Abstract
Epithelial cells from diverse tissues, including the enterocytes that line the intestinal tract, remodel their apical surface during differentiation to form a brush border: an array of actin-supported membrane protrusions known as microvilli that increases the functional capacity of the tissue. Although our understanding of how epithelial cells assemble, stabilize, and organize apical microvilli is still developing, investigations of the biochemical and physical underpinnings of these processes suggest that cells coordinate cytoskeletal remodeling, membrane-cytoskeleton cross-linking, and extracellular adhesion to shape the apical brush border domain.
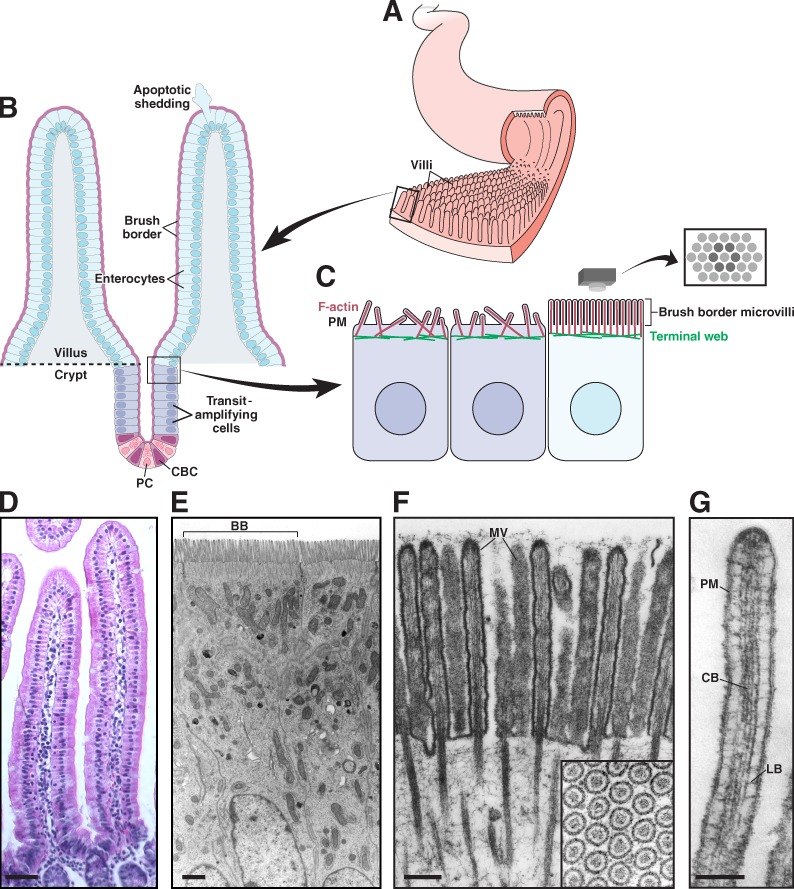
Biologists have long appreciated the intimate connection between morphology and function. Powerful examples are provided by the epithelial cells that line our hollow organs, including the gut, kidney, lung, and cochlea. The intestinal tract provides one of the most striking cases. Here, morphological adaptations at both the tissue and cellular level allow the intestinal epithelium to make close and prolonged contact with luminal contents, in turn promoting efficient uptake of available nutrients. The small intestinal mucosa features thousands of macroscopic (∼0.5 mm in length) fingerlike folds of tissue referred to as villi (Fig. 1, A, B, and D), which project into the lumen, increasing the tissue surface area available for nutrient absorption (Helander and Fändriks, 2014). The epithelium that lines each villus is composed of several cell types including goblet cells, enteroendocrine cells, Paneth cells, and enterocytes. Among these, enterocytes are by far the most abundant and are solely responsible for nutrient absorption. Because of the ever-present threat of chemical or physical damage, the intestinal epithelium is continuously renewed by stem cells found in the “crypts” at the base of each villus (Fig. 1, B and D; van der Flier and Clevers, 2009). As nascent epithelial cells emerge from the crypt, they undergo rapid differentiation (Fig. 1 C), and a subset takes on the attributes of mature enterocytes (Mariadason et al., 2005; Chang et al., 2008).
Figure 1.
Functional architecture of the intestinal epithelium. (A) The small intestinal epithelium is characterized by ubiquitous small folds of tissue known as villi. (B) Enterocytes, the most abundant cell type lining the villus, are generated in a stem cell niche composed of crypt base columnar (CBC) cells and flanking Paneth cells (PC), found in crypts near the base of each villus. CBC cells undergo asymmetric cell division, resulting in a new stem cell plus a committed daughter cell. Daughter cells undergo further division in the transit-amplifying region before differentiating into enterocytes and exiting the crypt. Enterocytes migrate up the crypt–villus axis over a period of 2–3 d. Once at the tip, cells undergo apoptosis and are extruded from the epithelium. (C) Apical surface organization of enterocytes before and after differentiation; brush border microvilli on the surface of differentiated cells are packed in tight, hexagonal arrays. (D) Villi from mouse small intestine. (E) Enterocytes from mouse small intestine; brush border (BB) from a single cell is indicated. (F) Brush border microvilli (MV) from chicken small intestine. Reproduced from Mooseker and Tilney (1975). The inset shows hexagonally packed microvilli in cross section. (G) A single microvillus. Plasma membrane (PM) is linked to the core bundle (CB) by lateral bridges (LB) that are formed at least in part by myo1a. Reproduced from Mooseker and Tilney (1975). Bars: (D) 50 µm; (E) 1 µm; (F) 0.2 µm; (G) 0.1 µm.
Fully differentiated enterocytes are characterized by a prominent brush border with two defining morphological features. First, microvilli demonstrate remarkably uniform length (Fig. 1 E), with little variability in dimensions within and between cells (∼100 nm diameter × ∼1–3 µm length depending on the region of the intestinal tract). Second, these protrusions exhibit highly ordered packing such that when the brush border is viewed en face hexagonal arrays of microvilli are observed (Fig. 1, C and F). The optimized packing of microvilli is a cellular level adaptation that further increases the surface area exposed to the lumen. Based on morphometric data obtained by light and electron microscopy, estimates suggest that microvilli amplify the surface area of the small intestine 9-16 fold (Helander and Fändriks, 2014). Thus, the numerous microvilli within the brush border support a membrane “reservoir,” which allows the enterocyte to enrich membrane-associated molecules needed for nutrient absorption (Maroux et al., 1988) and host defense (Koyama et al., 2002; Shifrin et al., 2012) on the apical surface.
Building core actin bundles
To generate microvilli, epithelial cells must overcome physical forces that oppose membrane deformation including membrane bending stiffness (κ, pN•nm), surface tension (Tm, pN/m), and membrane–cytoskeleton adhesion energy (γ, pN•nm/nm2; Sheetz, 2001; Nambiar et al., 2010). To this end, cells harness cytoskeletal dynamics to generate forces that “push” against cellular membranes and drive deformation (Theriot, 2000; Atilgan et al., 2006). In the case of intestinal microvilli, the origin of the deforming force remains unclear but is likely generated by the polymerization and bundling of actin filaments. In vitro biophysical studies indicate that actin filament elongation can generate forces in the pN range (Miyata et al., 1999; Cameron et al., 2000; Parekh et al., 2005; Footer et al., 2007). Interestingly, a classic set of experiments using native isolated brush borders revealed that actin polymerization preferentially occurs at the membrane-associated ends of core actin bundles (Pollard and Mooseker, 1981; Mooseker et al., 1982). In other early experiments, Tilney and Cardell (1970) observed the association of electron-dense foci just below the plasma membrane during the regrowth of microvilli that were destroyed by the application of hydrostatic pressure. Remarkably, actin filaments appear to originate from this material during microvillar regrowth. Similar structures have been observed at the tips of enterocyte microvilli (Mooseker and Tilney, 1975), stereocilia (Rzadzinska et al., 2004), and filopodia of motile cells (Svitkina et al., 2003). Although the composition of these structures is not well understood, they most likely represent protein complexes involved in the spatial and temporal control of actin polymerization, including actin nucleators and capping proteins, membrane bending and shaping proteins, and the signaling proteins or small GTPases that control them. However, the molecular machinery involved in controlling the polymerization of microvillar actin filaments in vivo is still unknown.
Because single actin filaments are not stiff enough to deform the membrane, microvillar protrusion requires that cells bundle numerous filaments together. This has the dual effect of increasing the number of putative force-producing elements (i.e., F-actin plus ends) per unit area of membrane and increasing the structural rigidity of the protrusion (Mogilner and Rubinstein, 2005; Claessens et al., 2006; Bathe et al., 2008). Recent electron tomography studies indicate that microvillar cores are composed of ∼30–40 bundled filaments that exhibit a slight clockwise-oriented twist when viewing distal tips en face (Ohta et al., 2012). Actin filaments within microvilli are bundled by villin (Bretscher and Weber, 1979; Mooseker et al., 1980), espin (Bartles et al., 1998), and fimbrin (Fig. 2 A and Fig. 3 A; Bretscher and Weber, 1980). Because the resulting bundles are composed of parallel actin filaments, these structures also hold the potential to serve as tracks for myosin motor proteins. In vivo studies examining the expression pattern of the microvillar core proteins revealed that villin, fimbrin, and actin localize to the apical cortex in enterocytes early during polarization, before the onset of protrusion (Heintzelman and Mooseker, 1990a,b). The role of filament bundling in microvillar protrusion was subsequently established in studies showing that exogenous villin could induce microvillar growth on the surface of cells that do not normally make microvilli (Franck et al., 1990). Later work demonstrated that overexpression of espin promoted microvillar elongation in an epithelial cell culture model system (Loomis et al., 2003). Fimbrin has also been implicated in physically linking microvillar rootlets to the dense network of cytokeratin filaments that comprise the terminal web (Grimm-Günter et al., 2009). Anchoring microvilli into the terminal web plays a crucial role in promoting the long-term stability of the brush border, as disruption of this functional connection leads to microvilli that are sensitive to biochemical manipulations and degradation (Grimm-Günter et al., 2009).
