Abstract
A large body of work demonstrates income-related disparities in access to coordinated preventive care in patients with diabetes and other chronic conditions. Much less information exists on associations between poverty and consequential negative health outcomes. Few studies have assessed geographic patterns linking household incomes to major, preventable complications of chronic diseases.
Using statewide facility discharge data for California during 2009, we identified 7,973 lower extremity amputations in 6,828 diabetic adults. We mapped amputation events based on residential zip codes, and used US census data to produce corresponding maps of poverty rate. Comparisons of the maps show amputation “hotspots” in lower income urban and rural regions of California. Prevalence-adjusted amputation rates varied ten-fold between high-income and low-income regions.
While our analysis does not support detailed causal inferences, our method for mapping complication “hot spots” using existing public data sources may help target interventions to communities most in need.
INTRODUCTION
For more than a century, detailed geographic analysis of illness patterns has underpinned major public health interventions, accounting in large part for the control of communicable diseases in developed countries. (1) Recently, investigators have begun to explore similar strategies to reduce the impact of chronic diseases. The advent of geographic information systems (GIS) and publically available, population-based databases has created new opportunities to better understand causes and target interventions for chronic illness using geographic pattern analysis. We applied this approach to explore the relationship between socioeconomic status and diabetic lower limb amputation in California.
Lower limb amputation is a debilitating, harrowing, but often avoidable complication of diabetes. A prolonged chain of events generally precedes amputation, beginning with chronic inadequate diabetes control, resulting in peripheral neuropathy and vascular disease that predispose patients to foot ulcers and infections that, if untreated, place the affected limb beyond salvage. (2)
Many opportunities exist to intervene along this pathway, and proactive team-based health care can substantially lower the incidence of amputations in diabetic patients. (3–5) The selection by the Agency for Healthcare Research and Quality of lower extremity amputation rate in diabetic patients as an indicator of preventive care quality reflects an emerging consensus that amputation is avoidable with good care. (6)
Despite general consensus regarding optimal diabetes care, disparities in access to and receipt of such care persist. (7, 8) Evidence shows that patients residing in low-income households receive lower quality diabetes care, even in settings where universal coverage for primary health care exists. (9, 10) Several studies have used mapping methods to assess diabetes-related processes and outcomes, including neurologic and renal complications (11), diabetes prevalence and treatment resources (12), and effectiveness of targeted programs to improve quality of diabetes care in zip codes with high minority populations (13). Other existing studies have analyzed disparities in diabetic amputations, but have generally focused on specific patient sub-groups or settings. (10, 14–17) No studies to our knowledge have assessed the relationship between socio-economic status and lower-limb amputation in the US. We sought to understand the relationship between socioeconomic status and amputation rate among all diabetic adults in California, using detailed geographic analysis of existing administrative data sets.
METHODS
To achieve our objective of creating detailed, neighborhood-level maps of prevalence-adjusted diabetic amputation rates for comparison with income data, we relied on zip code tabulation areas (ZCTAs) as the geographic unit of analysis. Defined by the U.S. Census Bureau, most ZCTA’s correspond closely to postal zip codes. (18) Our study’s primary outcome measure is the percentage of diabetics aged 45 or older residing in each ZCTA in California who underwent one or more non-traumatic lower extremity amputation during 2009. We chose this age cut-off to focus our analyses on the population at greatest risk of undergoing potentially preventable amputations from complications of diabetes.
We drew on three separate data sources to calculate this measure, as described in greater detail in our supplemental Online Appendix, Appendix Exhibit A1. (19) First, we identified non-traumatic amputation events associated with a diagnosis of diabetes in each zip code, using data from the California Office of Statewide Health Planning and Development’s Patient Discharge (20) and Ambulatory Surgery Center (21) Databases filtered by ICD-9 and CPT codes (Online Appendix section e-1). Second, to model diabetes prevalence, we used small area estimates from the California Health Interview Survey, which assesses the prevalence of diabetes and other chronic illnesses in zip code tabulation areas (ZCTAs). (22–24) Last, we used 2003–2009 American Community Survey pooled estimates of household income from the United States Census Bureau to obtain the percentage of households in each census tract reporting incomes below 200% of the Federal Poverty Level. (25)
To allow geographic linkage of the three different data sets, we used previously validated cross-walk algorithms to convert postal zip codes (26) and census tracts (27) to ZCTAs. To increase the stability of the amputation rate estimates, we merged adjacent, demographically similar ZCTAs with fewer than 3,000 diabetic adults aged 45 years and older. (Online Appendix section e-2 and Appendix Exhibit A1). We successfully merged 373 of 461 low-population ZCTAs into "multiZCTAs", dropping from the analysis 88 ZCTAS that could not be merged. We report all further analysis including map construction using this final geographic unit composed of single and merged ZCTAs, referred to henceforth as “neighborhoods.”
