Volatile anesthetics act directly on neuronal sodium channels, independently of effects on the lipid bilayer.
Abstract
Although general anesthetics are clinically important and widely used, their molecular mechanisms of action remain poorly understood. Volatile anesthetics such as isoflurane (ISO) are thought to alter neuronal function by depressing excitatory and facilitating inhibitory neurotransmission through direct interactions with specific protein targets, including voltage-gated sodium channels (Nav). Many anesthetics alter lipid bilayer properties, suggesting that ion channel function might also be altered indirectly through effects on the lipid bilayer. We compared the effects of ISO and of a series of fluorobenzene (FB) model volatile anesthetics on Nav function and lipid bilayer properties. We examined the effects of these agents on Nav in neuronal cells using whole-cell electrophysiology, and on lipid bilayer properties using a gramicidin-based fluorescence assay, which is a functional assay for detecting changes in lipid bilayer properties sensed by a bilayer-spanning ion channel. At clinically relevant concentrations (defined by the minimum alveolar concentration), both the FBs and ISO produced prepulse-dependent inhibition of Nav and shifted the voltage dependence of inactivation toward more hyperpolarized potentials without affecting lipid bilayer properties, as sensed by gramicidin channels. Only at supra-anesthetic (toxic) concentrations did ISO alter lipid bilayer properties. These results suggest that clinically relevant concentrations of volatile anesthetics alter Nav function through direct interactions with the channel protein with little, if any, contribution from changes in bulk lipid bilayer properties. Our findings further suggest that changes in lipid bilayer properties are not involved in clinical anesthesia.
INTRODUCTION
The public demonstration of the anesthetic properties of diethyl ether in 1846 initiated the era of modern anesthesia, enabling more complex and longer surgical procedures. Volatile anesthetics in current use are nonflammable fluorinated derivatives of ether that are capable of producing general anesthesia, a drug-induced coma-like state characterized by unconsciousness, amnesia, and immobility (Rudolph and Antkowiak, 2004; Hemmings et al., 2005). Despite their widespread clinical use, however, the molecular, cellular, and systems mechanisms underlying anesthesia remain incompletely understood.
For many years, general anesthetics were considered to produce their effects by altering membrane properties through unspecified interactions with the lipid bilayer, a concept rooted in the Meyer–Overton correlation between anesthetic potency and lipophilicity (Meyer, 1899; Overton, 1901). These nonspecific and usually undefined interactions with the lipid bilayer formed the basis for a lipid-based theory of anesthetic action (Perouansky, 2012). The demonstration that anesthetics bind directly to globular proteins in the absence of lipids (Ueda and Kamaya, 1973; Franks and Lieb, 1984), and that anesthetics produce measurable changes in lipid structure only at supra-anesthetic concentrations (Franks and Lieb, 1979), led to the search for relevant protein targets. Volatile anesthetics have since been shown to affect the function of γ-aminobutyric acid (GABA)A receptors (Zimmerman et al., 1994), NMDA receptors (Dickinson et al., 2007; Haseneder et al., 2008), two-pore domain potassium background channels (Patel and Honoré, 2001; Sirois et al., 2002), and voltage-gated calcium (Study, 1994; Nikonorov et al., 1998) and sodium (Herold and Hemmings, 2012) channels (Nav).
It is now generally accepted that general anesthetics alter neuronal function by modulating excitatory and inhibitory synaptic transmission (Rudolph and Antkowiak, 2004; Hemmings et al., 2005), and that anesthetics interact directly with proteins rather than acting solely through the lipid bilayer (Franks and Lieb, 1994; Eckenhoff and Johansson, 1997; Quillin et al., 2000). Indeed, prokaryotic ligand-gated channels of the Cys-loop family have been co-crystallized with anesthetics (Nury et al., 2011; Spurny et al., 2013), and anesthetic-binding sites in the bacterial sodium channel NaChBac have been inferred from molecular dynamics simulations (Raju et al., 2013). Although there is convincing evidence for the importance of direct anesthetic–protein interactions, it remains unclear if anesthesia can be ascribed solely to direct effects on membrane proteins or whether changes in lipid bilayer properties might also be involved. The diversity of targets that are sensitive to inhaled anesthetics (Eger et al., 2008), together with the inability to eliminate anesthesia in mice engineered to express volatile anesthetic-resistant mutant ion channels (Zeller et al., 2008; Werner et al., 2011), suggests that we need to look further—and modern theories of small-molecule interactions with lipid bilayers (Gruner and Shyamsunder, 1991; Cantor, 1997; Sonner and Cantor, 2013) provide a possible mechanism for lipid bilayer–based theories of anesthetic effects on ion channel function.
We therefore explored the effects of the family of fluorobenzene (FB) anesthetics (Solt et al., 2006), as well as the clinically used agent isoflurane (ISO), on the function of Nav expressed in neuronal cells and on lipid bilayer mechanical properties as sensed by prokaryotic gramicidin channels (Lundbæk et al., 2010b; compare Fig. 1 A).
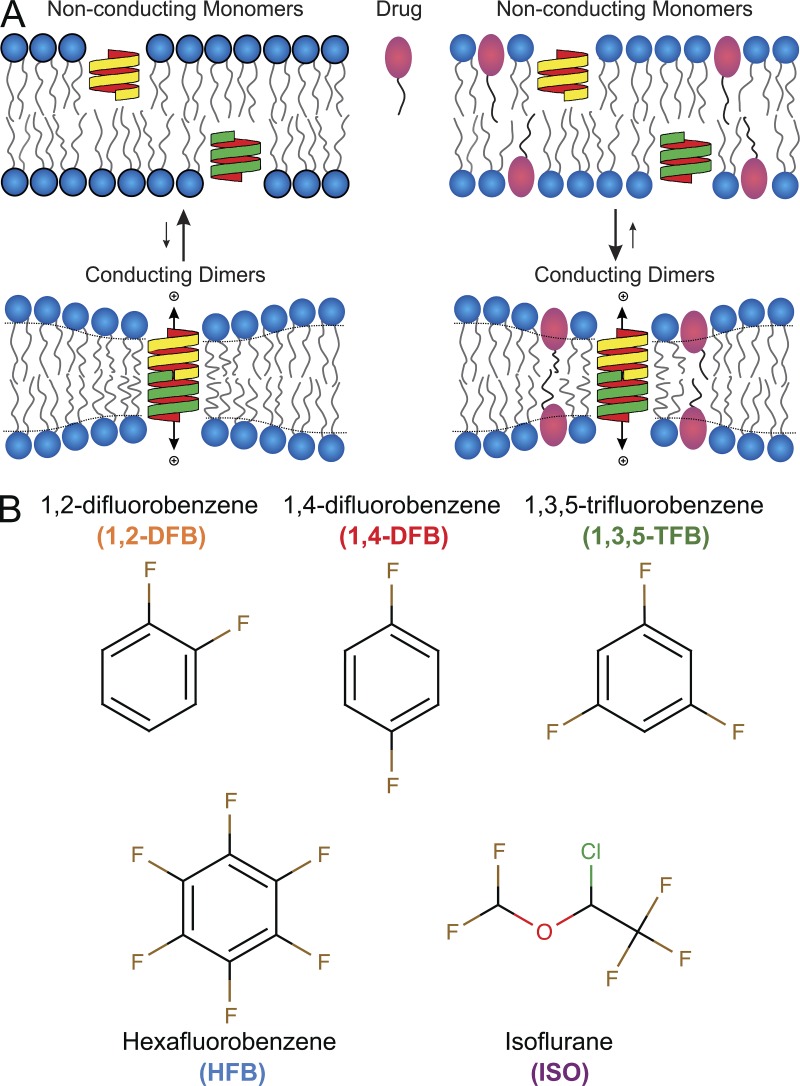
Figure 1.
Use of the gramicidin (gA) channel as a molecular force probe to detect changes in lipid bilayer properties. (A; left) Schematic of gA channel formation in the bilayer. Two nonconducting channel monomers (yellow and green structures) form a conducting dimer, enabling monovalent cations to pass through the pore. In the GBFA, changes in the gramicidin monomer↔dimer equilibrium are evaluated from changes in the rate of thallium (Tl+) influx into fluorophore-loaded LUVs where we exploit that Tl+ is a fluorescence quencher (see Materials and methods for details). (Right) gA dimerization is altered in the presence of a bilayer-modifying drug (red ovals). The drug partitions between the aqueous solution and the bilayer, and redistributes (between the aqueous solution and the membrane and in the plane of the membrane) in response to local bilayer deformation, which may increase the degrees of freedom available for the membrane to adapt to the bilayer deformation. (B) Chemical structures of the anesthetics used in this study. Their properties are summarized in Table 1.
The principles underlying the use of gramicidin channels to detect changes in bilayer properties are illustrated in Fig. 1. Gramicidin channels are dimers (D) formed by the trans-bilayer association of monomeric subunits (M) from each bilayer leaflet. The channel’s exterior is coupled to the lipid bilayer core through hydrophobic interactions and, as is the case for other membrane-spanning channels, gramicidin channels will locally reorganize the surrounding bilayer. Channel formation in a bilayer with a hydrophobic thickness (d0) that exceeds the channel hydrophobic length (l) involves a local bilayer deformation (thinning) (Huang, 1986; Lundbæk and Andersen, 1994; Nielsen et al., 1998; Harroun et al., 1999). This local bilayer deformation has an associated energetic cost, the bilayer deformation energy (), which varies as a function of the hydrophobic mismatch between the channel length (l) and the bilayer thickness (d0), and the channel radius and bilayer material properties —thickness, intrinsic curvature, and the associated elastic moduli (Huang, 1986; Nielsen et al., 1998; Nielsen and Andersen, 2000; Rusinova et al., 2011). Changes in bilayer properties will alter the bilayer deformation energy associated with the monomers and dimers, and , respectively, and thereby the bilayer contribution to the free energy of dimerization (, where denotes intrinsic contributions, such as the formation of the hydrogen bond–stabilized interface between the two monomers and ), and thus the gramicidin dimerization constant () and the number of conducting channels per unit area.
Gramicidin channels have been calibrated as probes of changes in lipid bilayer mechanical properties (Lundbæk and Andersen, 1994; Lundbæk et al., 1997; Goulian et al., 1998). In the present context, it is imperative that gramicidin channels sense changes in intrinsic lipid curvature—or lateral pressure profile (Andersen and Koeppe, 2007; Marsh, 2007). Gramicidin channels have proven useful for exploring how small molecules alter lipid bilayer properties that are relevant for altering the function of channels formed by integral membrane proteins (Lundbæk et al., 1996, 2004, 2005; Suchyna et al., 2004; Artigas et al., 2006; Søgaard et al., 2006; Rusinova et al., 2011; Ingólfsson et al., 2014). For the present study, it is important that Nav (Lundbæk et al., 2004, 2005; Rusinova et al., 2011) and GABAA channels (Søgaard et al., 2006; Chisari et al., 2010), both of which are important anesthetic targets (Hemmings et al., 2005), are modulated by amphiphiles at concentrations that alter lipid bilayer properties, as sensed by gramicidin channels.
We chose the FBs as model volatile anesthetics because of their closely related structures (Fig. 1 B) and physicochemical properties. These compounds were once considered for clinical use as anesthetics but were rejected in favor of modern inhaled anesthetics (Burns et al., 1961, 1964; Fang et al., 1996). They remain valuable model compounds for investigating molecular mechanisms of anesthetics (Solt et al., 2006). We find that at clinically relevant concentrations, the FBs and ISO inhibit Nav function with no obvious effects on gramicidin channel function, demonstrating that their effects are unlikely to involve alterations in lipid bilayer properties but rather result from direct interactions with Nav.
MATERIALS AND METHODS
Materials
1,2-difluorobenzene (DFB), 1,4-DFB, 1,3,5-trifluorobenzene (1,3,5-TFB), and hexafluorobenzene (HFB) were purchased from Sigma-Aldrich. ISO (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane) was from Abbott Laboratories. All other chemicals were from Sigma-Aldrich. Lipids were from Avanti Polar Lipids, Inc.