Figure 2.
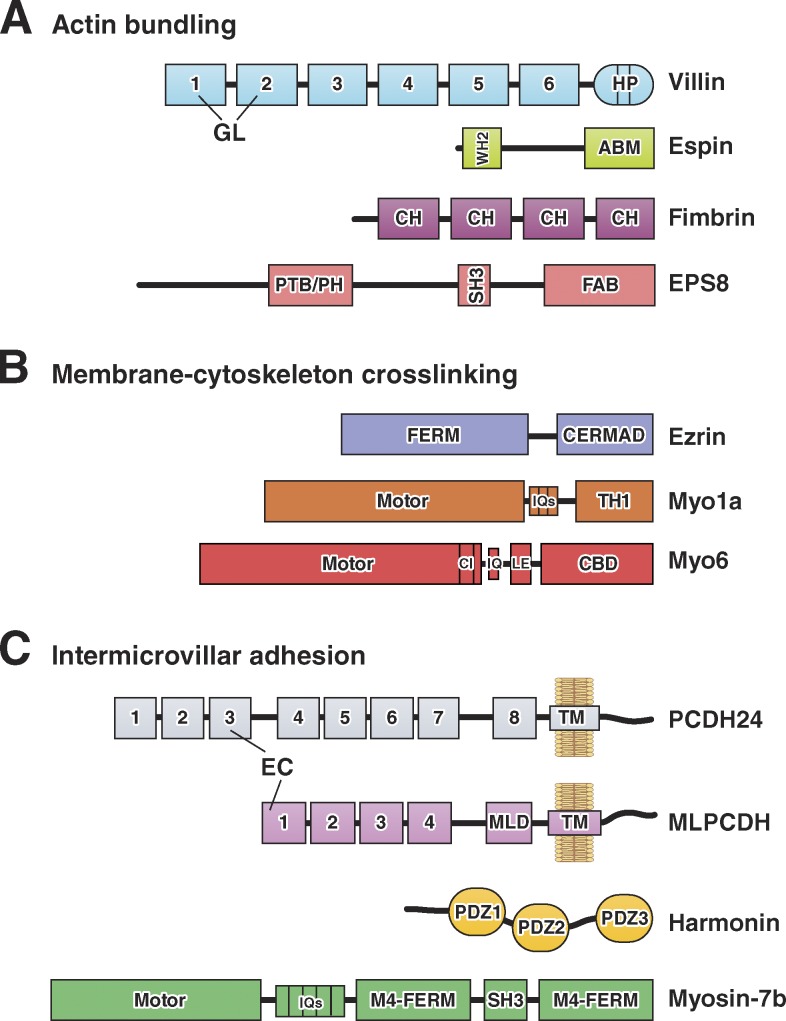
Domain organization of F-actin bundling, membrane–cytoskeleton cross-linking, and intermicrovillar adhesion molecules. (A) Actin-bundling. Villin is composed of multiple gelsolin-like (GL) domains and a C-terminal headpiece (HP) domain. Small espin contains a G-actin–binding Wiskott-Aldrich homology 2 (WH2) domain and an actin-bundling motif (ABM). Fimbrin contains tandem copies of a conserved calponin homology (CH) motif that binds actin. EPS8 contains phosphotyrosine-binding (PTB), pleckstrin homology (PH), SH3, and F-actin bundling (FAB) domains. (B) Membrane–cytoskeleton cross-linking. Ezrin contains a FERM domain and an ERM-association domain (C-ERMAD). The myo1a motor domain is linked to CaM-binding IQ motifs and a membrane-binding TH1 domain. The myo6 motor domain is linked to a converter insert (CI), an IQ motif, a lever arm extension (LE), and a cargo-binding domain (CBD). (C) Intermicrovillar adhesion. PCDH24 and MLPCDH contain multiple extracellular cadherin (EC) repeats; MLPCDH also contains a juxtamembrane mucin-like domain (MLD). Harmonin-a is composed of three PDZ domains. Myosin-7b contains an N-terminal motor domain, CaM-binding IQ motifs, and a tail composed of two MyTH4-FERM domains with an intervening SH3 domain.
Figure 3.
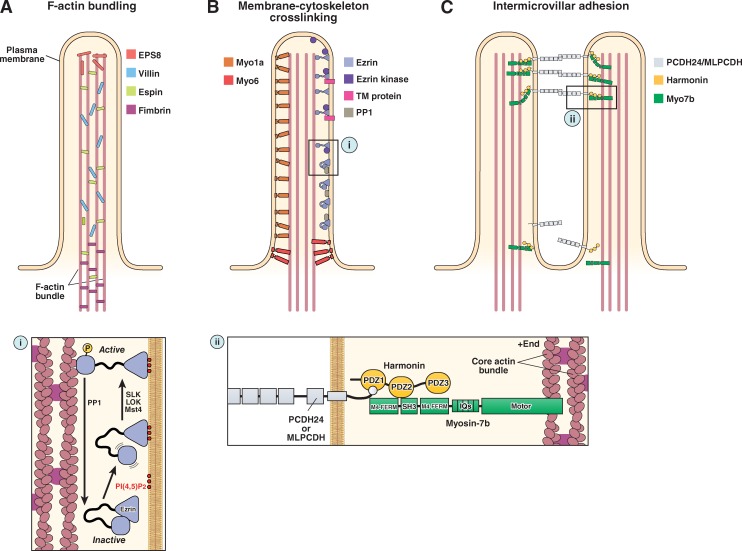
The molecular machinery of microvillar protrusion, stabilization, and organization. (A) Bundling of actin filaments in the microvillar actin core is performed through the collective and potentially redundant function of villin, espin, fimbrin, and EPS8. (B) Membrane–cytoskeleton cross-linking plays an important role in microvillar stabilization and is mediated by myo1a, myo6, and the active form of ezrin (i). (C) Extracellular adhesion between the distal tips of microvilli (i.e., intermicrovillar adhesion) is used to optimize the packing of these protrusions during brush border assembly. Intermicrovillar adhesion is mediated by a trans-heterophilic complex of PCDH24 and MLPCDH. Distal tip targeting of microvillar protocadherins requires interactions with harmonin and, potentially, myosin-7b (ii). See main text for details on the function of the other proteins depicted in the figure.
Although villin, espin, and fimbrin are acknowledged as the major F-actin bundling proteins in the intestinal brush border, mouse models lacking one or more of these proteins are still able to assemble microvilli. Indeed, deletion of villin in mice had a minimal impact on brush border organization under normal conditions (Ferrary et al., 1999). Even more remarkably, a mouse model lacking villin, espin, and fimbrin still assembled a functional brush border, although microvillar organization was perturbed (Revenu et al., 2012). These investigations strongly suggest that other atypical actin-bundling proteins may play a role in building core bundles. One possibility is the receptor tyrosine kinase substrate EPS8 (Fig. 2 A), which localizes to the tips of microvilli (Fig. 3 A) and is capable of both capping plus ends and bundling filaments (Croce et al., 2004; Hertzog et al., 2010). Interestingly, a knockout (KO) of EPS8 in mice led to microvilli that were shorter and more variable in length, but again, functional protrusions still formed (Tocchetti et al., 2010). Collectively, these studies indicate that brush border assembly is biologically robust, with highly effective compensatory mechanisms in place to ensure that microvillar core actin bundles are assembled during enterocyte differentiation.
Stabilizing protrusions
Although actin polymerization and bundling likely contribute forces that drive the protrusion of individual microvilli, additional physical obstacles arise when new protrusions are built in close proximity to each other. Unless prevented, membrane surface tension will promote the coalescence and fusion of adjacent protrusions to achieve an energetic minimum (Atilgan et al., 2006). This is a significant concern where the density of surface protrusions is exceedingly high, as in the intestinal brush border. To counteract such coalescence and stabilize protrusions, epithelial cells take advantage of molecules such as unconventional myosins and ERM (ezrin, radixin, moesin) family proteins, which cross-link the plasma membrane to the underlying actin cytoskeleton.
Myosin-1a.