We generated maps showing prevalence-adjusted amputation rates for comparison with maps showing high poverty rates (percent of households reporting incomes below 200% of FPL), by applying GIS analysis to the linked data sets at the neighborhood level. We constructed a map of California, and separate maps for four major urban areas: Los Angeles, Sacramento, San Diego, and San Francisco.
To complement the geographic analysis, we also used simple linear regression to model the relationship between amputation rate and poverty at the neighborhood level, weighted by neighborhood population size. We tested more complex modeling procedures and determined that simple linear regression was appropriate (Online Appendix section e-3 and Appendix Exhibit A8). The use of confidential data for the study protocol has been reviewed and approved by the institutional review boards at University of California Los Angeles and the California State Department of Health Care Services.
LIMITATIONS
Our analysis has several limitations. Its scope is limited to describing and quantifying the association between poverty and amputation rates based on geographic distribution. As an ecological study, we cannot link individual-level income and amputation event data. We did not apply multivariate methods to model causality, because available public data sets lack many potentially important explanatory variables at patient, provider and neighborhood levels. Neither the geographic analysis nor the regression results support direct inferences about the causes of observed higher amputation rates in lower income areas.
Each database used in our analysis has inherent limitations. The CHIS survey and the US Census Bureau American Community Survey have limitations in accuracy characteristic of large, population-based surveys. Furthermore, our diabetes prevalence estimates based on self-reported survey data likely undercount true diabetes rates due to undiagnosed cases. (28) Our tally of diabetic amputations using the OSHPD hospital discharge and ambulatory surgical data depends on accurate discharge coding by these facilities, which may vary.
Despite our effort to de-duplicate the data by limiting our analysis to the most anatomically proximal amputation for each individual, our de-duplication process was distinct for each OSPHD dataset; we therefore may have included individuals twice if they underwent amputations in both inpatient and ambulatory surgery settings. However, the overall contribution of ambulatory procedures was small relative to the total. Our analysis did not capture amputation procedures performed in Veteran's Administration hospitals and free-standing surgical centers not associated with hospitals, because these facilities are not included in the OSPHD data sets used. However, given patient demographics and procedure volumes at these centers, we believe the lack of data from these sources is unlikely to substantially bias our results.
In our regression analysis, we treated each neighborhood independently, not accounting for the potential correlation between neighborhoods that are in close geographic proximity. Finally, the existing crosswalk algorithms that allow conversion of postal ZIP codes and census tracts to ZCTAs may contain errors, though they have been validated in other settings. (26, 27)
RESULTS
We identified 7,973 diabetic lower extremity amputations in California during 2009; 7,205 took place during an inpatient hospitalization and 768 (9.6%) in hospital-affiliated outpatient surgery centers. From total events, we excluded 1,145 amputations in individuals who later experienced one or more repeat amputations during the same-year, retaining for analysis only the most recent, anatomically most proximal amputation in these individuals. This left 6,828 individuals who experienced at least one amputation due to diabetes (6,094 inpatient and 734 outpatient).
After merging adjacent small-population ZCTAs, we were left with 1,395 neighborhood-level units of analysis for mapping and regression. These neighborhoods contained a population of 1.867 million diabetic adults age 45 and older (80% of the California diabetic population (29)), and 6,763 individuals who underwent at least one amputation (we excluded 65 individuals who resided in small-population ZCTAs that could not be merged (Online Appendix section e-2)). Exhibit 1 displays the demographic characteristics of individuals who underwent at least one diabetes-related non-traumatic lower extremity amputation (“cases”) compared to the California general population aged 45 and greater with diabetes.
EXHIBIT 1.