Cell culture and electrophysiology
Na+ currents were recorded from rodent neuronal ND7/23 cells, which express endogenous tetrodotoxin-sensitive Na+ currents, using conventional whole-cell patch-clamp electrophysiology (Hamill et al., 1981). Cells were cultured on 12-mm glass coverslips in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco) at 37°C under 95% air plus 5% CO2. For recordings, coverslips were transferred into a small-volume laminar-flow perfusion chamber (Warner Instruments) and continuously perfused at 2 ml/min with extracellular solution containing (mM): 130 NaCl, 10 HEPES, 3.25 KCl, 2 MgCl2, 2 CaCl2, 0.1 CdCl2, 20 TEACl, and 5 d-glucose, adjusted to pH 7.4 (with NaOH) and 310 mOsm/kg H2O (with sucrose). Na+ currents were recorded in voltage-clamp mode at room temperature (23–24°C) using a patch-clamp amplifier (Axopatch 200B; Molecular Devices) with a 10-kHz low-pass filter at a sampling rate of 50 kHz. Recording pipettes were pulled from borosilicate glass capillaries (Sutter Instrument) on a horizontal puller (P-97; Sutter Instrument) and fire-polished before use. Pipettes were filled with internal solution containing (mM): 120 CsF, 10 NaCl, 10 HEPES, 10 EGTA, 10 TEACl, 1 CaCl2, and 1 MgCl2, adjusted to pH 7.3 (with CsOH) and 310 mOsm/kg H2O (with sucrose); pipette resistance was 1.5–2.5 MΩ. Access resistance (2–4 MΩ) was reduced using 75–85% series resistance correction. Initial whole-cell seal resistance was 2–4 GΩ; recordings were discarded if resistance dropped below 1 GΩ. Liquid-junction potentials were not corrected. Capacitive current transients were electronically cancelled with the internal amplifier circuitry, and leak currents were digitally subtracted online using the P/4 protocol (Bezanilla and Armstrong, 1977).
Before each experiment, stock solutions of FBs in DMSO were diluted with external perfusion buffer; both test and control solutions contained 0.14% (vol/vol) DMSO. ISO solutions were prepared as described previously (Herold et al., 2009). Clinical concentrations of volatile anesthetics are expressed relative to the minimum alveolar concentration (MAC; see Table 1), which is defined as the concentration necessary to inhibit movement in 50% of subjects in response to a painful stimulus (Eger et al., 1965). We corrected these MAC values for our experimental conditions of 25°C (Franks and Lieb, 1993). To reduce loss of volatile compounds, the perfusion system consisted of gastight glass syringes with Teflon tubing connected to a 0.2-mm diameter perfusion pipette positioned in close proximity to the patch-clamped cell with a perfusion rate of ∼0.2 ml/min controlled by a pressurized perfusion system (ALA Scientific Instruments). Anesthetic concentrations were determined using gas chromatography as described previously (Ratnakumari and Hemmings, 1998).
Table 1.
Properties of anesthetics
| Anesthetic | MAC at 25°C | cLogP | Membrane concentration (at 1 MAC) | Mole fraction (at 1 MAC) |
| mM | mM | |||
| 1,2-DFB | 0.70 | 2.2 | 106 | 0.087 |
| 1,4-DFB | 0.59 | 2.4 | 135 | 0.109 |
| 1,3,5-TFB | 0.60 | 2.5 | 174 | 0.136 |
| HFB | 0.25 | 2.4 | 57 | 0.049 |
| ISO | 0.30 | 2.3 | 57 | 0.049 |
MAC values from Solt et al. (2006); cLogP values were estimated using the ACD/Labs LogP algorithm on ChemSpider. Concentrations and mole fractions were calculated as described in Materials and methods.
Mole fractions of the anesthetics in the lipid bilayer were estimated following Bruno et al. (2007). Membrane concentrations of anesthetic [Drug]m were determined as:
where KP is the octanol/water partition coefficient, [Drug]nom is the nominal concentration of (added) drug in the aqueous solution, Vaq is the aqueous volume in the vesicle system, and Vlip is the lipid volume in the vesicle system; the second term on the right accounts for depletion caused by drug redistribution between aqueous and membrane phases. The mole fraction of anesthetic, mDrug, was determined as:
where [Lipid]m denotes the lipid concentration in the membrane phase (∼1 M). Given the relatively modest anesthetic hydrophobicity, aqueous concentrations will be close to nominal concentrations, and corrections for depletion that occur because of anesthetic redistribution between the aqueous and membrane phases are minimal. KP was estimated as 10cLogP, and cLogP was estimated using the ACD/Labs LogP algorithm (http://www.chemspider.com).
Stimulation protocols and data analysis
The holding potential (Vh) was −80 mV unless otherwise stated. Voltage-dependent inhibition of peak inward Na+ current (INa) was analyzed using a 10-ms test pulse to 0 mV, preceded by a 300-ms prepulse alternating between either −130 mV (V0) or the voltage of half-maximal inactivation (V1/2 = −69 ± 4 mV) applied every 5 s (Lundbæk et al., 2004; Rusinova et al., 2011). V1/2 was determined for each individual cell using the double-pulse protocol for steady-state inactivation described below.
Steady-state fast inactivation (h∞) was measured using a double-pulse protocol with a 300-ms prepulse ranging from −120 to −30 mV in 10-mV steps, followed by a 10-ms test pulse to 0 mV. Peak currents during the test pulse were normalized to the maximal current (I/Imax, where Imax is the maximal current that is elicited at the test potential), plotted against the prepulse potential (Vm), and fitted with a Boltzmann function (Rusinova et al., 2011):
where z1/2 and V1/2 denote the apparent gating valence and potential for half-maximal inactivation, respectively.
Recovery from fast inactivation was tested from a holding potential of −130 mV using a two-pulse protocol with two identical 10-ms pulses to 0 mV separated by an interpulse interval with increasing durations from 1 to 100 ms. Current amplitudes were normalized (Pulse2/Pulse1), plotted against interpulse interval, and fitted with the single-exponential equation to obtain the recovery time constants τ:
where Yt denotes the normalized current at time t, Y0 denotes the normalized current at zero time, and plateau denotes the normalized current at infinity.
Programs used for data acquisition and analysis were pClamp 10 (Molecular Devices), Excel (Microsoft), Prism 6.0 (GraphPad Software), and MATLAB (v7.4; The MathWorks, Inc.). Values are reported as mean ± SD unless otherwise stated. Statistical significance (P < 0.05) was assessed by two-tailed paired Student’s t test.
Fluorescence quench measurements of changes in lipid bilayer properties
To probe for FB- and ISO-dependent changes in lipid bilayer properties, as sensed by a bilayer-spanning ion channel, we used a gramicidin-based fluorescence assay (GBFA) (Ingólfsson and Andersen, 2010; Ingólfsson et al., 2010, 2014; Rusinova et al., 2011). This method allows determination of how experimental interventions, such as the addition of anesthetics, alter the equilibrium distribution between nonconducting gramicidin monomers and conducting gramicidin dimers (channels) based on changes in influx rate of a gramicidin-channel permeant heavy ion quencher (Tl+) (Fig. 1 A). In brief, 1,2-dierucoyl-sn-glycero-3-phosphocholine (DC22:1PC) without or with gramicidin (molar ratio of 1,000:1) in chloroform was dried under a nitrogen stream, desiccated overnight, and rehydrated in an aqueous solution containing 25 mM of the fluorophore 8-aminonaphthalene-1,3,6-trisulfonate (ANTS), 100 mM NaNO3, and 10 mM HEPES, pH 7. Experiments with cholesterol-containing vesicles were done using large unilamellar vesicles (LUVs) prepared from a 1:1 di-oleoyl-sn-3-phosphocholine (DC18:1PC) and cholesterol lipid mixture. LUVs were prepared by sonication, freeze-thawing, extrusion through a 100-nm polycarbonate membrane using a mini-extruder (Avanti Polar Lipids, Inc.), and elution on a desalting column to remove extravesicular ANTS. Changes in the gramicidin monomer↔dimer equilibrium were estimated from the rate of ANTS fluorescence quenching by the gramicidin channel–permeable quencher Tl+ using a stopped-flow spectrofluorometer (SX.20; Applied Photophysics) with a dead time of ∼1.5 ms. Samples were excited at 352 nm, and fluorescence emission above 455 nm was recorded. Vesicles were incubated with anesthetics for 10 min at 25°C before measuring fluorescence quenching rate.
Data were analyzed using MATLAB (v.7.9; The MathWorks, Inc.). Because of the unavoidable dispersion of LUV sizes, fluorescence quench curves cannot be described by single-exponential decays, so data were analyzed using a stretched exponential. This is a mathematically efficient way to express large sums of similar exponential decays (Peyron et al., 1996; Berberan-Santos et al., 2005). Fluorescence quench rates for the first 2–100 ms of the fluorescence time course for individual quench curves were fitted by the stretched exponential:
where F(t) denotes the fluorescence intensity as a function of time t, τ0 is a parameter with units of time, and β (0 < β ≤ 1) is a measure of sample dispersity. The quenching rate at 2 ms was determined as (Berberan-Santos et al., 2005):
Reported values are averages of quenching rates, normalized to the rate in the absence of anesthetic (n ≥ 3).
RESULTS
Inhibition of peak Na+ currents
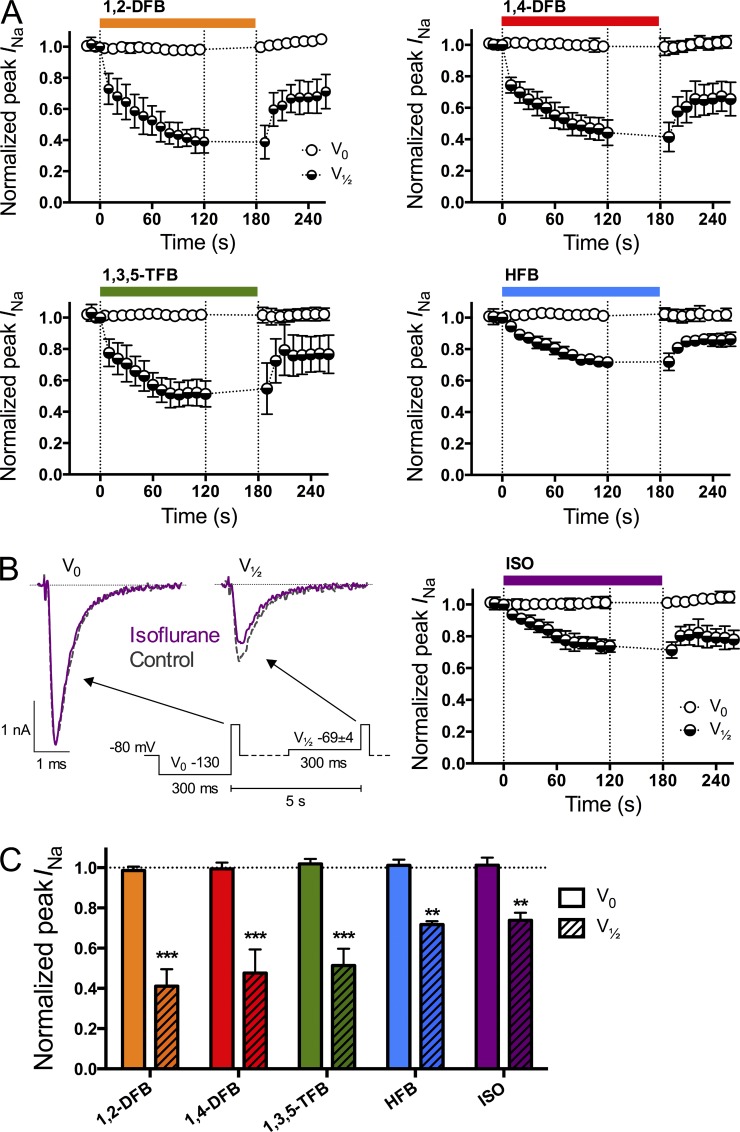
The effects of FBs and ISO on neuronal Na+ currents were tested during drug wash-in and wash-out using a periodic stimulation protocol that allows determination of voltage-dependent drug effects (Lundbæk et al., 2005; Rusinova et al., 2011; Fig. 2).
Figure 2.