Class I myosins are single-headed motor proteins that function at the actin cytoskeleton–plasma membrane interface in numerous cellular processes including endo- and exocytosis (Novak et al., 1995; Bose et al., 2004), the release of microvesicles (McConnell and Tyska, 2007), and the maintenance of membrane tension and cortical rigidity (Dai et al., 1999; Nambiar et al., 2009). The intestinal brush border is home to several class I myosins, with myosin-1a (myo1a) being by far the most abundant (Fig. 2 B and Fig. 3 B; McConnell et al., 2011). Expression of myo1a is limited to the intestinal tract, where it localizes almost exclusively to the brush border (Skowron et al., 1998; Skowron and Mooseker, 1999). Within microvilli, it forms the characteristic lateral bridges observed with transmission EM, which appear to cross-link the plasma membrane to the actin core (Fig. 1 G; Mooseker and Tilney, 1975; Howe and Mooseker, 1983). Myo1a is a slow motor (Wolenski et al., 1993a,b; Jontes et al., 1997) that interacts with membrane using a tail homology 1 (TH1) domain that exhibits moderate affinity for lipids with acidic head groups, such as phosphatidylserine or PI(4,5)P2 (Hayden et al., 1990; Mazerik and Tyska, 2012). TH1 contains two distinct membrane-binding motifs, the N-terminal and C-terminal targeting motifs (Mazerik and Tyska, 2012), which function to keep this motor in close proximity to the plasma membrane (Mazerik et al., 2014). Interestingly, mutations in the C-terminal motif have been linked to a loss of epithelial polarity and the development of colon cancer, which indicates that normal membrane binding is important for myo1a tumor suppressor function in vivo (Mazzolini et al., 2012). Kinetic studies indicate that the myo1a motor domain is a low-duty-ratio motor; i.e., it only spends a small fraction of its total ATPase cycle time bound to actin (Jontes et al., 1997). Although this kinetic limitation may appear to be inconsistent with a role in membrane–cytoskeleton cross-linking, each microvillus is home to thousands of asynchronously cycling myo1a molecules (Brown and McKnight, 2010), an ensemble large enough to ensure a continuous interaction between the plasma membrane and the actin core.
Mice lacking myo1a exhibit significant perturbations in brush border morphology including extensive membrane herniations and fused microvilli (Tyska et al., 2005). These abnormalities suggest that physical coupling between the plasma membrane and the actin core is disrupted in the absence of myo1a. Direct support for this proposal was eventually provided by optical trap-based measurements of membrane tension in isolated myo1a KO brush borders and in live cells where levels of myo1a were manipulated (Nambiar et al., 2009). Despite the absence of membrane–cytoskeleton cross-links, myo1a KO enterocytes still maintain functional microvilli (Tyska et al., 2005). Compensatory mechanisms in this case involve other class I myosins that redistribute to the brush border upon loss of myo1a, including myo1c and myo1d (Tyska et al., 2005; Benesh et al., 2010).
Disrupting the ability of myo1a to link plasma membrane to the actin core might also facilitate the infection of enteric pathogens. Enteropathogenic Escherichia coli (EPEC) colonize the intestinal mucosa by forming intimate attachments with enterocytes through the injection of virulence factors that remodel the apical surface (Rothbaum et al., 1983; Knutton et al., 1987). This remodeling destroys the brush border, resulting in severe watery diarrhea (Knutton et al., 1987). One such virulence factor, EspB, interacts with and inhibits actin binding of a variety of myosin family members, including myo1a (Iizumi et al., 2007). This raises the interesting possibility that EspB-mediated disruption of myo1a cross-links may be an important event in EPEC-induced remodeling of the apical surface. Indeed, a mutant strain of EPEC with a defective copy of EspB unable to interact with myosins exhibited reduced microvillar effacement in cultured cells and lower infection rates in mice (Iizumi et al., 2007). Interestingly, disrupting myo1a function during EPEC infection also prevents the shedding of antimicrobial vesicles from the tips of microvilli (Shifrin et al., 2012).
Myosin-6.
A second unconventional myosin, myosin-6 (myo6), also mediates membrane–cytoskeleton interactions in the brush border. Myo6 is unique among myosins in that it is the only minus-end-directed motor (Wells et al., 1999). Monomeric myo6 is thought to function as a mechanical tether in cells (Altman et al., 2004), whereas dimeric myo6 acts as an active transporter for numerous cargo (Buss and Kendrick-Jones, 2011). In enterocytes, myo6 localizes to the subapical terminal web region (Fig. 3 B), where it tethers the plasma membrane to the actin core bundle and regulates clathrin-dependent endocytosis (Ameen and Apodaca, 2007; Hegan et al., 2012). Loss of myo6 in mice results in lifting of the plasma membrane off the actin cytoskeleton and the fusion of adjacent microvilli (Hegan et al., 2012). Without motifs for direct membrane binding, how myo6 couples the actin cytoskeleton to the brush border membrane at the base of microvilli is unclear, but it might assemble into a multiprotein membrane-associated complex that localizes to this region. For example, in hair cells of the inner ear, myo6 exists as part of a complex containing chloride intracellular channel 5 (CLIC5), taperin, radixin, and protein tyrosine phosphatase receptor Q (PTPRQ). This complex localizes to the base of stereocilia (Salles et al., 2014), specialized actin-supported protrusions found on the apical surface of inner ear hair cells that play an essential role in hearing and balance. Stereocilia of mice null for myo6 become fused at their bases during development, resulting in profound deafness and vestibular dysfunction in these animals (Avraham et al., 1995; Self et al., 1999). A similar phenotype is observed in mice deficient in other components of this protein complex including CLIC5 (Salles et al., 2014), radixin (Kitajiri et al., 2004), and PTPRQ (Goodyear et al., 2003). Interestingly, myo6 is absent in brush borders of myo1a null mice, whereas myo1a levels are not perturbed in myo6 mutant mice (Tyska et al., 2005; Hegan et al., 2012). Whether this indicates that proper targeting of myo6 is uniquely sensitive to microvillar perturbations or if there exists a more complex functional interplay between these two motors is unknown, but further investigation will be needed to understand the significance and function of this relationship.
Ezrin.
Ezrin is the only ERM family member expressed in the intestinal epithelium (Bretscher, 1983; Berryman et al., 1993). Ezrin is composed of an N-terminal 4.1 ERM (FERM) domain that binds to membrane lipids including PI(4,5)P2 and a C-terminal ERM-association domain (C-ERMAD) that interacts with F-actin (Fig. 2 B; Algrain et al., 1993; Turunen et al., 1994; Niggli et al., 1995). The N-terminal FERM domain also contains binding pockets for a variety of protein interaction partners, including the membrane-associated scaffolding proteins ERM-binding protein 50 (EBP50; Reczek et al., 1997) and NHE3 kinase A regulatory protein (E3KARP; Yun et al., 1998). Interactions between the FERM and C-ERMAD domains hold ezrin in an inactive “closed” conformation (Fig. 3 B, i) that is unable to bind F-actin or membrane-associated binding partners (Gary and Bretscher, 1995). Activation of ezrin is proposed to occur sequentially. Ezrin first targets to the plasma membrane by interacting with PI(4,5)P2. This, in turn, leads to the phosphorylation of a key conserved threonine (T567) in the C-ERMAD, which disrupts the auto-inhibitory interaction between the N-terminal FERM and the C-ERMAD, thus activating the molecule and allowing for its interaction with F-actin and other membrane-associated binding partners (Fig. 3 B, i).
Phosphorylation of ezrin is a critical downstream event in the pathway involving the Lkb1/Strad-α/Mo25 polarization–signaling complex that is proposed to regulate the early events in brush border induction (ten Klooster et al., 2009). It is, however, still unclear which kinase is responsible for ezrin activation in intestinal brush borders, with several possible candidates including the STE20-like protein kinases Mst4, LOK, and SLK (ten Klooster et al., 2009; Viswanatha et al., 2012). In mature microvilli, ezrin undergoes constant phosphocycling, with the active phosphorylated form of the protein exhibiting a half-life of ∼2 min (Viswanatha et al., 2012). Activation of ezrin is proposed to occur at the distal tips of microvilli (Fig. 3 B; Hanono et al., 2006). While in this short-lived “on” state, ezrin functions to cross-link numerous transmembrane and membrane-associated proteins to the actin core bundle. Active ezrin eventually becomes dephosphorylated by microvillus-localized phosphatases (possibly protein phosphatase 1 [PP1]), which leads to its release from membrane and cytoskeleton (Viswanatha et al., 2012).
Several lines of evidence suggest that ezrin links the membrane to the actin cytoskeleton. Ezrin KO mice die soon after birth and exhibit defects in brush border formation, with short, fused microvilli that have disorganized rootlets (Saotome et al., 2004). Defects are even more severe when ezrin expression is lost in adult animals (Casaletto et al., 2011). Moreover, dephosphorylation of ezrin is observed as an early event in renal brush border breakdown during ischemia (Chen et al., 1995); this coincides with the dissociation of ezrin from the cytoskeleton and the appearance of apical membrane blebs from renal proximal tubule epithelial cells. Interestingly, brush borders from myo6 null mice exhibit higher levels of active ezrin, which might compensate for the loss of this myosin at the base of microvilli (Hegan et al., 2012). Studies in nonepithelial systems also suggest that ezrin stabilizes interactions between membrane and actin. Mouse lymphocytes engineered to express an active form of ezrin display an ∼70% increase in membrane tension, which leads to numerous perturbations in T lymphocyte function in vivo, including decreased migration and lower T cell count in efferent lymph (Liu et al., 2012).
In addition to linking the actin core to the overlying membrane in microvilli, ezrin plays other important roles in enterocyte polarization. During brush border formation, active ezrin functions as a signaling platform by promoting the apical localization of the Cdc42-specific guanine nucleotide exchange factor (GEF) Dbl3 (Zihni et al., 2014). Activation of Cdc42 at the apical cell margins drives the Par6–aPKC pathway toward apical differentiation and brush border induction. Coupling the activation of ezrin with the localization of a Cdc42-specific GEF involved in brush border induction might allow the early events of membrane protrusion (e.g., polymerization and bundling of actin filaments) to be coupled to the availability of membrane–cytoskeleton cross-linkers needed for stabilizing nascent protrusions.