Characteristics of Adults Age 45 and Older with Diabetes, California General Population versus Population with any Diabetic Amputation, 2009
| Characteristic | All people with diabetes (%) |
Cases (%) |
|---|---|---|
| Age (years) | ||
| 45–64 | 69.8 | 53.0 |
| 65–79 | 22.4 | 32.9 |
| 80 or more | 8.1 | 14.1 |
| Sex | ||
| Male | 49.6 | 68.6 |
| Race or ethnicity | ||
| White | 42.3 | 42.9 |
| Black | 5.6 | 12.6 |
| Hispanic | 36.8 | 36.7 |
| Asian or Pacific Islander | 12.4 | 4.8 |
| Native American | 0.6 | 0.6 |
| Other | 2.2 | 1.9 |
| Unknown | 0.0 | 0.5 |
| Language spoken | ||
| English | 86.2 | 79.1 |
| Spanish | 9.2 | 17.1 |
| Asian or Pacific Island language | 1.6 | 1.4 |
| Other | 3.0 | 0.9 |
| Unknown | 0.0 | 1.5 |
SOURCE: State of California estimates are from the California Health Interview Survey (CHIS) 2009; Cases are based on California Office of Statewide Health Planning and Development, Patient Discharge Data 2009.
NOTES: “All people with diabetes” are adults in California ages forty-five and older who have diabetes based on self-reported survey data. “Cases” are adults age 45 or greater with at least one non-traumatic lower extremity amputation associated with a diagnosis of diabetes in an inpatient hospital or ambulatory surgery center in California.