Inhibition of peak Na+ current (INa) by anesthetics. (A) Time course of the effects of wash-in and wash-out of the FBs and ISO at 1 MAC (see Table 1). (B; left) Macroscopic whole-cell current traces in the absence (gray discontinued traces) or presence (purple full traces) of ISO using a periodic stimulation protocol (inset) from a holding potential (Vh) of −80 mV. This alternating protocol was chosen to test voltage-dependent inhibition. A test pulse (10 ms at 0 mV) every 5 s to elicit peak INa was preceded by a 300-ms prepulse to either −130 mV (denoted “V0”; B, left, left traces) or to a voltage at which approximately half of the channels were in the fast-inactivated state (denoted “V1/2”; −69 ± 4 mV; B, left, right traces). Peak INa was normalized to control. (C) The values after 120 s of drug treatment. All five anesthetics significantly inhibited peak INa with a prepulse to V1/2 but had no effect with a prepulse to V0 (Table 2). The gap between 120 and 180 s was used to record steady-state inactivation (see Fig. 3). Data are mean ± SD; n = 6–9. **, P < 0.01 and ***, P < 0.001 versus control by paired two-tailed Student’s t test.
A depolarizing pulse to 0 mV was applied every 5 s to elicit peak Na+ currents (INa) after a 300-ms prepulse to either −130 mV (V0, at which most channels are in the resting state) or to the voltage of half-maximal steady-state inactivation (V1/2 = −69 ± 4 mV), which was determined for each cell under control conditions. Fig. 2 (A and B) shows the time course of peak INa inhibition for each anesthetic; the rather slow time course of inhibition most likely represents drug partitioning among different intracellular compartments, as opposed to an intrinsic slow rate of binding to the anesthetic target, whether Nav or the host bilayer (Gingrich et al., 2009). Fig. 2 C summarizes results for peak INa inhibition (after a wash-in period of 120 s to reach steady state) after a prepulse to either V0 or V1/2 (see also Table 2). Results are expressed as the ratio of peak INa in the presence of drug to control INa.
Table 2.
Anesthetic effects on peak Na+ current
| Anesthetic | Test pulse with prepulse to V0 | Test pulse with prepulse to V1/2 | n |
| 1,2-DFB | 0.99 ± 0.02 | 0.41 ± 0.08a | 7 |
| 1,4-DFB | 0.99 ± 0.03 | 0.48 ± 0.12a | 7 |
| 1,3,5-TFB | 1.02 ± 0.02 | 0.51 ± 0.08a | 7 |
| HFB | 1.01 ± 0.03 | 0.72 ± 0.02b | 7 |
| ISO | 1.01 ± 0.04 | 0.74 ± 0.04b | 9 |
P < 0.001 versus control by two-tailed paired Student’s t test.
P < 0.01 versus control by two-tailed paired Student’s t test.
With a prepulse to V0, none of the anesthetics inhibited peak INa; with a prepulse to V1/2, all five anesthetics produced significant inhibition. 1,2-DFB had the greatest effect, and HFB and ISO had the least (Table 2). Inhibition was reversible after a 1-min washout period, although peak INa did not completely recover to control values. This presumably reflects that the steady-state inactivation curves did not reverse fully (see next section); reversal is complete when Vh is corrected by the difference between the control and washout V1/2 (not depicted). Although not tested here, ethanol, at concentrations similar to that used as a positive control in the stopped-flow bilayer experiments (see below and Fig. 5), also inhibits INa (Frenkel et al., 1997; Wu and Kendig, 1998; Klein et al., 2007; Horishita and Harris, 2008; Xiao et al., 2008).
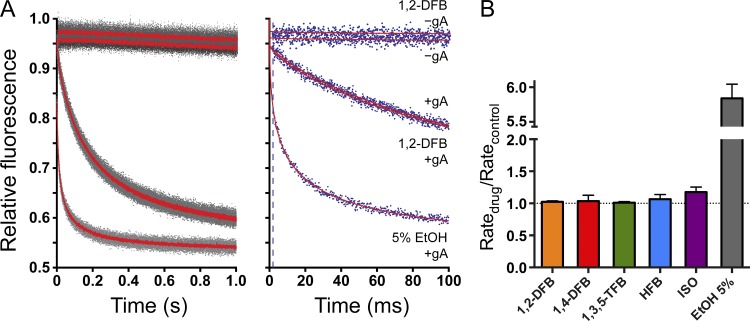
Figure 5.
Effects of anesthetics on lipid bilayer properties using a GBFA. (A) Examples of normalized fluorescence quench traces. (Left) Time course of normalized fluorescence decay over 1 s. Gray dots denote results from all repeats (more than five per condition), and red solid lines indicate the average of all repeats. (Right) The first 100 ms of the normalized fluorescence decay. Blue dots denote results from a single repeat for each condition; red lines correspond to the stretched exponential fits (2–100 ms) to those repeats; and the broken line marks the 2-ms time point, at which quenching rate is determined. In both panels, the top two traces show the results without gramicidin (gA) in the presence (1,2-DFB − gA) or absence (−gA) of 1,2-DFB. The next two overlapping red traces show control gA in the absence (+gA) or presence (1,2-DFB + gA) of 1,2-DFB. The bottom trace corresponds to gA-containing vesicles treated with 5% ethanol (5% EtOH + gA) as a positive control. (B) The rate of quencher influx for anesthetics at 1 MAC and ethanol. None of the anesthetics significantly altered lipid bilayer properties at clinically relevant concentrations. Data are mean ± SD; n = 3–4.
Effect on steady-state inactivation
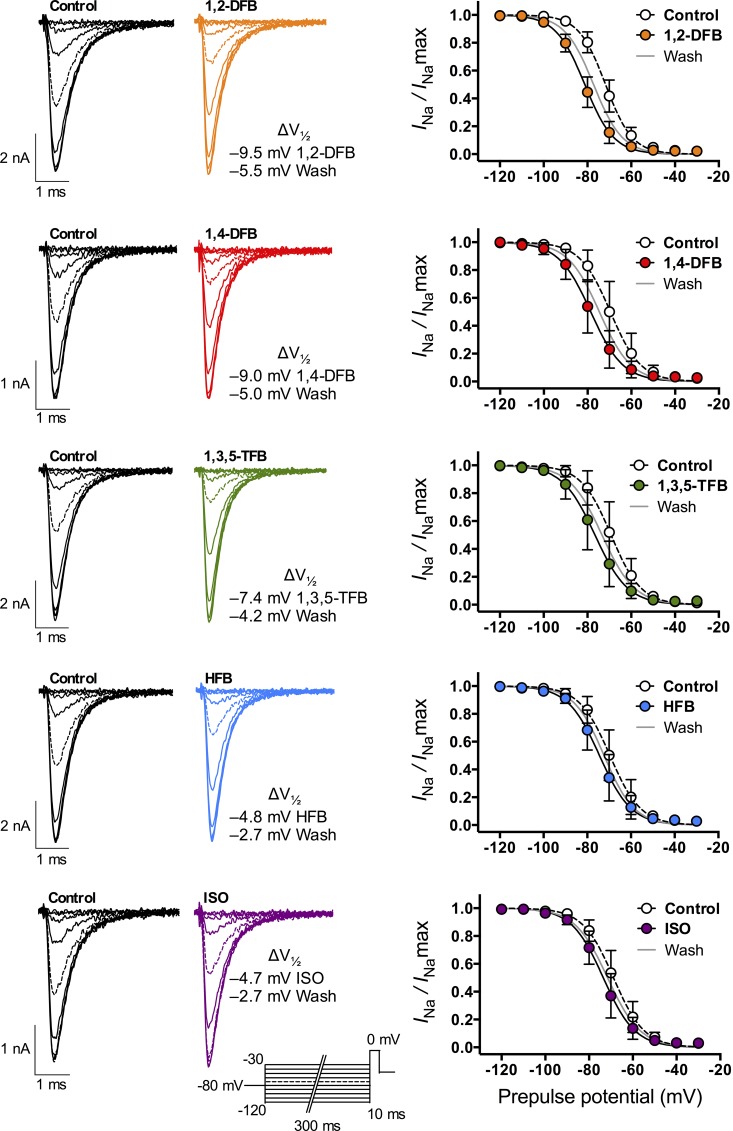
Inhibition of peak INa was observed only after a prepulse to V1/2, consistent with a preferential anesthetic interaction with an inactivated state of the channel (Herold and Hemmings, 2012). This was tested further by determining steady-state inactivation curves in the absence or presence (after 120-s superfusion) of anesthetic using a double-pulse protocol. All five anesthetics shifted V1/2 (steady-state inactivation) significantly toward more hyperpolarized potentials (Fig. 3 and Table 3). Again, 1,2-DFB had the greatest effect, and HFB and ISO had the least.
Figure 3.
Na+ channel steady-state inactivation. Na+ channel availability (or h∞) was tested using a double-pulse protocol with a 300-ms prepulse ranging from −120 to −30 mV in 10-mV steps, followed by a 10-ms test pulse to 0 mV (inset at ISO traces shows stimulation protocol). Peak INa was normalized (INa/INamax), plotted against prepulse potential, and fitted with a two-state Boltzmann distribution to calculate V1/2 (see Materials and methods for details). (Left) Families of Na+ current traces in the absence (black traces, control) or presence (colored traces, drug) of anesthetics at 1 MAC. (Right) Fitted data in the absence (open circles, dotted line) or presence (full colored circles, straight black line) of anesthetic, or after washout (straight gray line). All anesthetics caused a significant left-shift of V1/2 with values displayed next to each graph (see Table 3). Data are mean ± SD; n = 5–7.
Table 3.
Anesthetic effects on the voltage dependence of Nav inactivation
| Anesthetic | V1/2 control | V1/2 drug | V1/2 wash | ΔV1/2 | n |
| 1,2-DFB | −71.7 ± 2.8 | −81.1 ± 3.0a | −77.1 ± 3.6b | −9.4 ± 0.9 | 6 |
| 1,4-DFB | −69.5 ± 5.6 | −78.5 ± 5.4a | −74.6 ± 5.6a | −9.0 ± 1.0 | 6 |
| 1,3,5-TFB | −69.2 ± 5.4 | −76.5 ± 5.8a | −73.3 ± 5.9b | −7.4 ± 1.0 | 5 |
| HFB | −69.6 ± 4.9 | −74.3 ± 4.8b | −72.2 ± 5.0c | −4.8 ± 0.7 | 5 |
| ISO | −68.8 ± 4.2 | −73.4 ± 4.3a | −71.6 ± 3.3b | −4.7 ± 0.9 | 6 |
P < 0.0001 versus control by two-tailed paired Student’s t test.
P < 0.001 versus control by two-tailed paired Student’s t test.
P < 0.01 versus control by two-tailed paired Student’s t test.
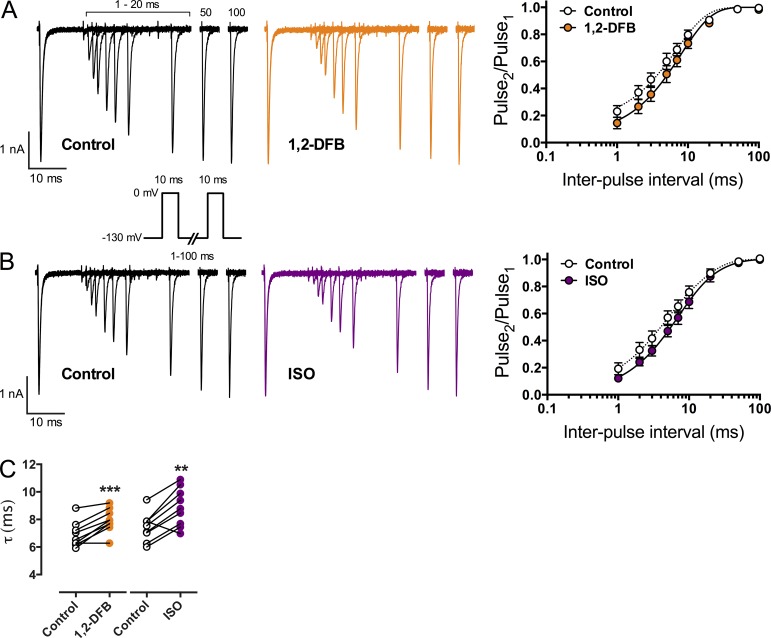
Recovery from inactivation
Recovery from inactivation was tested from a holding potential of −130 mV at which the majority of channels are in the resting, closed state. We compared 1,2-DFB, which showed the strongest effects in the previous experiments, to ISO (Fig. 4). Both anesthetics slowed recovery from inactivation; τ increased from 6.9 ± 0.9 ms to 7.9 ± 0.9 ms for 1,2-DFB and from 7.5 ± 1.0 ms to 8.9 ± 1.4 ms for ISO (Fig. 4 C; n = 9; mean ± SD; **, P < 0.01 and ***, P < 0.001 vs. control).