Putting the “order” in brush border
The highly ordered packing of brush border microvilli was first visualized in electron micrographs more than six decades ago (Granger and Baker, 1950). Analysis of the mechanism driving this organization was confounded for many years by the fact that most epithelial cell culture models do not recapitulate the apical surface organization observed in vivo. The CACO-2BBE cell line, however, has proven to be a valuable tool in recent studies. Derived from a human colonic adenocarcinoma (Fogh et al., 1977; Peterson and Mooseker, 1992), these cells establish a clearly defined apical–basal axis and assemble a well-ordered brush border when cultured for two to three weeks past confluency (Peterson and Mooseker, 1993). The resulting apical surface ultrastructure is comparable to that found in vivo. CACO-2BBE cells and native enterocytes also exhibit a similar gene expression profile during differentiation (Fleet et al., 2003).
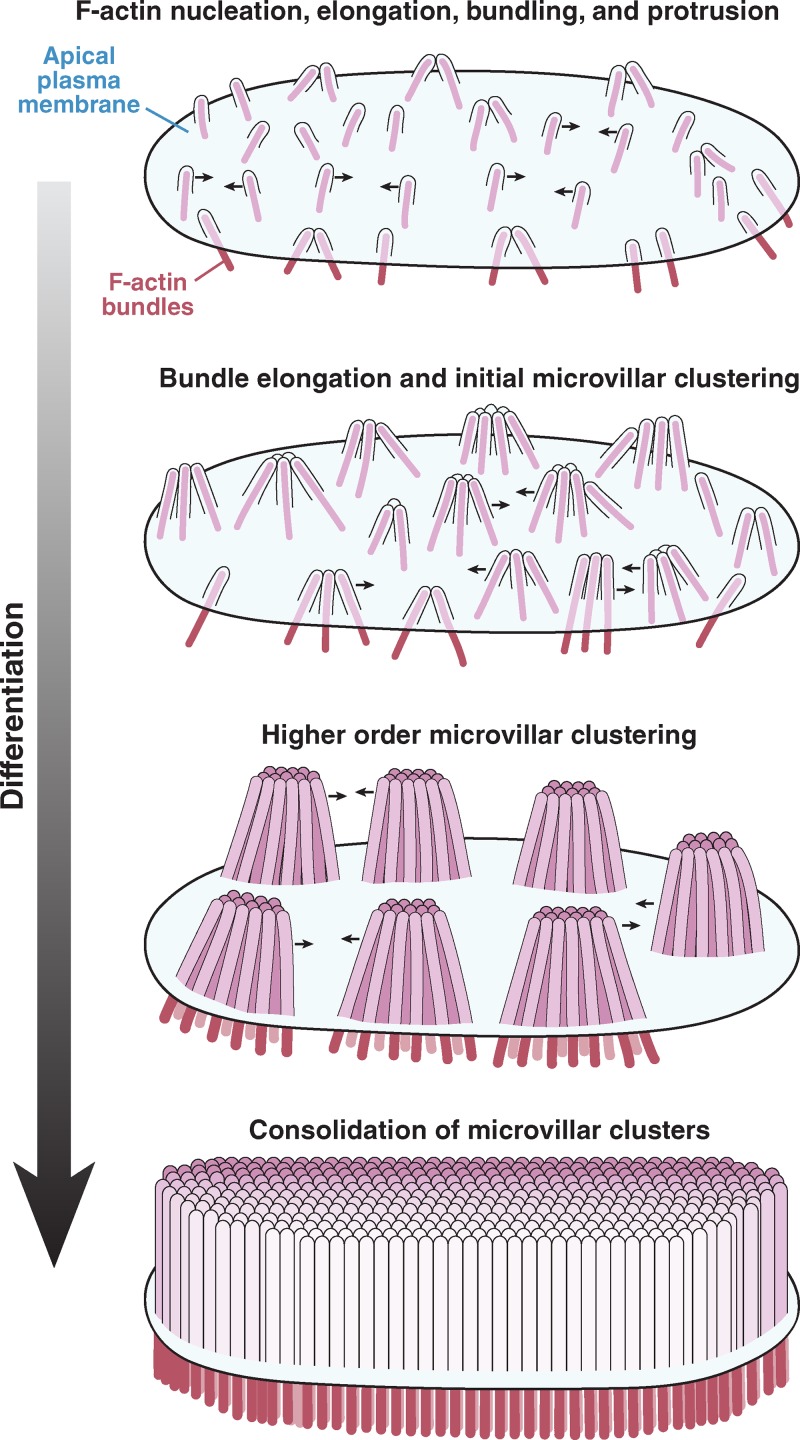
Using the CACO-2BBE model system, recent investigations demonstrated that extracellular adhesion molecules play a central role in organizing microvilli during brush border assembly. At early time points in differentiation, microvilli protrude from the cell surface and cluster together, exhibiting apparent adhesion between their distal tips (Fig. 4; Crawley et al., 2014). As brush border assembly progresses, intermicrovillar adhesion promotes the incorporation of additional microvilli into existing clusters as well as the coalescence of smaller clusters into larger structures. This process eventually leads to a single large-scale cluster on the apical surface, i.e., a mature brush border. These studies further revealed that clustering microvilli are physically connected by thread-like links composed of cadherin family members—protocadherin-24 (PCDH24) and mucin-like protocadherin (MLPCDH)—which form a trans-heterophilic adhesion complex (Fig. 2 C and Fig. 3 C; Crawley et al., 2014). Both protocadherins are highly expressed in tissues that build brush borders, including the intestine and kidney (Goldberg et al., 2002; Okazaki et al., 2002), where they localize to the tips of microvilli via interactions with the scaffolding protein, harmonin-a, and the molecular motor myosin-7b (Fig. 2 C and Fig. 3 C, ii; Crawley et al., 2014). Knockdown of PCDH24 or MLPCDH impairs brush border assembly, giving rise to apical domains with fewer protrusions that are more loosely packed and highly variable in length. Thus, although the emergence of microvilli is a stochastic process, adhesion between protrusions can be used to organize an otherwise disordered system.
Figure 4.
Remodeling of the apical surface during enterocyte differentiation. Microvilli initially appear as small membrane buds on the apical surface, formed by the polymerization and bundling of actin filaments beneath the membrane. From an early time point, these protrusions self-organize into distinct clusters, a process that is mediated by protocadherin-based adhesion between their distal tips. Distal tip adhesion promotes the highly ordered packing of microvilli, as well as uniformity in microvillar length. As maturation continues, clusters grow (in terms of number of microvilli incorporated) and continue to consolidate, ultimately resulting in a single large-scale cluster: a fully differentiated brush border.
Cadherins also play a crucial role in the organization and function of stereocilia of the inner ear. During hair cell development, stereocilia are organized into defined rows of graded height that form a characteristic “staircase” pattern, known as a hair bundle. Key to the assembly and maintenance of the hair bundle is a series of extracellular links that physically connect neighboring protrusions. Of particular importance are links comprised of two protocadherins—cadherin-23 and protocadherin-15—which form a strong trans-heterophilic adhesion complex (Kazmierczak et al., 2007). These protocadherins are highly expressed during early development and form transient lateral links that connect neighboring stereocilia, as well as kinociliary links that bridge the microtubule-based kinocilium to adjacent stereocilia (Siemens et al., 2004; Lagziel et al., 2005; Michel et al., 2005; Rzadzinska et al., 2005; Ahmed et al., 2006). Both forms of links have been shown to be important for the initial cohesion of the hair bundle during development (Alagramam et al., 2001; Di Palma et al., 2001). As development proceeds, these cadherins are lost from the base of hair bundle stereocilia and become restricted to the tip, forming the mature structure known as the tip link (Goodyear et al., 2005; Michel et al., 2005). Tip links connect neighboring stereocilia and directly mediate mechanotransduction (Müller, 2008). Analogous to intestinal intermicrovillar adhesion links that control brush border assembly, genetic disruption of the stereocilia adhesion links results in pronounced disorganization of hair bundles (Alagramam et al., 2001; Di Palma et al., 2001; Alagramam et al., 2011). Stereocilia protocadherins also associate with several cytosolic proteins, including the scaffolding proteins harmonin-b and sans, the calcium and integrin-binding protein CIB2, and the motor myosin-7a. Together, these molecules define the core set of genes associated with Type 1 Usher syndrome, the most common form of deafness/blindness in humans (Weil et al., 1995; Bitner-Glindzicz et al., 2000; Verpy et al., 2000; Bolz et al., 2001; Bork et al., 2001; Weil et al., 2003; Riazuddin et al., 2012). Currently, harmonin represents the only component that is common to the adhesion complexes found in the inner ear and the intestine (although different isoforms are expressed in the gut and cochlea). Interestingly, Usher syndrome patients who possess a large deletion mutation in their USH1C gene (the gene that encodes for harmonin) suffer from severe GI dysfunction in addition to the neurosensory deficits related to stereocilia dysfunction (Bitner-Glindzicz et al., 2000; Hussain et al., 2004). Consistent with this, Ush1c KO mice, which were developed to model Type 1 Usher syndrome, display significant perturbations in intestinal brush border morphology (Crawley et al., 2014). These findings provide strong support for the emerging paradigm that extracellular adhesion represents a conserved mechanism used to shape the surface of epithelial cells in tissues with divergent functions.