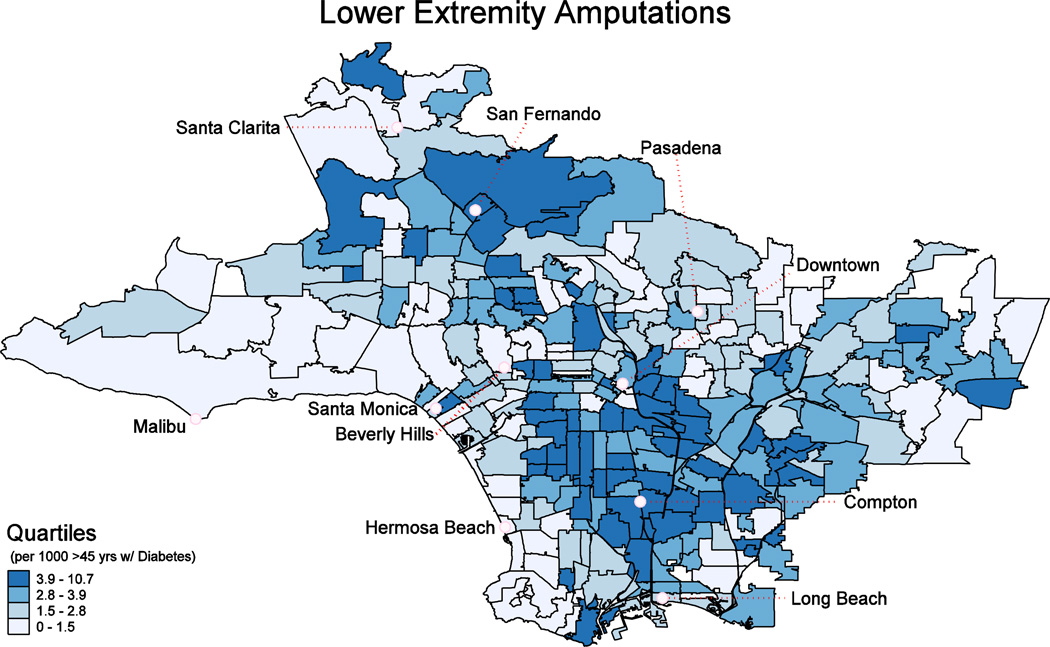
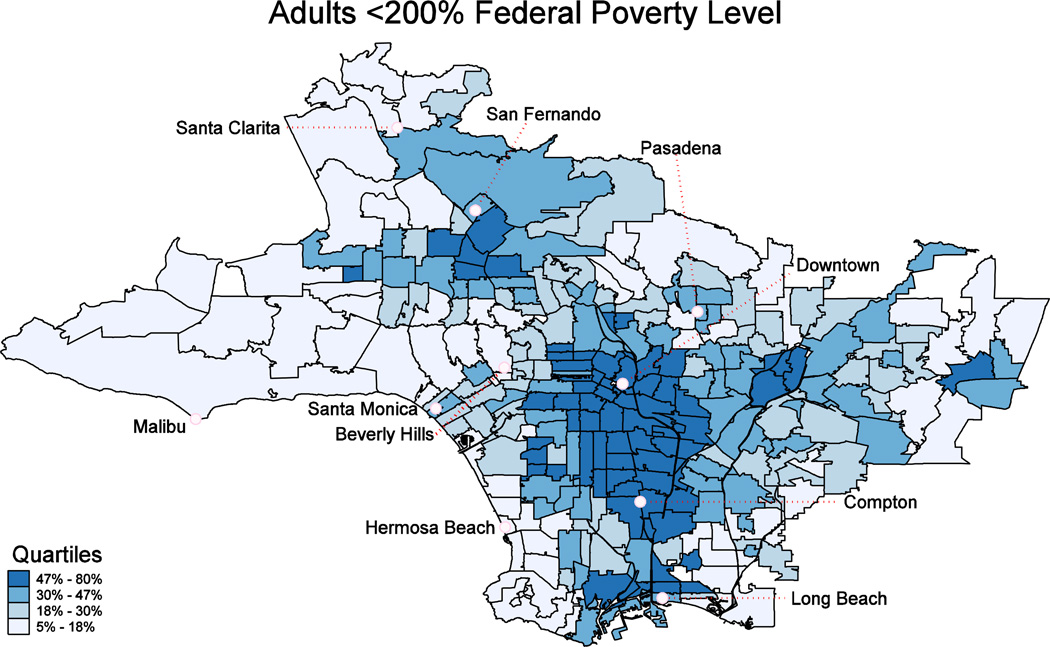
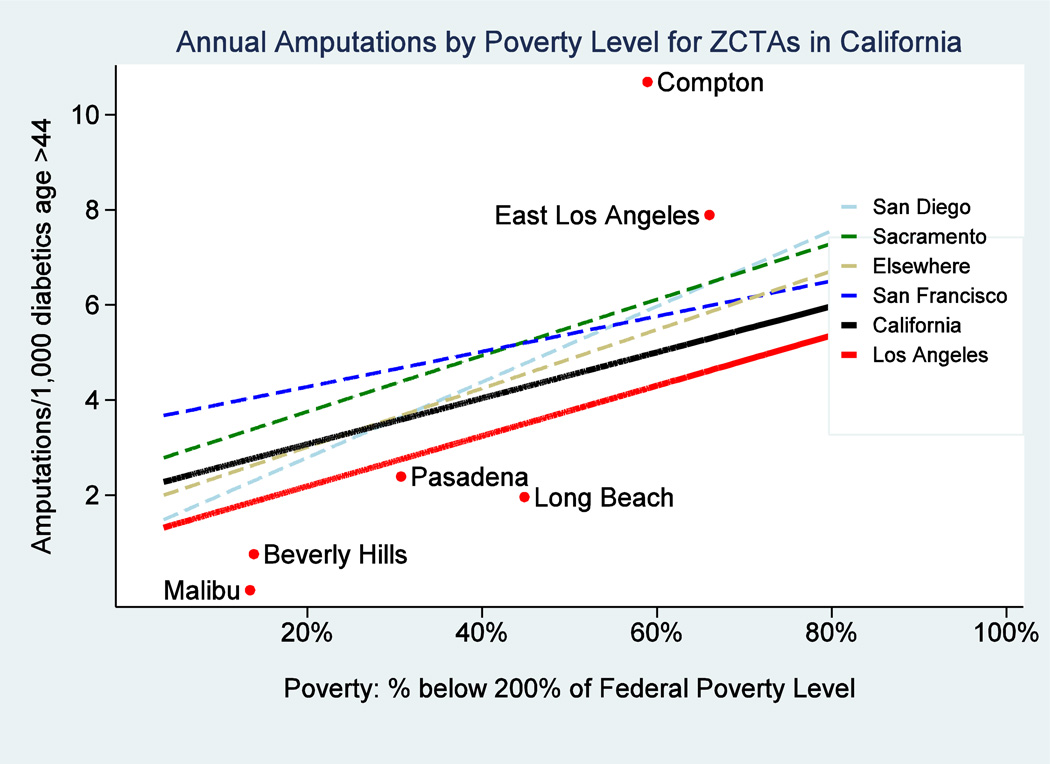
Maps showing neighborhood-level, prevalence adjusted amputation rates per 1,000 diabetic adults 45 and older (Exhibit 2) and low-income household density (Exhibit 3) revealed geographic “hotspots” in lower income areas (see also Appendix Exhibits A2–A5). For example, the Los Angeles maps identify clustering of diabetic lower extremity amputation hotspots in areas such as South Los Angeles, East Los Angeles, and the Northern San Fernando Valley. The amputation rate for diabetics in low income neighborhoods (where more than 40% of households have income below 200% FPL) is roughly double that of those in higher income neighborhoods (where less than 10% of households are below 200% FPL) (Exhibit 4). We found similar relationships in the state as a whole (Appendix Exhibit A2). These relationships did not appreciably change in sensitivity analyses that excluded toe amputations or considered only below-knee and above-knee amputations (Appendix Exhibits A6 & A7).