Figure 4.
Na+ channel recovery from inactivation. A two-pulse protocol was used from a holding potential (Vh) of −130 mV, with two 10-ms test pulses to 0 mV separated by an interpulse interval from 1 to 100 ms (inset shows stimulation protocol). Peak INa of the second test pulse was normalized to the first (Pulse2/Pulse1) and plotted against the interpulse interval. Data were fitted to a mono-exponential equation to calculate the time constant τ (see Materials and methods for details). (A and B; left) Overlaid macroscopic Na+ current traces of the two pulses with increasing interpulse durations in the absence (black traces, control) or presence (colored traces, drug) of anesthetic at 1 MAC. Peak INa of the second test pulse slowly recovers to control values with increasing interpulse durations. (A and B; right) Fitted data in the absence (open circles, dotted line) or presence (closed colored circles, straight line) of anesthetic. (C) Recovery time constant τ in the absence (open circles) or presence (colored circles) of anesthetic. Both anesthetics significantly increased τ, thereby slowing recovery from inactivation. Data are mean ± SD; n = 9. **, P < 0.01 and ***, P < 0.001 versus control by paired two-tailed Student’s t test.
Effects on lipid bilayer properties
Lipid bilayer–perturbing properties were tested using a GBFA. The time course of fluorescence quenching resulting from the influx of the heavy ion quencher Tl+ into LUVs that have been loaded with the water-soluble fluorophore ANTS is monitored (Ingólfsson and Andersen, 2010; Ingólfsson et al., 2010). Gramicidin channels are very permeable to Tl+, such that fluorescence quench rate varies with changes in the time-averaged number of conducting gramicidin channels in the LUV membrane, i.e., with changes in the dimerization constant (the free energy of dimerization). Gramicidin channels are sensitive reporters of changes in bilayer properties (Elliott et al., 1983; Lundbæk et al., 1997, 2010a) that are relevant for the function of voltage-gated calcium channels (Lundbæk et al., 1996) and Nav (Lundbæk et al., 2004, 2005; Rusinova et al., 2011).
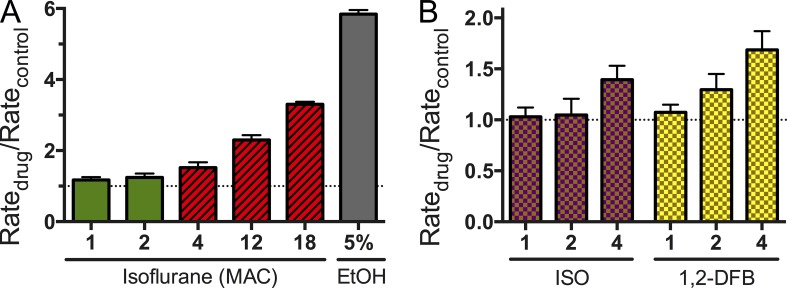
At 1 MAC, the FBs and ISO did not produce significant changes in quench rate (Fig. 5), meaning that they do not alter the gramicidin dimerization constant (or lipid bilayer properties that are sensed by gramicidin channels). This lack of effect should be compared with the changes produced by 5% ethanol (∼1 M; Fig. 5). For ISO, only minimal changes in bilayer properties were observed at the supratherapeutic concentration of 4 MAC (∼1.1 mM; Fig. 6 A). Only at extremely high concentrations of ISO (18 MAC or 5–6 mM, a lethal concentration) did ISO significantly alter fluorescence quench rate, and thus lipid bilayer properties as detected by the bilayer-spanning gramicidin channels (Fig. 6 A). We conclude that, at clinically relevant concentrations, the anesthetics tested cause minimal changes in the gramicidin monomer↔dimer equilibrium, and therefore produce minimal changes in lipid bilayer properties.
Figure 6.
Concentration dependence of the ISO effect on lipid bilayer properties. (A) At clinically relevant concentrations of 1 and 2 MAC (0.3 and 0.6 mM at 25°C; green columns), ISO did not significantly alter lipid bilayer properties. Even at the supratherapeutic concentration of 4 MAC (∼1.1 mM; red hashed column), only minimal changes were seen. Only at very high toxic concentrations of 12 and 18 MAC (1.1–5.4 mM; red hashed columns) did ISO alter lipid bilayer properties. 5% ethanol (∼1 M) was used as a positive control (gray column). (B) Effects of ISO and 1,2-DFB on lipid bilayer properties in the presence of cholesterol. Use of cholesterol-containing lipid bilayers (1:1 mixture of DC18:1PC and cholesterol) showed similar results to bilayers without cholesterol. Neither ISO nor 1,2-DFB altered lipid bilayer properties at clinically relevant concentrations of 1 and 2 MAC. Even at 4 MAC, only minimal changes were seen.
To explore whether anesthetic effects might be affected by lipid bilayer composition, we also performed experiments using cholesterol-containing lipid bilayers (1:1 mixture of DC18:1PC and cholesterol). The results were similar to those obtained in experiments without cholesterol, and confirmed that neither ISO nor 1,2-DFB produces significant changes in lipid bilayer properties at concentrations as high as four times MAC (Fig. 6 B).
DISCUSSION
Our key findings are that FB model anesthetics and the clinically used anesthetic ISO inhibit Nav function in neuronal cells at clinically relevant concentrations that do not perturb bulk lipid bilayer properties (as sensed by a bilayer-spanning reporter channel). These observations provide additional support for the notion that general anesthetics alter membrane protein function through direct (specific) interactions with ion channel proteins (in casu Nav) rather than indirect effects caused by altered lipid bilayer properties.
Direct anesthetic effects on membrane proteins
Several putative molecular targets for volatile anesthetics have been identified based on their sensitivities to anesthetics at clinical concentrations; these include multiple ligand-gated and voltage-gated ion channels (Hemmings et al., 2005). Direct anesthetic–protein interactions likely involve binding to hydrophobic cavities, such as those identified in soluble proteins (Dubois et al., 1993; Franks et al., 1998; Quillin et al., 2000; Liu et al., 2005). Direct anesthetic binding has been demonstrated in prokaryotic ion channels (Barber et al., 2011; Nury et al., 2011; Spurny et al., 2013) and in GABAA receptors by photolabeling experiments (Chen et al., 2012; Chiara et al., 2012; Yip et al., 2013). But a role for lipid bilayer–mediated effects on ion channel function has not been excluded. This is important because mice in which putative molecular targets have been genetically engineered to be resistant to volatile anesthetics remain sensitive to anesthetics (Liao et al., 2005; Sonner et al., 2007; Zeller et al., 2008; Werner et al., 2011).
One would expect anesthetic alteration of bilayer properties at higher concentrations (compare Franks and Lieb, 1979). Some anesthetics, including ISO (Haseneder et al., 2002) and pentobarbital (Gingrich et al., 2009), activate GABAA channels at supra-anesthetic concentrations (3–5 MAC for ISO) where they also alter lipid bilayer properties as sensed by gramicidin channels (Sun, Y.T., V. Jogini, and O.S. Andersen. 2002. Biophysical Society 46th Annual Meeting. Abstr. 550A; Fig. 6 A). It is in this context important that n-alkanols inhibit Nav function at concentrations that alter gramicidin channel function (compare Horishita and Harris, 2008; Ingólfsson and Andersen, 2011). Indeed, there is a remarkable correlation between alkanol inhibition of Nav function and alkanol-induced changes in bilayer properties as sensed by gramicidin channels (Ingólfsson and Andersen, 2011). These changes in gramicidin channel function occur at alcohol concentrations that are at the low end of the concentrations that alter lipid bilayer properties in micromechanical measurements (Ly and Longo, 2004), as would be expected for a functional assay of changes in bilayer properties.
In contrast, the anesthetics tested here do not alter lipid bilayer properties at clinical concentrations, as sensed by transmembrane gramicidin channels. It remains unclear whether anesthetic effects observed at supra-anesthetic concentrations, which would alter the function of numerous membrane proteins (and ion channels), involve changes in lipid bilayer properties. In this context, it is relevant that the bilayer-modifying potency (per molecule in the bilayer) varies considerably among different classes of amphiphiles. Bilayer-modifying effects occur at membrane mole fractions of ∼0.001 for troglitazone (Rusinova et al., 2011) to ∼0.01 for curcumin (Ingólfsson et al., 2007) and docosahexaenoic acid (Bruno et al., 2007), to ∼0.1 for n-alkanols (Ingólfsson and Andersen, 2011), whereas the intravenous anesthetic propofol is inert (Tibbs et al., 2013). The FBs, ISO, and propofol thus are notable for their remarkably low bilayer-perturbing effects at a mole fraction of about ∼0.1, although they do alter lipid bilayer properties at higher concentrations (membrane mole fractions).
Anesthetic effects on Nav
Inhibition of Nav has been implicated in the neurodepressant effects of general anesthetics (Herold and Hemmings, 2012), as well as in the action(s) of some antiepileptic drugs (Yang et al., 2009; Jo and Bean, 2014) and intoxicants (Harris and Bruno, 1985; Shiraishi and Harris, 2004). Blockade of presynaptic Nav results in reduced neurotransmitter release (Westphalen and Hemmings, 2006; Westphalen et al., 2010), thereby affecting synaptic transmission. Moreover, Nav inhibition has been implicated in anesthetic-induced immobilization, a cardinal feature of general anesthesia, as demonstrated in rat for ISO using an in vivo pharmacological approach (Zhang et al., 2007, 2010).
The electrophysiological effects of volatile anesthetics on Nav are characterized by voltage-dependent reduction in peak current largely caused by hyperpolarizing shifts in the voltage dependence of steady-state inactivation (Herold and Hemmings, 2012), as demonstrated here for a series of model FB anesthetics. The magnitude of the FBs’ effects varied among drugs tested at equi-anesthetic concentrations equivalent to 1 MAC, as expected if they altered the function of more than one target protein.
At the clinical concentration of 1 MAC, the estimated anesthetic concentrations in the bilayer are high (Table 1), varying between ∼60 mM (for HFB and ISO) and ∼170 mM (for 1,3,5-TFB), which suggests that it is the membrane-dissolved anesthetics that are relevant for Nav. In fact, molecular dynamics simulations of a homology model of the prokaryotic Nav homologue NaChBac (Raju et al., 2013) revealed that ISO was able to access the pore through hydrophobic side fenestrations that were observed in the crystal structure of NavAb (Payandeh et al., 2011). Although all four fenestrations were occupied by ISO, it is unclear whether other volatile anesthetics and larger molecules might do the same, or whether ISO binding in the fenestrations alters Nav function.
The agent-specific differences in FB effects on Nav are comparable to those shown previously for inhibition of NMDA-type glutamate receptors by structurally diverse inhaled anesthetics (Solt et al., 2006). The relative potencies of aromatic anesthetics for inhibition of NMDA receptors correlate well with their abilities to form cation–π interactions rather than with their hydrophobic properties (Raines et al., 2004). This is consistent with our observations that the anesthetic with strong cation–π interactions (1,2-DFB) had a strong effect on Nav.