In addition to promoting tight packing of microvilli, intermicrovillar adhesion might also be involved in unifying microvillar length. Brush borders lacking adhesion links produce microvilli that exhibit much greater variability in length (Crawley et al., 2014). One possible explanation is that intermicrovillar adhesion complex components directly influence the polymerization dynamics of the actin core. A second possibility is that intermicrovillar adhesion introduces physical constraints that prevent an individual protrusion from growing longer than its neighbors. In an interesting parallel to the brush border, components of the stereocilia tip complex also play a role in controlling the length of these specialized protrusions. Postnatal deletion of either cadherin-23 or sans results in the regression of stereocilia from the middle and lower rows of hair bundles in mice (Caberlotto et al., 2011). Additional studies will be needed to determine the models alluded to here, or other models that might explain protrusion length control, such as the balance point model proposed to regulate the length of cilia (Chan and Marshall, 2012), are active in microvilli and stereocilia.
Microvilli in mature brush borders are interconnected by a vast network of intermicrovillar links (Crawley et al., 2014). This suggests that intermicrovillar adhesion is needed not only for brush border assembly, but also for the long-term maintenance of this organelle. Whether microvillar protocadherins play a role beyond shaping and stabilizing the apical domain, e.g., in promoting barrier function, is currently unknown. However, one can easily envision a scenario where intermicrovillar adhesion contributes to the formation of a physical barrier against luminal microbes that seek to gain access to the apical surface, such as EPEC (Nougayrède et al., 2003). Both the high packing density and the uniform length of microvilli could play into this by preventing the formation of spaces or gaps in the brush border that could act as protective niches for growth of these microbes. Adherence and internalization of typically noninvasive luminal bacteria into enterocytes can also play a role in intestinal disease and has been documented in patients with inflammatory bowel disease (Kleessen et al., 2002; Swidsinski et al., 2002) and celiac disease (Forsberg et al., 2004). This form of microbial invasion is thought to occur when the brush border barrier becomes disrupted as a result of microvillar “fanning,” a condition caused by interferon-γ–induced myosin II–dependent contraction of the terminal web (Wu et al., 2014). Brush border fanning allows bacteria access to the base of microvilli, where they are internalized by lipid raft–dependent endocytosis (Clark et al., 2005; Wu et al., 2014). One might expect intermicrovillar links to resist such mechanical disruption, but additional studies will be needed to confirm the function of brush border protocadherins in this context.
Future directions
Investigators have made significant progress over the course of the last several decades toward understanding the molecular basis of brush border assembly, yet several key questions remain unanswered. One fundamental question is how cells control microvillar dimensions such as length and diameter (i.e., the number of bundled actin filaments). Although recent discoveries of intermicrovillar adhesion provide a molecular basis for relative length control, bringing uniformity to microvillar length, the matter of how absolute protrusion length is determined is still an open question. Recent studies suggest that cells grapple with an actin allocation problem that arises from a limited pool of G-actin, which in turn limits the amount of actin available for assembling cytoskeletal structures (Burke et al., 2014). It will be interesting to determine if microvillar growth is sensitive to the size of the actin pool, and if so, how enterocytes determine how much actin they can allocate to brush border assembly. With regard to filament number in the core actin bundle, this parameter is mostly likely controlled by the nucleation machinery that polymerizes microvillar actin filaments. To date, the nucleator responsible for brush border assembly has yet to be identified. Proteomic analyses of isolated brush borders identified three candidates: cordon-bleu, diaphanous homologue 1, and the Arp2/3 complex (McConnell et al., 2011; Revenu et al., 2012). Cordon-bleu is an interesting possibility; this protein was originally characterized as a linear actin nucleator controlling neuronal morphology and development (Ahuja et al., 2007). More recently, cordon-bleu was reported to localize near the base of microvilli in JEG-3 cells (a choriocarcinoma cell line derived from human placenta), although it did not appear to regulate microvillar formation in this context (Wayt and Bretscher, 2014). Future studies will need to focus on identifying the nucleator responsible for polymerizing F-actin in the intestinal brush border.
Although this review focuses on molecules that play direct roles in shaping the brush border domain, other cellular pathways must be involved in the regulation of these components and their coordination in space and time. Broadening our understanding along these lines will require development of new model systems, which are amenable to time-lapse imaging so that the temporal component of enterocyte differentiation can be studied. Recent advances in tissue-derived cell culture models represent a promising avenue for such investigations (Sato and Clevers, 2013). Indeed, stem cell–containing crypts can be isolated from intestinal epithelial tissues and expanded in culture. In the presence of appropriate growth factors, crypts will differentiate into “mini-gut” structures that exhibit crypt–villus organization like that observed in vivo (Sato et al., 2009). Intestinal organoids have yet to be used for studies of brush border assembly and cytoskeletal dynamics, but because this model is amenable to both live cell imaging and genetic manipulation, we expect it to provide an unprecedented opportunity to probe the molecular underpinnings of enterocyte differentiation.
Future studies must also focus on clarifying mechanisms of human diseases characterized by perturbations in brush border morphology. Loss of microvilli leads to nutrient malabsorption and osmotic diarrhea, common features of several intestinal diseases that pose significant threats to human health. These include infections with attaching and effacing bacteria (EPEC), celiac disease, Usher syndrome, and microvillus inclusion disease (MVID; Bailey et al., 1989; Bitner-Glindzicz et al., 2000; Wilson et al., 2001; Vallance et al., 2002; Khubchandani et al., 2011; Crawley et al., 2014). MVID in particular has received a great deal of experimental attention recently; this inherited disease is typically observed in infants, who present with unremitting diarrhea that can only be treated with total parenteral nutrition (Davidson et al., 1978). Mutations in myosin-5b and syntaxin-3 are now established as drivers of MVID (Erickson et al., 2008; Müller et al., 2008; Wiegerinck et al., 2014). Both molecules function in the apical recycling system, which recent work suggests is required for maintenance of the brush border domain (Dhekne et al., 2014; Knowles et al., 2014; Wiegerinck et al., 2014). Thus, another exciting direction for the future will be investigating the coordination between the trafficking machinery that delivers membrane to the apical surface and cytoskeletal components and adhesion molecules that build, stabilize, and organize brush border microvilli.
Acknowledgments
The authors thank all members of the Tyska laboratory for feedback and advice.
This work was supported by National Institutes of Health grants DK075555 and DK095811 (to M.J. Tyska).
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- EPEC
- enteropathogenic Escherichia coli
- KO
- knockout
References
- Ahmed Z.M., Goodyear R., Riazuddin S., Lagziel A., Legan P.K., Behra M., Burgess S.M., Lilley K.S., Wilcox E.R., Riazuddin S., et al. 2006. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J. Neurosci. 26:7022–7034 10.1523/JNEUROSCI.1163-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja R., Pinyol R., Reichenbach N., Custer L., Klingensmith J., Kessels M.M., and Qualmann B.. 2007. Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell. 131:337–350 10.1016/j.cell.2007.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam K.N., Murcia C.L., Kwon H.Y., Pawlowski K.S., Wright C.G., and Woychik R.P.. 2001. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet. 27:99–102 10.1038/83837 [DOI] [PubMed] [Google Scholar]
- Alagramam K.N., Goodyear R.J., Geng R., Furness D.N., van Aken A.F., Marcotti W., Kros C.J., and Richardson G.P.. 2011. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS ONE. 6:e19183 10.1371/journal.pone.0019183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algrain M., Turunen O., Vaheri A., Louvard D., and Arpin M.. 1993. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J. Cell Biol. 120:129–139 10.1083/jcb.120.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D., Sweeney H.L., and Spudich J.A.. 2004. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 116:737–749 10.1016/S0092-8674(04)00211-9 [DOI] [PubMed] [Google Scholar]
- Ameen N., and Apodaca G.. 2007. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic. 8:998–1006 10.1111/j.1600-0854.2007.00587.x [DOI] [PubMed] [Google Scholar]
- Atilgan E., Wirtz D., and Sun S.X.. 2006. Mechanics and dynamics of actin-driven thin membrane protrusions. Biophys. J. 90:65–76 10.1529/biophysj.105.071480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham K.B., Hasson T., Steel K.P., Kingsley D.M., Russell L.B., Mooseker M.S., Copeland N.G., and Jenkins N.A.. 1995. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 11:369–375 10.1038/ng1295-369 [DOI] [PubMed] [Google Scholar]