EXHIBIT 2.
Rate of Lower Extremity Diabetic Amputations per 1000 Adults Age 45 and Older with Diabetes, Los Angeles County, 2009
SOURCE: The numerator is based on analysis of the California Office of Statewide Health Planning and Development, 2009 Patient Discharge Data and 2009 Ambulatory Surgery Center Data. The denominator is based on the 2009 California Health Interview Survey.
NOTES: Data are mapped at the ZCTA/multiZCTA level, and represent the rate of non-traumatic lower extremity amputations associated with a diagnosis of diabetes per 1000 diabetic adults age 45 and older. The rate is presented in quartiles for the region.
EXHIBIT 3.
Proportion of Households with Income Below 200% of the Federal Poverty Level, Los Angeles County, 2003–2009
SOURCE: 2003–2009 American Community Survey pooled estimates of household income from the United States Census Bureau.
NOTES: Data are mapped at the ZCTA/multiZCTA level, and represent proportion of households with income below 200% of the Federal Poverty Level. The rate is presented in quartiles for the region.
EXHIBIT 4.
Association between Proportion of the Population with Low Income and Amputation Rate Among Diabetic Adults, California Overall and Four Urban Areas, 2009
SOURCE: Amputation data are based on California Office of Statewide Health Planning and Development, 2009 Patient Discharge Data and 2009 Ambulatory Surgery Center Data and the 2009 California Health Interview Survey. Poverty data are based on 2003–2009 American Community Survey pooled estimates of household income from the United States Census Bureau.
NOTES: This is a plot of amputation rate and proportion of the population below 200% of the federal poverty level by ZCTA/multiZCTA. Fitted lines are provided for each geographic region of interest. This plot represents a simple association and does not adjust for possible confounders.
DISCUSSION
Neighborhoods with high amputation rates often cluster geographically into amputation hotspots that correspond with areas having a high concentration of low-income households. The regression analysis confirms the patterns observed on the maps, showing a strong association between diabetic lower extremity amputation rate and neighborhood density of low-income households in urban and rural California. Amputation rates varied ten-fold between the highest and lowest income neighborhoods in California.
Our results parallel the findings of a recent study that demonstrated that poverty and diabetes each independently contribute to vision loss in the United States. (30) They are also similar to findings from a recent observational study in Finland that demonstrated a significant association between diabetic lower extremity amputation rate and socioeconomic status, with an approximately two-fold increase from lowest to highest socioeconomic status strata, despite universal access to health services. (10)
We cannot determine the relative contributions of the many possible explanatory factors for the observed disparities, and future research should explore the underlying causes. Many possible sources of outcome disparities exist, including differences in patient (31) or provider (8) beliefs, behaviors and characteristics, health system factors (31, 32), and social determinants of health (33).
A substantial literature suggests that impaired access to ambulatory systems that provide comprehensive chronic disease care constitutes an important contributing factor to less favorable outcomes among low-income populations. (34) Patients living in low-income neighborhoods are more likely to be treated at safety-net hospitals, which have been found to provide lower quality of care across many measures. (32) Furthermore, there is some evidence of differing practice patterns by physicians caring for lower income patients, such as greater reliance on amputation as compared to less invasive, limb-sparing treatment approaches. (35)
Downward secular trends in diabetes complications observed over the past two decades demonstrate that substantial gains are possible in reducing diabetes-related morbidity. (36) However, while a recent analysis found a reduction in amputation rates in the US from 58 per 10,000 diabetics in 1990 to 28 in 2010 (37), it is apparent that this decrease has not resolved disparities in this debilitating outcome.