Modulation of ion channel function by anesthetics
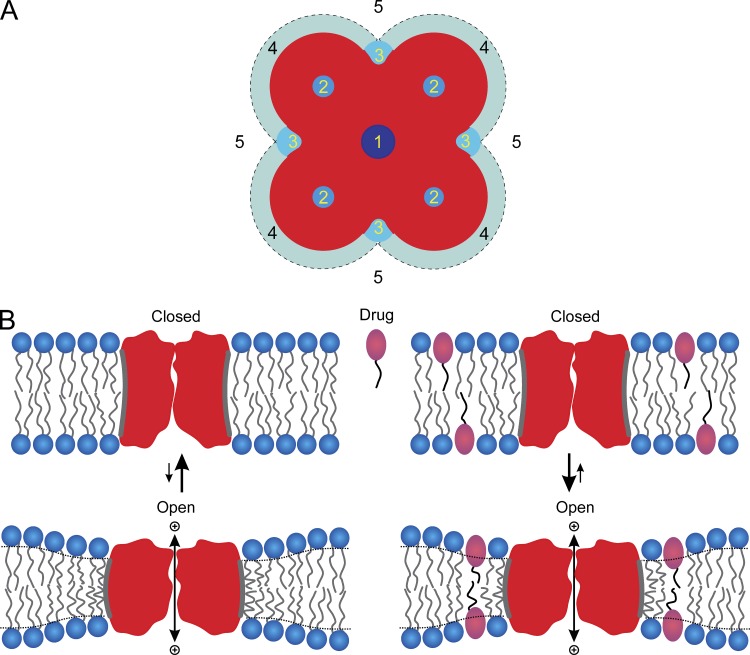
The FB model anesthetics have qualitatively similar effects on Nav as clinically used halogenated anesthetics such as ISO, sevoflurane, and desflurane, all of which produce voltage-dependent inhibition of Nav function (Rehberg et al., 1996; Stadnicka et al., 1999; Horishita et al., 2008; Ouyang et al., 2009). These effects on Nav function could result from both lipid bilayer–mediated and/or direct protein-mediated mechanisms, and the relative contributions of these mechanisms to anesthetic-induced changes in ion channel function remain uncertain (Yamakura et al., 2001; Hemmings et al., 2005; Eger et al., 2008; Perouansky, 2012; Sonner and Cantor, 2013). It thus becomes important to consider the different mechanisms by which anesthetics can modify protein (e.g., ion channel) function. Fig. 7 A shows a schematic representation of five major mechanisms by which volatile anesthetics could plausibly alter protein function.
Figure 7.
Schematic representation of potential sites for drug–ion channel interactions. (A) 1, binding in the pore to block ion movement; 2, binding to sites formed by the channel to modify the free energy difference between different conformational states; 3, binding to specific sites composed of both the protein and bilayer lipids to alter the bilayer deformation energy contribution to the free energy difference between conformational states; 4, accumulation of drug at the protein–bilayer interface to alter local lipid packing; 5, partitioning into the lipid bilayer–solution interface to alter bulk lipid bilayer properties, which will alter the bilayer deformation energy associated with the channel conformational changes (adapted from Andersen, 2008). (B) Membrane protein conformational changes in the absence and presence of an amphiphile. (Left) Hydrophobic coupling between a channel protein and the host bilayer causes membrane protein conformational changes that involve the protein’s bilayer-spanning domain to alter the packing of the adjacent membrane lipids. This bilayer deformation has an associated energetic cost () that contributes to the total free energy of the protein conformational change. (Right) Amphiphiles partition between the aqueous solution and the bilayer, and will redistribute (between the aqueous solution and the membrane and in the plane of the membrane) in response to the local protein-induced bilayer deformation, which may increase the degrees of freedom available for the membrane to adapt to the protein and thus the value of (adapted from Ingólfsson and Andersen, 2010).
Whatever the mechanism, or combination of mechanisms, anesthetics alter the distribution among various membrane protein conformations, i.e., shift the equilibrium between resting and inactivated Nav toward the inactivated state(s). The free energy difference for conformational transitions in membrane proteins (in our case, ) is the sum of contributions from rearrangements within the protein () and within the surrounding bilayer (the difference in the bilayer deformation energy associated with the two conformations, (). Anesthetic-induced changes in channel function could result from direct binding to the channel protein (sites 2 and 3 in Fig. 7 A), as a result of differences in binding energy to different channel states (Monod et al., 1965; Jackson et al., 1989) included in , as well as from drug-induced changes in lipid bilayer properties included in . Changes in are expected to vary as a saturating function of anesthetic concentration, whereas changes in are expected to vary as a linear function of anesthetic concentration (Alejo et al., 2013). Thus, as anesthetic concentration increases, not only will the function of an increasing number of membrane proteins be altered because of direct anesthetic–protein interactions (of decreasing specificity), but indiscriminate changes in the function of many membrane proteins will occur caused by changes in lipid bilayer properties that increase the magnitude of the contribution to the energetics of conformational changes in any membrane protein.
Bilayer-mediated effects arise because hydrophobic interactions between a bilayer-spanning protein and the lipid bilayer in which it is embedded cause the relatively soft lipid bilayer to adapt itself to the exterior surface of the protein bilayer–spanning domain (Fig. 7 B, left). As is the case for gramicidin channels (Fig. 1), this bilayer adaptation (deformation) is associated with an energetic cost, the bilayer deformation energy (). When an amphiphilic drug partitions into the bilayer (Fig. 7 B, right), it may increase the number of degrees of freedom that are available to the bilayer as it adapts to the protein, which in general reduces the energetic cost of bilayer deformation.
But the structure of the amphiphile is important. Experiments with n-alcohols of varying length show that short- and long-chain alcohols have opposite effects on chain ordering (Pope and Dubro, 1986) and on partial molar volume changes that occur upon partitioning into lipid bilayers (Aagaard et al., 2006). These compounds similarly have opposite effects on the energetics of the metarhodopsin I↔metarhodopsin II equilibrium (Mitchell et al., 1996) and the gramicidin monomer↔dimer equilibrium (Ingólfsson and Andersen, 2011). Although amphiphile-induced increases in lipid bilayer elasticity appear to be quite general (Lundbæk et al., 2010b), the sign of the change in bilayer deformation energy also reflects the ordering/disordering effects that amphiphiles impose on the acyl chains.
Bilayer deformation energy can be expressed using the theory of elastic bilayer deformations (Nielsen and Andersen, 2000; Rusinova et al., 2011):
where l and d0 denote protein hydrophobic length and average thickness of the bilayer hydrophobic core, respectively; c0 is intrinsic curvature of the bilayer-forming lipids; is the energetic cost of embedding a protein into a bilayer with no hydrophobic mismatch (l − d0 = 0 and c0 = 0), such as the loss of conformational entropy by the acyl chains adjacent to the protein (Fattal and Ben-Shaul, 1993); and the H coefficients are functions of bilayer thickness, protein shape (for cylindrical inclusions, the inclusion radius), and the elastic area compression and bending moduli (Ka and Kc, respectively). Membrane proteins are not smooth cylinders, however, and there might not be perfect hydrophobic adaption between a protein and bilayer lipids, in which case there can be an additional residual exposure contribution, , to the energetics of protein-imposed bilayer perturbations (Mondal et al., 2011).
Ka, Kc, c0 (and d0) are functions of the lateral pressure profile across each bilayer leaflet (Helfrich, 1981; Szleifer et al., 1990; Marsh, 2007), which in turn means that the H coefficients in the expression for , as well as c0 and d0, vary with changes in lateral pressure profile. That is, the bilayer contribution to the free energy of gramicidin dimerization (and thus the average number of conducting channels per vesicle) will vary as a function of changes in the lateral pressure profile. The above analysis does not consider explicitly the ΔA · p contribution, where A is the channel’s cross-sectional area and p is the lateral pressure, to the bilayer deformation energy (Cantor, 1997, 1999). This is likely to be minimal in the case of gramicidin channels but does not imply that gramicidin channels do not report changes in lateral pressure profile.
Early studies of anesthetic–lipid interactions tended to focus on poorly defined lipid bilayer–mediated effects (Perouansky, 2012). Overt changes in lipid bilayer properties occur only at very high concentrations (Franks and Lieb, 1979), and the magnitude of anesthetic-induced changes in bilayer properties appeared insufficient to explain the clinical effects of anesthetics (Yamakura et al., 2001). More recently, it was proposed that anesthetics could alter membrane protein function by altering more subtle bilayer properties, such as intrinsic lipid curvature (Gruner and Shyamsunder, 1991) or lateral pressure profile (Cantor, 1997; Sonner and Cantor, 2013), two equivalent descriptors of membrane protein–lipid bilayer interactions (Andersen and Koeppe, 2007; Marsh, 2007), which also could alter the free energy difference between different protein conformations. Whether or not the energetic consequences of changes in curvature or lateral pressure are sufficient to alter membrane protein function remains controversial (Lee, 2004; Marsh, 2007).
There is considerable experimental evidence that lateral pressure variations can be important for MscL (Martinac et al., 1990) and MscS (Kamaraju and Sukharev, 2008; Kamaraju et al., 2011) gating, and Teague et al. (2013) have demonstrated that rhodopsin is regulated by changes in lipid bilayer curvature under conditions where lipid bilayer thickness is maintained constant. Moreover, lipid bilayers respond to mechanical deformation and stress (stretch) by membrane area expansion (or thinning), which produces altered function of embedded proteins such as ion channels (Hamill and Martinac, 2001; Markin and Sachs, 2007; Morris and Juranka, 2007). However, experiments with Nav (Lundbæk et al., 2005; Rusinova et al., 2011) and GABAA (Søgaard et al., 2006; Chisari et al., 2010) channels show that amphiphiles that produce positive and negative changes in curvature—and thus rather different changes in lateral pressure profile, Marsh (2007)—produce similar changes in channel function. This suggests that the energetic consequences of changes in lateral pressure profile (or curvature) are dominated by other contributions. One such contribution could be changes in bilayer elasticity (Evans et al., 1995; Zhelev, 1998; Bruno et al., 2013) that arise from reversible partitioning of amphiphiles between the aqueous solution and the bilayer–solution interface, as well as any lateral redistribution in the plane of the membrane (Bruno et al., 2007), as shown schematically in Fig. 7 B (right). It is, in fact, possible to predict changes in Nav gating under situations where changes in bilayer elasticity were evaluated using gramicidin channels of different lengths (Lundbæk et al., 2010b).
Lack of anesthetic-induced changes in lipid bilayer properties
Although anesthetic potency correlates with lipid solubility, the FBs and ISO had no discernable effects on lipid bilayer properties at clinical concentrations. At higher supratherapeutic and toxic (potentially lethal) concentrations, however, anesthetics can alter bilayer properties (Franks and Lieb, 1978, 1979; Goodman et al., 1996; Pickholz et al., 2005; Finol-Urdaneta et al., 2010; Cascales et al., 2011; Weinrich et al., 2012). Such changes, however, would be associated with promiscuous changes in membrane protein function because changes in lipid bilayer properties would alter the lipid bilayer contribution to the free energy of many membrane protein conformational transitions (Lundbæk et al., 2010b; Rusinova et al., 2011). These effects therefore are unlikely to be relevant for their desired clinical effects, but might account for their toxic effects.
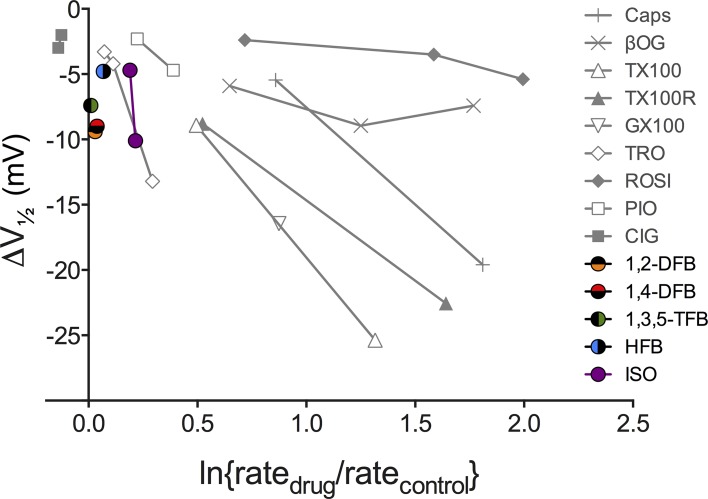
Experiments with gramicidin channels in single-component bilayers have proven to be very sensitive to changes in lipid bilayer properties (Ingólfsson and Andersen, 2010; Ingólfsson et al., 2010; Rusinova et al., 2011). Amphiphile-induced changes in Nav function (the hyperpolarizing shift in the inactivation curve) can be predicted from changes in gramicidin single-channel lifetimes (Lundbæk et al., 2004, 2005; Rusinova et al., 2011). Fig. 8 (gray symbols) shows a similar relationship when the changes in Nav function (V1/2) are plotted against gramicidin channel function determined using the GBFA, meaning that gramicidin channels indeed sense changes in bilayer properties that are relevant for Nav function. In contrast, anesthetic-induced shifts in V1/2 occur in the absence of changes in fluorescence quench rate, i.e., in the absence of changes in lipid bilayer properties detected as changes in gramicidin channel function (Fig. 8, colored symbols).
