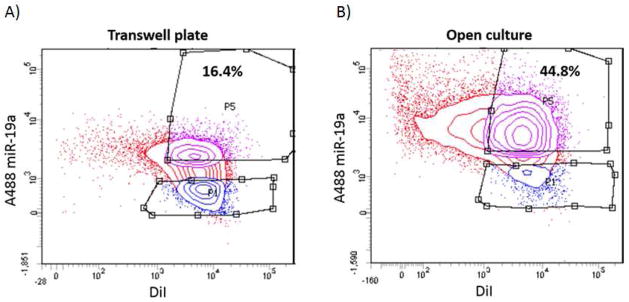
Adapting our model of in vitro cell culture of osteosarcoma cells and osteoblast cells, we used modified Boyden chambers including membrane filters with the smallest commercially available pore-size (0.4 μm, or 400 nm). At 48 hours, cells in the bottom chambers were collected and sorted using fluorescence-activated cell sorting (FACS); this step separated out single-positive non-recipient cells that were excluded from further analysis. (A) FACS analysis of double-positive MC3T3 cells following transwell assay for 48 hours, versus (B) FACS analysis of double-positive MC3T3 cells in open co-culture with K7M2 for 48 hours (without membrane barrier separation). The difference in these results was statistically significant with a p-value of 0.0002; the standard deviation was 2.15 for the transwell plate and 3.50 for the open culture experiments.
Discussion
Intercellular communication is a basic but critical process for cancer cell clustering, organization, synchronization, and coordinated invasion of surrounding stroma. It is well-established that exosomes can transport miRNAs between cells[7]. A recent study also strongly suggested that the intercellular transfer of miRNAs via contact-dependent mechanisms (gap junction channels) from macrophages to malignant cells significantly affects posttranscriptional gene regulation and thus alters cellular proliferation[8]. We recently demonstrated that exosomes co-localized with TnTs spatially and that they stimulated an increased rate of TnT formation[9]. Our findings of intercellular transfer of miRNAs via TnTs in the current study are also consistent with a prior report in which mRNA-containing organelles were transferred between rat spermatids via what seem to be TnTs (stable cytoplasmic bridges, also called ring canals).[10]
Our studies demonstrate direct cell-to-cell transfer of genetic material between tumor and stromal cells via TnT conduits. This finding has potential relevance to the emerging paradigm that intratumoral heterogeneity and tumor-stroma interactions are important properties of tumor formation, progression, and recurrence. This is especially the case for aggressive, incurable cancers that remain difficult to treat in human patients. TnTs represent a potential therapeutic target for cancers through disruption of intercellular transfer of miRNAs and other vital stimulants of malignant progression and proliferation.
Supplementary Material
Arrows indicate nanotubes. Scale bar = 10 μm.
In this example, the TnT acts as a conduit for miRNA transfer from the malignant, chemoresistant (SKOV3) cell to the non-malignant, epithelial (IOSE) cell. For live video of this experiment, please see Supplemental Movie 3.
Time-lapse movie of experiment depicted in Figure 1F in the main text, demonstrating a miR-19a-expressing K7M2 cell (green) extending a long TnT that connects with and transfers miR-19a to another K7M2 cell.
Time-lapse movie of experiment depicted in Figure 1G in the main text, demonstrating a miR-19a-expressing K7M2 cell (green) extending a long TnT that connects with an MC3T3 OB cell marked by fluorescent DiI (red). The K7M2 forms a TnT connection which then allows transfer of the miRNA cargo (miR-19a) to the OB cell.
Time-lapse movie demonstration of TnT-mediated intercellular transfer of miR-199a from an miR-199a-marked SKOV3 cell (green) connected to DiI-stained IOSE cell (red). In this example, the TnT acts as a conduit for miRNA transfer from the malignant, chemoresistant (SKOV3) cell to the non-malignant, epithelial (IOSE) cell.
Brief Commentary.