- Bailey D.S., Freedman A.R., Price S.C., Chescoe D., and Ciclitira P.J.. 1989. Early biochemical responses of the small intestine of coeliac patients to wheat gluten. Gut. 30:78–85 10.1136/gut.30.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J.R., Zheng L., Li A., Wierda A., and Chen B.. 1998. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J. Cell Biol. 143:107–119 10.1083/jcb.143.1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathe M., Heussinger C., Claessens M.M., Bausch A.R., and Frey E.. 2008. Cytoskeletal bundle mechanics. Biophys. J. 94:2955–2964 10.1529/biophysj.107.119743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesh A.E., Nambiar R., McConnell R.E., Mao S., Tabb D.L., and Tyska M.J.. 2010. Differential localization and dynamics of class I myosins in the enterocyte microvillus. Mol. Biol. Cell. 21:970–978 10.1091/mbc.E09-07-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman M., Franck Z., and Bretscher A.. 1993. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 105:1025–1043. [DOI] [PubMed] [Google Scholar]
- Bitner-Glindzicz M., Lindley K.J., Rutland P., Blaydon D., Smith V.V., Milla P.J., Hussain K., Furth-Lavi J., Cosgrove K.E., Shepherd R.M., et al. 2000. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat. Genet. 26:56–60 10.1038/79178 [DOI] [PubMed] [Google Scholar]
- Bolz H., von Brederlow B., Ramírez A., Bryda E.C., Kutsche K., Nothwang H.G., Seeliger M., del C-Salcedó Cabrera M., Vila M.C., Molina O.P., et al. 2001. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat. Genet. 27:108–112 10.1038/83667 [DOI] [PubMed] [Google Scholar]
- Bork J.M., Peters L.M., Riazuddin S., Bernstein S.L., Ahmed Z.M., Ness S.L., Polomeno R., Ramesh A., Schloss M., Srisailpathy C.R., et al. 2001. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am. J. Hum. Genet. 68:26–37 10.1086/316954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Robida S., Furcinitti P.S., Chawla A., Fogarty K., Corvera S., and Czech M.P.. 2004. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol. Cell. Biol. 24:5447–5458 10.1128/MCB.24.12.5447-5458.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A.1983. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J. Cell Biol. 97:425–432 10.1083/jcb.97.2.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., and Weber K.. 1979. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc. Natl. Acad. Sci. USA. 76:2321–2325 10.1073/pnas.76.5.2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., and Weber K.. 1980. Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J. Cell Biol. 86:335–340 10.1083/jcb.86.1.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.W., and McKnight C.J.. 2010. Molecular model of the microvillar cytoskeleton and organization of the brush border. PLoS ONE. 5:e9406 10.1371/journal.pone.0009406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T.A., Christensen J.R., Barone E., Suarez C., Sirotkin V., and Kovar D.R.. 2014. Homeostatic actin cytoskeleton networks are regulated by assembly factor competition for monomers. Curr. Biol. 24:579–585 10.1016/j.cub.2014.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F., and Kendrick-Jones J.. 2011. Multifunctional myosin VI has a multitude of cargoes. Proc. Natl. Acad. Sci. USA. 108:5927–5928 10.1073/pnas.1103086108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto E., Michel V., Foucher I., Bahloul A., Goodyear R.J., Pepermans E., Michalski N., Perfettini I., Alegria-Prévot O., Chardenoux S., et al. 2011. Usher type 1G protein sans is a critical component of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc. Natl. Acad. Sci. USA. 108:5825–5830 10.1073/pnas.1017114108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L.A., Giardini P.A., Soo F.S., and Theriot J.A.. 2000. Secrets of actin-based motility revealed by a bacterial pathogen. Nat. Rev. Mol. Cell Biol. 1:110–119 10.1038/35040061 [DOI] [PubMed] [Google Scholar]
- Casaletto J.B., Saotome I., Curto M., and McClatchey A.I.. 2011. Ezrin-mediated apical integrity is required for intestinal homeostasis. Proc. Natl. Acad. Sci. USA. 108:11924–11929 10.1073/pnas.1103418108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y.H., and Marshall W.F.. 2012. How cells know the size of their organelles. Science. 337:1186–1189 10.1126/science.1223539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Chance M.R., Nicholas C., Ahmed N., Guilmeau S., Flandez M., Wang D., Byun D.S., Nasser S., Albanese J.M., et al. 2008. Proteomic changes during intestinal cell maturation in vivo. J. Proteomics. 71:530–546 10.1016/j.jprot.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Cohn J.A., and Mandel L.J.. 1995. Dephosphorylation of ezrin as an early event in renal microvillar breakdown and anoxic injury. Proc. Natl. Acad. Sci. USA. 92:7495–7499 10.1073/pnas.92.16.7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens M.M., Bathe M., Frey E., and Bausch A.R.. 2006. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat. Mater. 5:748–753 10.1038/nmat1718 [DOI] [PubMed] [Google Scholar]
- Clark E., Hoare C., Tanianis-Hughes J., Carlson G.L., and Warhurst G.. 2005. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 128:1258–1267 10.1053/j.gastro.2005.01.046 [DOI] [PubMed] [Google Scholar]
- Crawley S.W., Shifrin D.A. Jr, Grega-Larson N.E., McConnell R.E., Benesh A.E., Mao S., Zheng Y., Zheng Q.Y., Nam K.T., Millis B.A., et al. 2014. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell. 157:433–446 10.1016/j.cell.2014.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce A., Cassata G., Disanza A., Gagliani M.C., Tacchetti C., Malabarba M.G., Carlier M.F., Scita G., Baumeister R., and Di Fiore P.P.. 2004. A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat. Cell Biol. 6:1173–1179 10.1038/ncb1198 [DOI] [PubMed] [Google Scholar]
- Dai J., Ting-Beall H.P., Hochmuth R.M., Sheetz M.P., and Titus M.A.. 1999. Myosin I contributes to the generation of resting cortical tension. Biophys. J. 77:1168–1176 10.1016/S0006-3495(99)76968-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G.P., Cutz E., Hamilton J.R., and Gall D.G.. 1978. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology. 75:783–790. [PubMed] [Google Scholar]
- Dhekne H.S., Hsiao N.H., Roelofs P., Kumari M., Slim C.L., Rings E.H., and van Ijzendoorn S.C.. 2014. Myosin Vb and Rab11a regulate phosphorylation of ezrin in enterocytes. J. Cell Sci. 127:1007–1017 10.1242/jcs.137273 [DOI] [PubMed] [Google Scholar]
- Di Palma F., Holme R.H., Bryda E.C., Belyantseva I.A., Pellegrino R., Kachar B., Steel K.P., and Noben-Trauth K.. 2001. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat. Genet. 27:103–107 10.1038/83660 [DOI] [PubMed] [Google Scholar]
- Erickson R.P., Larson-Thomé K., Valenzuela R.K., Whitaker S.E., and Shub M.D.. 2008. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am. J. Med. Genet. A. 146A:3117–3119 10.1002/ajmg.a.32605 [DOI] [PubMed] [Google Scholar]
- Ferrary E., Cohen-Tannoudji M., Pehau-Arnaudet G., Lapillonne A., Athman R., Ruiz T., Boulouha L., El Marjou F., Doye A., Fontaine J.J., et al. 1999. In vivo, villin is required for Ca2+-dependent F-actin disruption in intestinal brush borders. J. Cell Biol. 146:819–830 10.1083/jcb.146.4.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet J.C., Wang L., Vitek O., Craig B.A., and Edenberg H.J.. 2003. Gene expression profiling of Caco-2 BBe cells suggests a role for specific signaling pathways during intestinal differentiation. Physiol. Genomics. 13:57–68. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J.M., and Orfeo T.. 1977. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 59:221–226. [DOI] [PubMed] [Google Scholar]
- Footer M.J., Kerssemakers J.W., Theriot J.A., and Dogterom M.. 2007. Direct measurement of force generation by actin filament polymerization using an optical trap. Proc. Natl. Acad. Sci. USA. 104:2181–2186 10.1073/pnas.0607052104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg G., Fahlgren A., Hörstedt P., Hammarström S., Hernell O., and Hammarström M.L.. 2004. Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am. J. Gastroenterol. 99:894–904 10.1111/j.1572-0241.2004.04157.x [DOI] [PubMed] [Google Scholar]
- Franck Z., Footer M., and Bretscher A.. 1990. Microinjection of villin into cultured cells induces rapid and long-lasting changes in cell morphology but does not inhibit cytokinesis, cell motility, or membrane ruffling. J. Cell Biol. 111:2475–2485 10.1083/jcb.111.6.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R., and Bretscher A.. 1995. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell. 6:1061–1075 10.1091/mbc.6.8.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Wei M., Tycko B., Falikovich I., and Warburton D.. 2002. Identification and expression analysis of the human mu-protocadherin gene in fetal and adult kidneys. Am. J. Physiol. Renal Physiol. 283:F454–F463. [DOI] [PubMed] [Google Scholar]
- Goodyear R.J., Legan P.K., Wright M.B., Marcotti W., Oganesian A., Coats S.A., Booth C.J., Kros C.J., Seifert R.A., Bowen-Pope D.F., and Richardson G.P.. 2003. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J. Neurosci. 23:9208–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear R.J., Marcotti W., Kros C.J., and Richardson G.P.. 2005. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J. Comp. Neurol. 485:75–85 10.1002/cne.20513 [DOI] [PubMed] [Google Scholar]
- Granger B., and Baker R.F.. 1950. Electron microscope investigation of the striated border of intestinal epithelium. Anat. Rec. 107:423–441 10.1002/ar.1091070409 [DOI] [PubMed] [Google Scholar]