Figure 8.
Relation between bilayer-modifying effects and alteration of Nav function for various drugs and other amphiphiles. Plot of amphiphile-induced changes in the midpoints of the Nav inactivation curve (V1/2) against changes in the fluorescence quench rate measured using the GBFA. The gray points denote results for bilayer-modifying amphiphiles that have been examined in previous studies (Lundbæk et al., 2005; Rusinova et al., 2011). The colored points show results for the anesthetics from this study. The amphiphile-induced shifts in V1/2 are from Rusinova et al. (2011); the second ISO concentration is from Ouyang et al. (2009); except for the results for Genapol X-100 and reduced Triton X-100, which were done as part of this study, the amphiphile-induced changes in quench rates are from Ingólfsson and Andersen (2010) and Rusinova et al. (2011). Caps, capsaicin; βOG, β-octyl-glucoside; TX100, Triton X-100; TX100R, reduced Triton X-100; GX100, Genapol X-100; TRO, troglitazone; ROSI, rosiglitazone; PIO, pioglitazone; CIG, ciglitazone.
The question remains as to whether anesthetics might alter some bilayer property that is not sensed by gramicidin channels. This is unlikely because gramicidin channels are sensitive to changes in intrinsic curvature (Lundbæk et al., 1997), lateral stress (Goulian et al., 1998), and bilayer thickness (Lundbæk and Andersen, 1994). The lateral stress- and bilayer thickness–dependent changes in gramicidin channel function agree with predictions based on the theory of elastic bilayer deformations (compare Huang, 1986; Helfrich and Jakobsson, 1990; Nielsen et al., 1998). Changes in the elastic moduli and intrinsic curvature arise from changes in the lateral pressure profile across the bilayer (Helfrich, 1981; Szleifer et al., 1990; Marsh, 2007), meaning that gramicidin channels sense changes in lateral pressure profile, as shown experimentally (Lundbæk et al., 1997; Goulian et al., 1998). Given that gramicidin channels, if anything, are more sensitive probes of changes in lipid bilayer properties than micromechanical measurements (compare Ly and Longo, 2004; Ingólfsson and Andersen, 2011), we conclude that it is most unlikely that anesthetics alter some bilayer property not reported by gramicidin channels.
We also examined the bilayer-modifying effects of anesthetics in LUVs formed from cholesterol-containing lipid mixtures (1:1 mixture of DC18:1PC and cholesterol). In these mixed LUVs, their bilayer-modifying effects were, if anything, less, which strengthens our conclusion that anesthetics have minimal effects on lipid bilayer properties at anesthetic concentrations. Our results do not exclude the possibility that there might exist membranes with a lipid composition that makes them especially sensitive to anesthetics. We are not aware of evidence for such specialized membranes, and the generality of anesthetic effects across organisms ranging from microbes to man (Sonner and Cantor, 2013) makes the existence of such specialized domains unlikely.
Conclusions
Model aromatic FB anesthetics and the clinical anesthetic ISO inhibit Nav at clinically relevant concentrations at which they do not alter lipid bilayer properties, as sensed by gramicidin channel function. We therefore conclude that that these anesthetics alter Nav function through direct interactions with the ion channel protein rather than through alterations of lipid bilayer properties. These results provide strong support for the notion that the functionally relevant targets of volatile anesthetics are membrane proteins rather than the lipid bilayer itself.
Acknowledgments
The authors thank the reviewers of the manuscript for their incisive comments.
This work was supported by National Institutes of Health grants GM058055 (to H.C. Hemmings Jr.) and GM021347 (to O.S. Andersen), and Deutsche Forschungsgemeinschaft (German Research Foundation) grant HE4554/5-1 (to K.F. Herold).
The authors declare no competing financial interests.
Sergei Sukharev served as guest editor.
Footnotes
Abbreviations used in this paper:
- ANTS
- 8-aminonaphthalene-1,3,6-trisulfonate
- DFB
- difluorobenzene
- GABA
- γ-aminobutyric acid
- GBFA
- gramicidin-based fluorescence assay
- HFB
- hexafluorobenzene
- ISO
- isoflurane
- LUV
- large unilamellar vesicle
- MAC
- minimum alveolar concentration
- Nav
- voltage-gated sodium channel(s)
- TFB
- trifluorobenzene
References
- Aagaard T.H., Kristensen M.N., and Westh P.. 2006. Packing properties of 1-alkanols and alkanes in a phospholipid membrane. Biophys. Chem. 119:61–68 10.1016/j.bpc.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Alejo J.L., Blanchard S.C., and Andersen O.S.. 2013. Small-molecule photostabilizing agents are modifiers of lipid bilayer properties. Biophys. J. 104:2410–2418 10.1016/j.bpj.2013.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O.S.2008. Perspectives on how to drug an ion channel. J. Gen. Physiol. 131:395–397 10.1085/jgp.200810012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O.S., and Koeppe R.E. II. 2007. Bilayer thickness and membrane protein function: An energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36:107–130 10.1146/annurev.biophys.36.040306.132643 [DOI] [PubMed] [Google Scholar]
- Artigas P., Al’aref S.J., Hobart E.A., Díaz L.F., Sakaguchi M., Straw S., and Andersen O.S.. 2006. 2,3-butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: The role of bilayer material properties. Mol. Pharmacol. 70:2015–2026 10.1124/mol.106.026070 [DOI] [PubMed] [Google Scholar]
- Barber A.F., Liang Q., Amaral C., Treptow W., and Covarrubias M.. 2011. Molecular mapping of general anesthetic sites in a voltage-gated ion channel. Biophys. J. 101:1613–1622 10.1016/j.bpj.2011.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberan-Santos M.N., Bodunov E.N., and Valeur B.. 2005. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential). Chem. Phys. 315:171–182 10.1016/j.chemphys.2005.04.006 [DOI] [Google Scholar]
- Bezanilla F., and Armstrong C.M.. 1977. Inactivation of the sodium channel. I. Sodium current experiments. J. Gen. Physiol. 70:549–566 10.1085/jgp.70.5.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M.J., Koeppe R.E. II, and Andersen O.S.. 2007. Docosahexaenoic acid alters bilayer elastic properties. Proc. Natl. Acad. Sci. USA. 104:9638–9643 10.1073/pnas.0701015104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M.J., Rusinova R., Gleason N.J., Koeppe R.E. II, and Andersen O.S.. 2013. Interactions of drugs and amphiphiles with membranes: modulation of lipid bilayer elastic properties by changes in acyl chain unsaturation and protonation. Faraday Discuss. 161:461–480 10.1039/c2fd20092a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns T.H., Hall J.M., Bracken A., and Gouldstone G.. 1961. An investigation of new fluorine compounds in anaesthesia. (3) The anaesthetic properties of hexafluorobenzene. Anaesthesia. 16:333–339 10.1111/j.1365-2044.1961.tb13832.x [DOI] [PubMed] [Google Scholar]
- Burns T.H., Hall J.M., Bracken A., and Gouldstone G.. 1964. Fluorine compounds in anaesthesia. 6. Examination of fourteen heavily halogenated ring compounds. Anaesthesia. 19:167–176 10.1111/j.1365-2044.1964.tb00364.x [DOI] [PubMed] [Google Scholar]
- Cantor R.S.1997. The lateral pressure profile in membranes: A physical mechanism of general anesthesia. Biochemistry. 36:2339–2344 10.1021/bi9627323 [DOI] [PubMed] [Google Scholar]
- Cantor R.S.1999. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids. 101:45–56 10.1016/S0009-3084(99)00054-7 [DOI] [PubMed] [Google Scholar]
- Cascales J.J., Costa S.D., and Porasso R.D.. 2011. Thermodynamic study of benzocaine insertion into different lipid bilayers. J. Chem. Phys. 135:135103 10.1063/1.3643496 [DOI] [PubMed] [Google Scholar]
- Chen Z.W., Manion B., Townsend R.R., Reichert D.E., Covey D.F., Steinbach J.H., Sieghart W., Fuchs K., and Evers A.S.. 2012. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABAA receptor. Mol. Pharmacol. 82:408–419 10.1124/mol.112.078410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara D.C., Dostalova Z., Jayakar S.S., Zhou X., Miller K.W., and Cohen J.B.. 2012. Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [³H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 51:836–847 10.1021/bi201772m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M., Shu H.J., Taylor A., Steinbach J.H., Zorumski C.F., and Mennerick S.. 2010. Structurally diverse amphiphiles exhibit biphasic modulation of GABAA receptors: similarities and differences with neurosteroid actions. Br. J. Pharmacol. 160:130–141 10.1111/j.1476-5381.2010.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R., Peterson B.K., Banks P., Simillis C., Martin J.C., Valenzuela C.A., Maze M., and Franks N.P.. 2007. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: Evidence from molecular modeling and electrophysiology. Anesthesiology. 107:756–767 10.1097/01.anes.0000287061.77674.71 [DOI] [PubMed] [Google Scholar]
- Dubois B.W., Cherian S.F., and Evers A.S.. 1993. Volatile anesthetics compete for common binding sites on bovine serum albumin: a 19F-NMR study. Proc. Natl. Acad. Sci. USA. 90:6478–6482 10.1073/pnas.90.14.6478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff R.G., and Johansson J.S.. 1997. Molecular interactions between inhaled anesthetics and proteins. Pharmacol. Rev. 49:343–367. [PubMed] [Google Scholar]
- Eger E.I. II, Saidman L.J., and Brandstater B.. 1965. Minimum alveolar anesthetic concentration: A standard of anesthetic potency. Anesthesiology. 26:756–763 10.1097/00000542-196511000-00010 [DOI] [PubMed] [Google Scholar]
- Eger E.I. II, Raines D.E., Shafer S.L., Hemmings H.C. Jr, and Sonner J.M.. 2008. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth. Analg. 107:832–848 10.1213/ane.0b013e318182aedb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J.R., Needham D., Dilger J.P., and Haydon D.A.. 1983. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim. Biophys. Acta. 735:95–103 10.1016/0005-2736(83)90264-X [DOI] [PubMed] [Google Scholar]
- Evans E., Rawicz W., and Hofmann A.F.. 1995. Lipid bilayer expansion and mechanical disruption in solutions of water-soluble bile acid. In Bile Acids in Gastroenterology Basic and Clinical Advances Hofmann A.F., Paumgartner G., and Stiehl A., Kluwer Academic Publishers, Dordrecht, Netherlands: 59–68. [Google Scholar]
- Fang Z., Sonner J., Laster M.J., Ionescu P., Kandel L., Koblin D.D., Eger E.I. II, and Halsey M.J.. 1996. Anesthetic and convulsant properties of aromatic compounds and cycloalkanes: Implications for mechanisms of narcosis. Anesth. Analg. 83:1097–1104. [DOI] [PubMed] [Google Scholar]
- Fattal D.R., and Ben-Shaul A.. 1993. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys. J. 65:1795–1809 10.1016/S0006-3495(93)81249-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finol-Urdaneta R.K., McArthur J.R., Juranka P.F., French R.J., and Morris C.E.. 2010. Modulation of KvAP unitary conductance and gating by 1-alkanols and other surface active agents. Biophys. J. 98:762–772 10.1016/j.bpj.2009.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.P., and Lieb W.R.. 1978. Where do general anaesthetics act? Nature. 274:339–342 10.1038/274339a0 [DOI] [PubMed] [Google Scholar]
- Franks N.P., and Lieb W.R.. 1979. The structure of lipid bilayers and the effects of general anaesthetics. An x-ray and neutron diffraction study. J. Mol. Biol. 133:469–500 10.1016/0022-2836(79)90403-0 [DOI] [PubMed] [Google Scholar]