Background
Tunneling nanotubes (TnTs) represent a novel mechanism by which intercellular components such as proteins, Golgi vesicles, and mitochondria can be transferred from cell to cell in the complex tumor microenvironment.
Translational Significance
Here, we report data showing that microRNAs (miRNAs) are transferred through TnTs in osteosarcoma and ovarian cancer as in vitro model systems. This finding has potential relevance to the emerging paradigm that tumor-stroma interactions are important properties of tumor formation, progression, and recurrence and that miRNAs play an important role in regulating these processes. TnTs represent a potential therapeutic target for cancers through disruption of intercellular transfer of miRNAs and other vital stimulants of malignant progression and proliferation.
Acknowledgments
We thank Michael Franklin, M.S. for assistance with editing and critical review of this manuscript.
Research Funding
This research was kindly funded and supported in part by Minnesota Masonic Charities, Minnesota Medical Foundation, Institutional Research Grant #118198-IRG-58-001-52-IRG94 and Research Grant RSG-13-381-01 from the American Cancer Society, the Deborah Powell Center for Women’s Health at the University of Minnesota, the National Pancreas Foundation, Karen Wycoff Rein in Sarcoma Foundation, and the UMN Clinical and Translational Science Institute KL2 Scholar Award (to E.L.). Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114.
Footnotes
The authors have no conflicts-of-interest to declare. All authors have read the journal’s policy on disclosure of potential conflicts of interest and authorship.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mesnil M, Yamasaki H. Cell-cell communication and growth control of normal and cancer cells: evidence and hypothesis. Molecular carcinogenesis. 1993;7:14–7. doi: 10.1002/mc.2940070103. [DOI] [PubMed] [Google Scholar]
- 2.Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular ‘debris’. Seminars in immunopathology. 2011;33:455–67. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 3.Lou E, Fujisawa S, Barlas A, Romin Y, Manova-Todorova K, Moore MA, et al. Tunneling Nanotubes: A new paradigm for studying intercellular communication and therapeutics in cancer. Communicative & integrative biology. 2012;5:399–403. doi: 10.4161/cib.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PloS one. 2012;7:e33093. doi: 10.1371/journal.pone.0033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thayanithy V, Sarver AL, Kartha RV, Li L, Angstadt AY, Breen M, et al. Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma. Bone. 2012;50:171–81. doi: 10.1016/j.bone.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Molecular therapy Nucleic acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 8.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250–60. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thayanithy V, Babatunde V, Dickson EL, Wong P, Oh S, Ke X, et al. Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Experimental cell research. 2014;323:178–88. doi: 10.1016/j.yexcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventela S, Toppari J, Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing. Molecular biology of the cell. 2003;14:2768–80. doi: 10.1091/mbc.E02-10-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arrows indicate nanotubes. Scale bar = 10 μm.
In this example, the TnT acts as a conduit for miRNA transfer from the malignant, chemoresistant (SKOV3) cell to the non-malignant, epithelial (IOSE) cell. For live video of this experiment, please see Supplemental Movie 3.
Time-lapse movie of experiment depicted in Figure 1F in the main text, demonstrating a miR-19a-expressing K7M2 cell (green) extending a long TnT that connects with and transfers miR-19a to another K7M2 cell.
Time-lapse movie of experiment depicted in Figure 1G in the main text, demonstrating a miR-19a-expressing K7M2 cell (green) extending a long TnT that connects with an MC3T3 OB cell marked by fluorescent DiI (red). The K7M2 forms a TnT connection which then allows transfer of the miRNA cargo (miR-19a) to the OB cell.
Time-lapse movie demonstration of TnT-mediated intercellular transfer of miR-199a from an miR-199a-marked SKOV3 cell (green) connected to DiI-stained IOSE cell (red). In this example, the TnT acts as a conduit for miRNA transfer from the malignant, chemoresistant (SKOV3) cell to the non-malignant, epithelial (IOSE) cell.