- Grimm-Günter E.M., Revenu C., Ramos S., Hurbain I., Smyth N., Ferrary E., Louvard D., Robine S., and Rivero F.. 2009. Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol. Biol. Cell. 20:2549–2562 10.1091/mbc.E08-10-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanono A., Garbett D., Reczek D., Chambers D.N., and Bretscher A.. 2006. EPI64 regulates microvillar subdomains and structure. J. Cell Biol. 175:803–813 10.1083/jcb.200604046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden S.M., Wolenski J.S., and Mooseker M.S.. 1990. Binding of brush border myosin I to phospholipid vesicles. J. Cell Biol. 111:443–451 10.1083/jcb.111.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegan P.S., Giral H., Levi M., and Mooseker M.S.. 2012. Myosin VI is required for maintenance of brush border structure, composition, and membrane trafficking functions in the intestinal epithelial cell. Cytoskeleton (Hoboken). 69:235–251 10.1002/cm.21018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzelman M.B., and Mooseker M.S.. 1990a. Assembly of the brush border cytoskeleton: changes in the distribution of microvillar core proteins during enterocyte differentiation in adult chicken intestine. Cell Motil. Cytoskeleton. 15:12–22 10.1002/cm.970150104 [DOI] [PubMed] [Google Scholar]
- Heintzelman M.B., and Mooseker M.S.. 1990b. Structural and compositional analysis of early stages in microvillus assembly in the enterocyte of the chick embryo. Differentiation. 43:175–182 10.1111/j.1432-0436.1990.tb00444.x [DOI] [PubMed] [Google Scholar]
- Helander H.F., and Fändriks L.. 2014. Surface area of the digestive tract - revisited. Scand. J. Gastroenterol. 49:681–689 10.3109/00365521.2014.898326 [DOI] [PubMed] [Google Scholar]
- Hertzog M., Milanesi F., Hazelwood L., Disanza A., Liu H., Perlade E., Malabarba M.G., Pasqualato S., Maiolica A., Confalonieri S., et al. 2010. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol. 8:e1000387 10.1371/journal.pbio.1000387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C.L., and Mooseker M.S.. 1983. Characterization of the 110-kdalton actin-calmodulin-, and membrane-binding protein from microvilli of intestinal epithelial cells. J. Cell Biol. 97:974–985 10.1083/jcb.97.4.974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K., Bitner-Glindzicz M., Blaydon D., Lindley K.J., Thompson D.A., Kriss T., Rajput K., Ramadan D.G., Al-Mazidi Z., Cosgrove K.E., et al. 2004. Infantile hyperinsulinism associated with enteropathy, deafness and renal tubulopathy: clinical manifestations of a syndrome caused by a contiguous gene deletion located on chromosome 11p. J. Pediatr. Endocrinol. Metab. 17:1613–1621. [DOI] [PubMed] [Google Scholar]
- Iizumi Y., Sagara H., Kabe Y., Azuma M., Kume K., Ogawa M., Nagai T., Gillespie P.G., Sasakawa C., and Handa H.. 2007. The enteropathogenic E. coli effector EspB facilitates microvillus effacing and antiphagocytosis by inhibiting myosin function. Cell Host Microbe. 2:383–392 10.1016/j.chom.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Jontes J.D., Milligan R.A., Pollard T.D., and Ostap E.M.. 1997. Kinetic characterization of brush border myosin-I ATPase. Proc. Natl. Acad. Sci. USA. 94:14332–14337 10.1073/pnas.94.26.14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P., Sakaguchi H., Tokita J., Wilson-Kubalek E.M., Milligan R.A., Müller U., and Kachar B.. 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 449:87–91 10.1038/nature06091 [DOI] [PubMed] [Google Scholar]
- Khubchandani S.R., Vohra P., Chitale A.R., and Sidana P.. 2011. Microvillous inclusion disease—an ultrastructural diagnosis: with a review of the literature. Ultrastruct. Pathol. 35:87–91 10.3109/01913123.2010.537438 [DOI] [PubMed] [Google Scholar]
- Kitajiri S., Fukumoto K., Hata M., Sasaki H., Katsuno T., Nakagawa T., Ito J., Tsukita S., and Tsukita S.. 2004. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J. Cell Biol. 166:559–570 10.1083/jcb.200402007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleessen B., Kroesen A.J., Buhr H.J., and Blaut M.. 2002. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand. J. Gastroenterol. 37:1034–1041 10.1080/003655202320378220 [DOI] [PubMed] [Google Scholar]
- Knowles B.C., Roland J.T., Krishnan M., Tyska M.J., Lapierre L.A., Dickman P.S., Goldenring J.R., and Shub M.D.. 2014. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J. Clin. Invest. 124:2947–2962 10.1172/JCI71651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D.R., and McNeish A.S.. 1987. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect. Immun. 55:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama I., Matsunaga T., Harada T., Hokari S., and Komoda T.. 2002. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin. Biochem. 35:455–461 10.1016/S0009-9120(02)00330-2 [DOI] [PubMed] [Google Scholar]
- Lagziel A., Ahmed Z.M., Schultz J.M., Morell R.J., Belyantseva I.A., and Friedman T.B.. 2005. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev. Biol. 280:295–306 10.1016/j.ydbio.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Liu Y., Belkina N.V., Park C., Nambiar R., Loughhead S.M., Patino-Lopez G., Ben-Aissa K., Hao J.J., Kruhlak M.J., Qi H., et al. 2012. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood. 119:445–453 10.1182/blood-2011-07-368860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis P.A., Zheng L., Sekerková G., Changyaleket B., Mugnaini E., and Bartles J.R.. 2003. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol. 163:1045–1055 10.1083/jcb.200309093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariadason J.M., Nicholas C., L’Italien K.E., Zhuang M., Smartt H.J., Heerdt B.G., Yang W., Corner G.A., Wilson A.J., Klampfer L., et al. 2005. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology. 128:1081–1088 10.1053/j.gastro.2005.01.054 [DOI] [PubMed] [Google Scholar]
- Maroux S., Coudrier E., Feracci H., Gorvel J.P., and Louvard D.. 1988. Molecular organization of the intestinal brush border. Biochimie. 70:1297–1306 10.1016/0300-9084(88)90198-8 [DOI] [PubMed] [Google Scholar]
- Mazerik J.N., and Tyska M.J.. 2012. Myosin-1A targets to microvilli using multiple membrane binding motifs in the tail homology 1 (TH1) domain. J. Biol. Chem. 287:13104–13115 10.1074/jbc.M111.336313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerik J.N., Kraft L.J., Kenworthy A.K., and Tyska M.J.. 2014. Motor and tail homology 1 (Th1) domains antagonistically control myosin-1 dynamics. Biophys. J. 106:649–658 10.1016/j.bpj.2013.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini R., Dopeso H., Mateo-Lozano S., Chang W., Rodrigues P., Bazzocco S., Alazzouzi H., Landolfi S., Hernández-Losa J., Andretta E., et al. 2012. Brush border myosin Ia has tumor suppressor activity in the intestine. Proc. Natl. Acad. Sci. USA. 109:1530–1535 10.1073/pnas.1108411109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R.E., and Tyska M.J.. 2007. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J. Cell Biol. 177:671–681 10.1083/jcb.200701144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R.E., Benesh A.E., Mao S., Tabb D.L., and Tyska M.J.. 2011. Proteomic analysis of the enterocyte brush border. Am. J. Physiol. Gastrointest. Liver Physiol. 300:G914–G926 10.1152/ajpgi.00005.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V., Goodyear R.J., Weil D., Marcotti W., Perfettini I., Wolfrum U., Kros C.J., Richardson G.P., and Petit C.. 2005. Cadherin 23 is a component of the transient lateral links in the developing hair bundles of cochlear sensory cells. Dev. Biol. 280:281–294 10.1016/j.ydbio.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Miyata H., Nishiyama S., Akashi K., and Kinosita K. Jr. 1999. Protrusive growth from giant liposomes driven by actin polymerization. Proc. Natl. Acad. Sci. USA. 96:2048–2053 10.1073/pnas.96.5.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner A., and Rubinstein B.. 2005. The physics of filopodial protrusion. Biophys. J. 89:782–795 10.1529/biophysj.104.056515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M.S., and Tilney L.G.. 1975. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Biol. 67:725–743 10.1083/jcb.67.3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M.S., Graves T.A., Wharton K.A., Falco N., and Howe C.L.. 1980. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J. Cell Biol. 87:809–822 10.1083/jcb.87.3.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M.S., Pollard T.D., and Wharton K.A.. 1982. Nucleated polymerization of actin from the membrane-associated ends of microvillar filaments in the intestinal brush border. J. Cell Biol. 95:223–233 10.1083/jcb.95.1.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U.2008. Cadherins and mechanotransduction by hair cells. Curr. Opin. Cell Biol. 20:557–566 10.1016/j.ceb.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Hess M.W., Schiefermeier N., Pfaller K., Ebner H.L., Heinz-Erian P., Ponstingl H., Partsch J., Röllinghoff B., Köhler H., et al. 2008. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 40:1163–1165 10.1038/ng.225 [DOI] [PubMed] [Google Scholar]
- Nambiar R., McConnell R.E., and Tyska M.J.. 2009. Control of cell membrane tension by myosin-I. Proc. Natl. Acad. Sci. USA. 106:11972–11977 10.1073/pnas.0901641106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar R., McConnell R.E., and Tyska M.J.. 2010. Myosin motor function: the ins and outs of actin-based membrane protrusions. Cell. Mol. Life Sci. 67:1239–1254 10.1007/s00018-009-0254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli V., Andréoli C., Roy C., and Mangeat P.. 1995. Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett. 376:172–176 10.1016/0014-5793(95)01270-1 [DOI] [PubMed] [Google Scholar]
- Nougayrède J.P., Fernandes P.J., and Donnenberg M.S.. 2003. Adhesion of enteropathogenic Escherichia coli to host cells. Cell. Microbiol. 5:359–372 10.1046/j.1462-5822.2003.00281.x [DOI] [PubMed] [Google Scholar]