- Franks N.P., and Lieb W.R.. 1984. Do general anaesthetics act by competitive binding to specific receptors? Nature. 310:599–601 10.1038/310599a0 [DOI] [PubMed] [Google Scholar]
- Franks N.P., and Lieb W.R.. 1993. Selective actions of volatile general anaesthetics at molecular and cellular levels. Br. J. Anaesth. 71:65–76 10.1093/bja/71.1.65 [DOI] [PubMed] [Google Scholar]
- Franks N.P., and Lieb W.R.. 1994. Molecular and cellular mechanisms of general anaesthesia. Nature. 367:607–614 10.1038/367607a0 [DOI] [PubMed] [Google Scholar]
- Franks N.P., Jenkins A., Conti E., Lieb W.R., and Brick P.. 1998. Structural basis for the inhibition of firefly luciferase by a general anesthetic. Biophys. J. 75:2205–2211 10.1016/S0006-3495(98)77664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C., Wartenberg H.C., Rehberg B., and Urban B.W.. 1997. Interactions of ethanol with single human brain sodium channels. Neurosci. Res. Commun. 20:113–120 http://onlinelibrary.wiley.com/doi/10.1002/%28SICI%291520-6769%28199703%2920:2%3C113::AID-NRC192%3E3.0.CO;2-6/abstract [Google Scholar]
- Gingrich K.J., Burkat P.M., and Roberts W.A.. 2009. Pentobarbital produces activation and block of α1β2γ2S GABAA receptors in rapidly perfused whole cells and membrane patches: Divergent results can be explained by pharmacokinetics. J. Gen. Physiol. 133:171–188 10.1085/jgp.200810081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D.M., Nemoto E.M., Evans R.W., and Winter P.M.. 1996. Anesthetics modulate phospholipase C hydrolysis of monolayer phospholipids by surface pressure. Chem. Phys. Lipids. 84:57–64 10.1016/S0009-3084(96)02618-7 [DOI] [PubMed] [Google Scholar]
- Goulian M., Mesquita O.N., Fygenson D.K., Nielsen C., Andersen O.S., and Libchaber A.. 1998. Gramicidin channel kinetics under tension. Biophys. J. 74:328–337 10.1016/S0006-3495(98)77790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S.M., and Shyamsunder E.. 1991. Is the mechanism of general anesthesia related to lipid membrane spontaneous curvature? Ann. NY Acad. Sci. 625:685–697 10.1111/j.1749-6632.1991.tb33902.x [DOI] [PubMed] [Google Scholar]
- Hamill O.P., and Martinac B.. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740. [DOI] [PubMed] [Google Scholar]
- Hamill O.P., Marty A., Neher E., Sakmann B., and Sigworth F.J.. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100 10.1007/BF00656997 [DOI] [PubMed] [Google Scholar]
- Harris R.A., and Bruno P.. 1985. Effects of ethanol and other intoxicant-anesthetics on voltage-dependent sodium channels of brain synaptosomes. J. Pharmacol. Exp. Ther. 232:401–406. [PubMed] [Google Scholar]
- Harroun T.A., Heller W.T., Weiss T.M., Yang L., and Huang H.W.. 1999. Experimental evidence for hydrophobic matching and membrane-mediated interactions in lipid bilayers containing gramicidin. Biophys. J. 76:937–945 10.1016/S0006-3495(99)77257-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseneder R., Rammes G., Zieglgänsberger W., Kochs E., and Hapfelmeier G.. 2002. GABAA receptor activation and open-channel block by volatile anaesthetics: a new principle of receptor modulation? Eur. J. Pharmacol. 451:43–50 10.1016/S0014-2999(02)02194-5 [DOI] [PubMed] [Google Scholar]
- Haseneder R., Kratzer S., Kochs E., Eckle V.S., Zieglgänsberger W., and Rammes G.. 2008. Xenon reduces N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiology. 109:998–1006 10.1097/ALN.0b013e31818d6aee [DOI] [PubMed] [Google Scholar]
- Helfrich W.1981. Amphiphilic mesophases made of defects. In Physique des Deìfauts (Physics of Defects) Balian R., Kleìman M., and Poirier J.-P., North-Holland Publishing Company, Amsterdam: 716–755. [Google Scholar]
- Helfrich P., and Jakobsson E.. 1990. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophys. J. 57:1075–1084 10.1016/S0006-3495(90)82625-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings H.C. Jr, Akabas M.H., Goldstein P.A., Trudell J.R., Orser B.A., and Harrison N.L.. 2005. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 26:503–510 10.1016/j.tips.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Herold K.F., and Hemmings H.C. Jr. 2012. Sodium channels as targets for volatile anesthetics. Front Pharmacol. 3:50 10.3389/fphar.2012.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold K.F., Nau C., Ouyang W., and Hemmings H.C. Jr. 2009. Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology. 111:591–599 10.1097/ALN.0b013e3181af64d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horishita T., and Harris R.A.. 2008. n-Alcohols inhibit voltage-gated Na+ channels expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 326:270–277 10.1124/jpet.108.138370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horishita T., Eger E.I. II, and Harris R.A.. 2008. The effects of volatile aromatic anesthetics on voltage-gated Na+ channels expressed in Xenopus oocytes. Anesth. Analg. 107:1579–1586 10.1213/ane.0b013e318184b966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.W.1986. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 50:1061–1070 10.1016/S0006-3495(86)83550-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson H.I., and Andersen O.S.. 2010. Screening for small molecules’ bilayer-modifying potential using a gramicidin-based fluorescence assay. Assay Drug Dev. Technol. 8:427–436 10.1089/adt.2009.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson H.I., and Andersen O.S.. 2011. Alcohol’s effects on lipid bilayer properties. Biophys. J. 101:847–855 10.1016/j.bpj.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson H.I., Koeppe R.E. II, and Andersen O.S.. 2007. Curcumin is a modulator of bilayer material properties. Biochemistry. 46:10384–10391 10.1021/bi701013n [DOI] [PubMed] [Google Scholar]
- Ingólfsson H.I., Sanford R.L., Kapoor R., and Andersen O.S.. 2010. Gramicidin-based fluorescence assay; for determining small molecules potential for modifying lipid bilayer properties. J. Vis. Exp. 44:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson H.I., Thakur P., Herold K.F., Hobart E.A., Ramsey N.B., Periole X., de Jong D.H., Zwama M., Yilmaz D., Hall K., et al. 2014. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 9:1788–1798 10.1021/cb500086e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M., Haris P.I., and Chapman D.. 1989. Fourier transform infrared spectroscopic studies of lipids, polypeptides and proteins. J. Mol. Struct. 214:329–355 10.1016/0022-2860(89)80021-3 [DOI] [Google Scholar]
- Jo S., and Bean B.P.. 2014. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol. Pharmacol. 85:381–387 10.1124/mol.113.090472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaraju K., and Sukharev S.. 2008. The membrane lateral pressure-perturbing capacity of parabens and their effects on the mechanosensitive channel directly correlate with hydrophobicity. Biochemistry. 47:10540–10550 10.1021/bi801092g [DOI] [PubMed] [Google Scholar]
- Kamaraju K., Smith J., Wang J., Roy V., Sintim H.O., Bentley W.E., and Sukharev S.. 2011. Effects on membrane lateral pressure suggest permeation mechanisms for bacterial quorum signaling molecules. Biochemistry. 50:6983–6993 10.1021/bi200684z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Gardiwal A., Schaefer A., Panning B., and Breitmeier D.. 2007. Effect of ethanol on cardiac single sodium channel gating. Forensic Sci. Int. 171:131–135 10.1016/j.forsciint.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Lee A.G.2004. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta. 1666:62–87 10.1016/j.bbamem.2004.05.012 [DOI] [PubMed] [Google Scholar]
- Liao M., Sonner J.M., Jurd R., Rudolph U., Borghese C.M., Harris R.A., Laster M.J., and Eger E.I. II. 2005. Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth. Analg. 101:412–418 10.1213/01.ANE.0000154196.86587.35 [DOI] [PubMed] [Google Scholar]
- Liu R., Loll P.J., and Eckenhoff R.G.. 2005. Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle protein. FASEB J. 19:567–576 10.1096/fj.04-3171com [DOI] [PubMed] [Google Scholar]
- Lundbæk J.A., and Andersen O.S.. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673 10.1085/jgp.104.4.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J.A., Birn P., Girshman J., Hansen A.J., and Andersen O.S.. 1996. Membrane stiffness and channel function. Biochemistry. 35:3825–3830 10.1021/bi952250b [DOI] [PubMed] [Google Scholar]
- Lundbæk J.A., Maer A.M., and Andersen O.S.. 1997. Lipid bilayer electrostatic energy, curvature stress, and assembly of gramicidin channels. Biochemistry. 36:5695–5701 10.1021/bi9619841 [DOI] [PubMed] [Google Scholar]
- Lundbæk J.A., Birn P., Hansen A.J., Søgaard R., Nielsen C., Girshman J., Bruno M.J., Tape S.E., Egebjerg J., Greathouse D.V., et al. 2004. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 123:599–621 10.1085/jgp.200308996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J.A., Birn P., Tape S.E., Toombes G.E.S., Søgaard R., Koeppe R.E. II, Gruner S.M., Hansen A.J., and Andersen O.S.. 2005. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol. Pharmacol. 68:680–689. [DOI] [PubMed] [Google Scholar]
- Lundbæk J.A., Collingwood S.A., Ingólfsson H.I., Kapoor R., and Andersen O.S.. 2010a. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J. R. Soc. Interface. 7:373–395 10.1098/rsif.2009.0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J.A., Koeppe R.E. II, and Andersen O.S.. 2010b. Amphiphile regulation of ion channel function by changes in the bilayer spring constant. Proc. Natl. Acad. Sci. USA. 107:15427–15430 10.1073/pnas.1007455107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly H.V., and Longo M.L.. 2004. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 87:1013–1033 10.1529/biophysj.103.034280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin V.S., and Sachs F.. 2007. Thermodynamics of mechanosensitivity. Curr. Top. Membr. 58:87–119 10.1016/S1063-5823(06)58004-4 [DOI] [Google Scholar]
- Marsh D.2007. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys. J. 93:3884–3899 10.1529/biophysj.107.107938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Adler J., and Kung C.. 1990. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 348:261–263 10.1038/348261a0 [DOI] [PubMed] [Google Scholar]
- Meyer H.1899. Zur theorie der alkoholnarkose. Archiv fur experimentelle Pathologie und Pharmakologie. 42:109–118. [Google Scholar]
- Mitchell D.C., Lawrence J.T., and Litman B.J.. 1996. Primary alcohols modulate the activation of the G protein-coupled receptor rhodopsin by a lipid-mediated mechanism. J. Biol. Chem. 271:19033–19036 10.1074/jbc.271.32.19033 [DOI] [PubMed] [Google Scholar]
- Mondal S., Khelashvili G., Shan J., Andersen O.S., and Weinstein H.. 2011. Quantitative modeling of membrane deformations by multihelical membrane proteins: application to G-protein coupled receptors. Biophys. J. 101:2092–2101 10.1016/j.bpj.2011.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J., Wyman J., and Changeux J.P.. 1965. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12:88–118 10.1016/S0022-2836(65)80285-6 [DOI] [PubMed] [Google Scholar]
- Morris C.E., and Juranka P.F.. 2007. Lipid stress at play: mechanosensitivity of voltage-gated channels. Curr. Top. Membr. 59:297–338. [DOI] [PubMed] [Google Scholar]