- Novak K.D., Peterson M.D., Reedy M.C., and Titus M.A.. 1995. Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J. Cell Biol. 131:1205–1221 10.1083/jcb.131.5.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Higashi R., Sawaguchi A., and Nakamura K.. 2012. Helical arrangement of filaments in microvillar actin bundles. J. Struct. Biol. 177:513–519 10.1016/j.jsb.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Okazaki N., Takahashi N., Kojima S., Masuho Y., and Koga H.. 2002. Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation. Carcinogenesis. 23:1139–1148 10.1093/carcin/23.7.1139 [DOI] [PubMed] [Google Scholar]
- Parekh S.H., Chaudhuri O., Theriot J.A., and Fletcher D.A.. 2005. Loading history determines the velocity of actin-network growth. Nat. Cell Biol. 7:1219–1223 10.1038/ncb1336 [DOI] [PubMed] [Google Scholar]
- Peterson M.D., and Mooseker M.S.. 1992. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J. Cell Sci. 102:581–600. [DOI] [PubMed] [Google Scholar]
- Peterson M.D., and Mooseker M.S.. 1993. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J. Cell Sci. 105:445–460. [DOI] [PubMed] [Google Scholar]
- Pollard T.D., and Mooseker M.S.. 1981. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J. Cell Biol. 88:654–659 10.1083/jcb.88.3.654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D., Berryman M., and Bretscher A.. 1997. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 139:169–179 10.1083/jcb.139.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C., Ubelmann F., Hurbain I., El-Marjou F., Dingli F., Loew D., Delacour D., Gilet J., Brot-Laroche E., Rivero F., et al. 2012. A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol. Biol. Cell. 23:324–336 10.1091/mbc.E11-09-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S., Belyantseva I.A., Giese A.P., Lee K., Indzhykulian A.A., Nandamuri S.P., Yousaf R., Sinha G.P., Lee S., Terrell D., et al. 2012. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat. Genet. 44:1265–1271 10.1038/ng.2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum R.J., Partin J.C., Saalfield K., and McAdams A.J.. 1983. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct. Pathol. 4:291–304 10.3109/01913128309140582 [DOI] [PubMed] [Google Scholar]
- Rzadzinska A.K., Schneider M.E., Davies C., Riordan G.P., and Kachar B.. 2004. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J. Cell Biol. 164:887–897 10.1083/jcb.200310055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska A.K., Derr A., Kachar B., and Noben-Trauth K.. 2005. Sustained cadherin 23 expression in young and adult cochlea of normal and hearing-impaired mice. Hear. Res. 208:114–121 10.1016/j.heares.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Salles F.T., Andrade L.R., Tanda S., Grati M., Plona K.L., Gagnon L.H., Johnson K.R., Kachar B., and Berryman M.A.. 2014. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton (Hoboken). 71:61–78 10.1002/cm.21159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I., Curto M., and McClatchey A.I.. 2004. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell. 6:855–864 10.1016/j.devcel.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Sato T., and Clevers H.. 2013. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 340:1190–1194 10.1126/science.1234852 [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., and Clevers H.. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Self T., Sobe T., Copeland N.G., Jenkins N.A., Avraham K.B., and Steel K.P.. 1999. Role of myosin VI in the differentiation of cochlear hair cells. Dev. Biol. 214:331–341 10.1006/dbio.1999.9424 [DOI] [PubMed] [Google Scholar]
- Sheetz M.P.2001. Cell control by membrane-cytoskeleton adhesion. Nat. Rev. Mol. Cell Biol. 2:392–396 10.1038/35073095 [DOI] [PubMed] [Google Scholar]
- Shifrin D.A. Jr, McConnell R.E., Nambiar R., Higginbotham J.N., Coffey R.J., and Tyska M.J.. 2012. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr. Biol. 22:627–631 10.1016/j.cub.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J., Lillo C., Dumont R.A., Reynolds A., Williams D.S., Gillespie P.G., and Müller U.. 2004. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 428:950–955 10.1038/nature02483 [DOI] [PubMed] [Google Scholar]
- Skowron J.F., and Mooseker M.S.. 1999. Cloning and characterization of mouse brush border myosin-I in adult and embryonic intestine. J. Exp. Zool. 283:242–257 [DOI] [PubMed] [Google Scholar]
- Skowron J.F., Bement W.M., and Mooseker M.S.. 1998. Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil. Cytoskeleton. 41:308–324 [DOI] [PubMed] [Google Scholar]
- Svitkina T.M., Bulanova E.A., Chaga O.Y., Vignjevic D.M., Kojima S., Vasiliev J.M., and Borisy G.G.. 2003. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160:409–421 10.1083/jcb.200210174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A., Ladhoff A., Pernthaler A., Swidsinski S., Loening-Baucke V., Ortner M., Weber J., Hoffmann U., Schreiber S., Dietel M., and Lochs H.. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology. 122:44–54 10.1053/gast.2002.30294 [DOI] [PubMed] [Google Scholar]
- ten Klooster J.P., Jansen M., Yuan J., Oorschot V., Begthel H., Di Giacomo V., Colland F., de Koning J., Maurice M.M., Hornbeck P., and Clevers H.. 2009. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell. 16:551–562 10.1016/j.devcel.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Theriot J.A.2000. The polymerization motor. Traffic. 1:19–28 10.1034/j.1600-0854.2000.010104.x [DOI] [PubMed] [Google Scholar]
- Tilney L.G., and Cardell R.R.. 1970. Factors controlling the reassembly of the microvillous border of the small intestine of the salamander. J. Cell Biol. 47:408–422 10.1083/jcb.47.2.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchetti A., Soppo C.B., Zani F., Bianchi F., Gagliani M.C., Pozzi B., Rozman J., Elvert R., Ehrhardt N., Rathkolb B., et al. 2010. Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS ONE. 5:e9468 10.1371/journal.pone.0009468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen O., Wahlström T., and Vaheri A.. 1994. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J. Cell Biol. 126:1445–1453 10.1083/jcb.126.6.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska M.J., Mackey A.T., Huang J.D., Copeland N.G., Jenkins N.A., and Mooseker M.S.. 2005. Myosin-1a is critical for normal brush border structure and composition. Mol. Biol. Cell. 16:2443–2457 10.1091/mbc.E04-12-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance B.A., Chan C., Robertson M.L., and Finlay B.B.. 2002. Enteropathogenic and enterohemorrhagic Escherichia coli infections: emerging themes in pathogenesis and prevention. Can. J. Gastroenterol. 16:771–778. [DOI] [PubMed] [Google Scholar]
- van der Flier L.G., and Clevers H.. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71:241–260 10.1146/annurev.physiol.010908.163145 [DOI] [PubMed] [Google Scholar]
- Verpy E., Leibovici M., Zwaenepoel I., Liu X.Z., Gal A., Salem N., Mansour A., Blanchard S., Kobayashi I., Keats B.J., et al. 2000. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat. Genet. 26:51–55 10.1038/79171 [DOI] [PubMed] [Google Scholar]
- Viswanatha R., Ohouo P.Y., Smolka M.B., and Bretscher A.. 2012. Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 199:969–984 10.1083/jcb.201207047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayt J., and Bretscher A.. 2014. Cordon Bleu serves as a platform at the basal region of microvilli, where it regulates microvillar length through its WH2 domains. Mol. Biol. Cell. 25:2817–2827 10.1091/mbc.E14-06-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D., Blanchard S., Kaplan J., Guilford P., Gibson F., Walsh J., Mburu P., Varela A., Levilliers J., Weston M.D., et al. 1995. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 374:60–61 10.1038/374060a0 [DOI] [PubMed] [Google Scholar]
- Weil D., El-Amraoui A., Masmoudi S., Mustapha M., Kikkawa Y., Lainé S., Delmaghani S., Adato A., Nadifi S., Zina Z.B., et al. 2003. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum. Mol. Genet. 12:463–471 10.1093/hmg/ddg051 [DOI] [PubMed] [Google Scholar]
- Wells A.L., Lin A.W., Chen L.Q., Safer D., Cain S.M., Hasson T., Carragher B.O., Milligan R.A., and Sweeney H.L.. 1999. Myosin VI is an actin-based motor that moves backwards. Nature. 401:505–508 10.1038/46835 [DOI] [PubMed] [Google Scholar]
- Wiegerinck C.L., Janecke A.R., Schneeberger K., Vogel G.F., van Haaften-Visser D.Y., Escher J.C., Adam R., Thöni C.E., Pfaller K., Jordan A.J., et al. 2014. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology. 147:65–: e10. 10.1053/j.gastro.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Wilson W., Scott R.B., Pinto A., and Robertson M.A.. 2001. Intractable diarrhea in a newborn infant: microvillous inclusion disease. Can. J. Gastroenterol. 15:61–64. [DOI] [PubMed] [Google Scholar]
- Wolenski J.S., Cheney R.E., Forscher P., and Mooseker M.S.. 1993a. In vitro motilities of the unconventional myosins, brush border myosin-I, and chick brain myosin-V exhibit assay-dependent differences in velocity. J. Exp. Zool. 267:33–39 10.1002/jez.1402670106 [DOI] [PubMed] [Google Scholar]
- Wolenski J.S., Hayden S.M., Forscher P., and Mooseker M.S.. 1993b. Calcium-calmodulin and regulation of brush border myosin-I MgATPase and mechanochemistry. J. Cell Biol. 122:613–621 10.1083/jcb.122.3.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.L., Peng W.H., Kuo W.T., Huang C.Y., Ni Y.H., Lu K.S., Turner J.R., and Yu L.C.. 2014. Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-γ. Am. J. Pathol. 184:2260–2274 10.1016/j.ajpath.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C.H., Lamprecht G., Forster D.V., and Sidor A.. 1998. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J. Biol. Chem. 273:25856–25863 10.1074/jbc.273.40.25856 [DOI] [PubMed] [Google Scholar]
- Zihni C., Munro P.M., Elbediwy A., Keep N.H., Terry S.J., Harris J., Balda M.S., and Matter K.. 2014. Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J. Cell Biol. 204:111–127 10.1083/jcb.201304064 [DOI] [PMC free article] [PubMed] [Google Scholar]