- Nielsen C., and Andersen O.S.. 2000. Inclusion-induced bilayer deformations: Effects of monolayer equilibrium curvature. Biophys. J. 79:2583–2604 10.1016/S0006-3495(00)76498-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C., Goulian M., and Andersen O.S.. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74:1966–1983 10.1016/S0006-3495(98)77904-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonorov I.M., Blanck T.J., and Recio-Pinto E.. 1998. The effects of halothane on single human neuronal L-type calcium channels. Anesth. Analg. 86:885–895. [DOI] [PubMed] [Google Scholar]
- Nury H., Van Renterghem C., Weng Y., Tran A., Baaden M., Dufresne V., Changeux J.P., Sonner J.M., Delarue M., and Corringer P.J.. 2011. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 469:428–431 10.1038/nature09647 [DOI] [PubMed] [Google Scholar]
- Ouyang W., Herold K.F., and Hemmings H.C. Jr. 2009. Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 110:582–590 10.1097/ALN.0b013e318197941e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton C.1901. Studien über die Narkose zugleich ein Beitrag zur allgemeinen Pharmakologie. Verlag von Gustav Fischer, Jena, Germany. 195 pp. [Google Scholar]
- Patel A.J., and Honoré E.. 2001. Anesthetic-sensitive 2P domain K+ channels. Anesthesiology. 95:1013–1021 10.1097/00000542-200110000-00034 [DOI] [PubMed] [Google Scholar]
- Payandeh J., Scheuer T., Zheng N., and Catterall W.A.. 2011. The crystal structure of a voltage-gated sodium channel. Nature. 475:353–358 10.1038/nature10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perouansky M.2012. The quest for a unified model of anesthetic action: A century in Claude Bernard’s shadow. Anesthesiology. 117:465–474 10.1097/ALN.0b013e318264492e [DOI] [PubMed] [Google Scholar]
- Peyron M., Pierens G.K., Lucas A.J., Hall L.D., and Stewart R.C.. 1996. The modified stretched-exponential model for characterization of NMR relaxation in porous media. J. Magn. Reson. A. 118:214–220 10.1006/jmra.1996.0029 [DOI] [Google Scholar]
- Pickholz M., Saiz L., and Klein M.L.. 2005. Concentration effects of volatile anesthetics on the properties of model membranes: A coarse-grain approach. Biophys. J. 88:1524–1534 10.1529/biophysj.104.044354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J.M., and Dubro D.W.. 1986. The interaction of n-alkanes and n-alcohols with lipid bilayer membranes: a 2H-NMR study. Biochim. Biophys. Acta. 858:243–253 10.1016/0005-2736(86)90329-9 [DOI] [PubMed] [Google Scholar]
- Quillin M.L., Breyer W.A., Griswold I.J., and Matthews B.W.. 2000. Size versus polarizability in protein-ligand interactions: binding of noble gases within engineered cavities in phage T4 lysozyme. J. Mol. Biol. 302:955–977 10.1006/jmbi.2000.4063 [DOI] [PubMed] [Google Scholar]
- Raines D.E., Gioia F., Claycomb R.J., and Stevens R.J.. 2004. The N-methyl-d-aspartate receptor inhibitory potencies of aromatic inhaled drugs of abuse: Evidence for modulation by cation-pi interactions. J. Pharmacol. Exp. Ther. 311:14–21 10.1124/jpet.104.069930 [DOI] [PubMed] [Google Scholar]
- Raju S.G., Barber A.F., LeBard D.N., Klein M.L., and Carnevale V.. 2013. Exploring volatile general anesthetic binding to a closed membrane-bound bacterial voltage-gated sodium channel via computation. PLOS Comput. Biol. 9:e1003090 10.1371/journal.pcbi.1003090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnakumari L., and Hemmings H.C. Jr. 1998. Inhibition of presynaptic sodium channels by halothane. Anesthesiology. 88:1043–1054 10.1097/00000542-199804000-00025 [DOI] [PubMed] [Google Scholar]
- Rehberg B., Xiao Y.H., and Duch D.S.. 1996. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology. 84:1223–1233 10.1097/00000542-199605000-00025 [DOI] [PubMed] [Google Scholar]
- Rudolph U., and Antkowiak B.. 2004. Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci. 5:709–720 10.1038/nrn1496 [DOI] [PubMed] [Google Scholar]
- Rusinova R., Herold K.F., Sanford R.L., Greathouse D.V., Hemmings H.C. Jr, and Andersen O.S.. 2011. Thiazolidinedione insulin sensitizers alter lipid bilayer properties and voltage-dependent sodium channel function: implications for drug discovery. J. Gen. Physiol. 138:249–270 10.1085/jgp.201010529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi M., and Harris R.A.. 2004. Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels. J. Pharmacol. Exp. Ther. 309:987–994 10.1124/jpet.103.064063 [DOI] [PubMed] [Google Scholar]
- Sirois J.E., Lynch C. III, and Bayliss D.A.. 2002. Convergent and reciprocal modulation of a leak K+ current and Ih by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones. J. Physiol. 541:717–729 10.1113/jphysiol.2002.018119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard R., Werge T.M., Bertelsen C., Lundbye C., Madsen K.L., Nielsen C.H., and Lundbaek J.A.. 2006. GABA(A) receptor function is regulated by lipid bilayer elasticity. Biochemistry. 45:13118–13129 10.1021/bi060734+ [DOI] [PubMed] [Google Scholar]
- Solt K., Eger E.I. II, and Raines D.E.. 2006. Differential modulation of human N-methyl-d-aspartate receptors by structurally diverse general anesthetics. Anesth. Analg. 102:1407–1411 10.1213/01.ane.0000204252.07406.9f [DOI] [PubMed] [Google Scholar]
- Sonner J.M., and Cantor R.S.. 2013. Molecular mechanisms of drug action: An emerging view. Annu Rev Biophys. 42:143–167 10.1146/annurev-biophys-083012-130341 [DOI] [PubMed] [Google Scholar]
- Sonner J.M., Werner D.F., Elsen F.P., Xing Y., Liao M., Harris R.A., Harrison N.L., Fanselow M.S., Eger E.I. II, and Homanics G.E.. 2007. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology. 106:107–113 10.1097/00000542-200701000-00019 [DOI] [PubMed] [Google Scholar]
- Spurny R., Billen B., Howard R.J., Brams M., Debaveye S., Price K.L., Weston D.A., Strelkov S.V., Tytgat J., Bertrand S., et al. 2013. Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC). J. Biol. Chem. 288:8355–8364 10.1074/jbc.M112.424507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnicka A., Kwok W.M., Hartmann H.A., and Bosnjak Z.J.. 1999. Effects of halothane and isoflurane on fast and slow inactivation of human heart hH1a sodium channels. Anesthesiology. 90:1671–1683 10.1097/00000542-199906000-00024 [DOI] [PubMed] [Google Scholar]
- Study R.E.1994. Isoflurane inhibits multiple voltage-gated calcium currents in hippocampal pyramidal neurons. Anesthesiology. 81:104–116 10.1097/00000542-199407000-00016 [DOI] [PubMed] [Google Scholar]
- Suchyna T.M., Tape S.E., Koeppe R.E. II, Andersen O.S., Sachs F., and Gottlieb P.A.. 2004. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 430:235–240 10.1038/nature02743 [DOI] [PubMed] [Google Scholar]
- Szleifer I., Kramer D., Ben-Shaul A., Gelbart W.M., and Safran S.A.. 1990. Molecular theory of curvature elasticity in surfactant films. J. Chem. Phys. 92:6800–6817 10.1063/1.458267 [DOI] [Google Scholar]
- Teague W.E. Jr, Soubias O., Petrache H., Fuller N., Hines K.G., Rand R.P., and Gawrisch K.. 2013. Elastic properties of polyunsaturated phosphatidylethanolamines influence rhodopsin function. Faraday Discuss. 161:383–395 10.1039/c2fd20095c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbs G.R., Rowley T.J., Sanford R.L., Herold K.F., Proekt A., Hemmings H.C. Jr, Andersen O.S., Goldstein P.A., and Flood P.D.. 2013. HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain. J. Pharmacol. Exp. Ther. 345:363–373 10.1124/jpet.113.203620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda I., and Kamaya H.. 1973. Kinetic and thermodynamic aspects of the mechanism of general anesthesia in a model system of firefly luminescence in vitro. Anesthesiology. 38:425–436 10.1097/00000542-197305000-00002 [DOI] [PubMed] [Google Scholar]
- Weinrich M., Nanda H., Worcester D.L., Majkrzak C.F., Maranville B.B., and Bezrukov S.M.. 2012. Halothane changes the domain structure of a binary lipid membrane. Langmuir. 28:4723–4728 10.1021/la204317k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D.F., Swihart A., Rau V., Jia F., Borghese C.M., McCracken M.L., Iyer S., Fanselow M.S., Oh I., Sonner J.M., et al. 2011. Inhaled anesthetic responses of recombinant receptors and knockin mice harboring α2(S270H/L277A) GABA(A) receptor subunits that are resistant to isoflurane. J. Pharmacol. Exp. Ther. 336:134–144 10.1124/jpet.110.170431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen R.I., and Hemmings H.C. Jr. 2006. Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: Basal release. J. Pharmacol. Exp. Ther. 316:208–215 10.1124/jpet.105.090647 [DOI] [PubMed] [Google Scholar]
- Westphalen R.I., Yu J., Krivitski M., Jih T.Y., and Hemmings H.C. Jr. 2010. Regional differences in nerve terminal Na+ channel subtype expression and Na+ channel-dependent glutamate and GABA release in rat CNS. J. Neurochem. 113:1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.V., and Kendig J.J.. 1998. Differential sensitivities of TTX-resistant and TTX-sensitive sodium channels to anesthetic concentrations of ethanol in rat sensory neurons. J. Neurosci. Res. 54:433–443 http://onlinelibrary.wiley.com/doi/10.1002/%28SICI%291097-4547%2819981115%2954:4%3C433::AID-JNR1%3E3.0.CO;2-A/abstract [DOI] [PubMed] [Google Scholar]
- Xiao Z., Lu Z., Liu Z., Liu W., Li L., Yin S., Yu S., Dong H., and Zhu F.. 2008. Ethanol inhibits voltage-gated sodium channels in cultured superior cervical ganglion neurons. Neuroreport. 19:1773–1776 10.1097/WNR.0b013e328318ed9a [DOI] [PubMed] [Google Scholar]
- Yamakura T., Bertaccini E., Trudell J.R., and Harris R.A.. 2001. Anesthetics and ion channels: Molecular models and sites of action. Annu. Rev. Pharmacol. Toxicol. 41:23–51 10.1146/annurev.pharmtox.41.1.23 [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Hsieh J.Y., and Kuo C.C.. 2009. The external pore loop interacts with S6 and S3-S4 linker in domain 4 to assume an essential role in gating control and anticonvulsant action in the Na+ channel. J. Gen. Physiol. 134:95–113 10.1085/jgp.200810158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip G.M., Chen Z.W., Edge C.J., Smith E.H., Dickinson R., Hohenester E., Townsend R.R., Fuchs K., Sieghart W., Evers A.S., and Franks N.P.. 2013. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat. Chem. Biol. 9:715–720 10.1038/nchembio.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller A., Jurd R., Lambert S., Arras M., Drexler B., Grashoff C., Antkowiak B., and Rudolph U.. 2008. Inhibitory ligand-gated ion channels as substrates for general anesthetic actions. In Modern Anesthetics Schüttler J. and Schwilden H., Springer, Berlin: 31–51 10.1007/978-3-540-74806-9_2 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Laster M.J., Eger E.I. II, Sharma M., and Sonner J.M.. 2007. Lidocaine, MK-801, and MAC. Anesth. Analg. 104:1098–1102 10.1213/01.ane.0000260318.60504.a9 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guzinski M., Eger E.I. II, Laster M.J., Sharma M., Harris R.A., and Hemmings H.C. Jr. 2010. Bidirectional modulation of isoflurane potency by intrathecal tetrodotoxin and veratridine in rats. Br. J. Pharmacol. 159:872–878 10.1111/j.1476-5381.2009.00583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev D.V.1998. Material property characteristics for lipid bilayers containing lysolipid. Biophys. J. 75:321–330 10.1016/S0006-3495(98)77516-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S.A., Jones M.V., and Harrison N.L.. 1994. Potentiation of gamma-aminobutyric acidA receptor Cl− current correlates with in vivo anesthetic potency. J. Pharmacol. Exp. Ther. 270:987–991. [PubMed] [Google Scholar]