POLICY IMPLICATIONS
The finding that residents living in lower income areas bear a disproportionate share of disability and disfigurement from amputations is deeply disturbing in a society that espouses equality and outspends all other nations on health care for its more affluent citizens. We believe our findings dictate a vigorous response from the health policy community. Like the complex web of demographic and delivery system factors that underlie higher amputation rates in poor communities, a successful policy response will likely need to employ multiple strategies, including addressing social determinants of health, engaging patients, and deploying multidisciplinary primary care facilities to improve access in underserved urban and rural communities.
The recent expansion of both private insurance and Medicaid enrollment under the Affordable Care Act addresses one dimension of access disparity, but the potential benefits may be blunted by the undersupply of primary care providers in low-income neighborhoods. (38) A recent experiment in Oregon found substantial increases in primary care and pharmaceutical utilization among new Medicaid beneficiaries, though did not demonstrate improvements in health outcomes. (39, 40)
Our study contributes to a small but growing literature demonstrating the utility of GIS in combination with public data for identifying preventable disease hot spots, and focusing interventions on these communities. In addition, it lends urgency to the search for neighborhood-level solutions to reduce the disproportionate burden of lower extremity amputation in low income communities.
CONCLUSION
Diabetic individuals who live in lower-income neighborhoods in California have higher rates of lower extremity amputation than those residing in more affluent areas. Our hotspot method of displaying complication rates may assist providers and public health agencies in targeting interventions to the most impacted populations.
Supplementary Material
Acknowledgments
David Schriger received support for the research described in this article from the Korein Foundation. Anna Davis and David Zingmond received support for the research described in this article from a grant from the National Center for Advancing Translational Science of the National Institutes of Health to the University of California, Los Angeles, (UCLA) Clinical and Translational Science Institute (Grant No. TL1TR000121). The authors gratefully acknowledge the work of E. Richard Brown (1942–2012), the founder and former principal investigator for the California Health Interview Survey (CHIS) and a professor of health policy and management in the UCLA Fielding School of Public Health. His tireless advocacy for local data collection and leadership in creating the CHIS was vital in completing this study. The authors thank Ninez Ponce (the new CHIS principal investigator) and David Grant (director of the CHIS) for their efforts to continue the survey and provide the small-area estimates used in this analysis. The authors also thank the California Office of Statewide Health Planning and Development for providing easy-to-use publicly available data.
Contributor Information
Carl D. Stevens, Email: carlstevens@mednet.ucla.edu, David Geffen School of Medicine, University of California, Los Angeles (UCLA).
David L. Schriger, Emergency Medicine Center, UCLA.
Brian Raffetto, Department of Emergency Medicine, Keck School of Medicine, University of Southern California, in Los Angeles.
Anna C. Davis, Department of Health Policy and Management, Fielding School of Public Health, UCLA.
David Zingmond, Division of General Internal Medicine and Health Services Research, David Geffen School of Medicine, UCLA.
Dylan H. Roby, Department of Health Policy and Management, Fielding School of Public Health, UCLA, and director of health economics and evaluation research, UCLA Center for Health Policy Research.
REFERENCES
- 1.Johnson S. The ghost map: The story of London's most terrifying epidemic--and how it changed science, cities, and the modern world. New York: Penguin; 2006. [Google Scholar]
- 2.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990 May;13(5):513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 3.Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000 Sep-Oct;16(Suppl 1):S75–S83. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr139>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes Care. 2003 Jan;26(Suppl 1):S78–S79. doi: 10.2337/diacare.26.2007.s78. [DOI] [PubMed] [Google Scholar]
- 5.Patout CA, Jr, Birke JA, Horswell R, Williams D, Cerise FP. Effectiveness of a comprehensive diabetes lower-extremity amputation prevention program in a predominantly low-income African-American population. Diabetes Care. 2000 Sep;23(9):1339–1342. doi: 10.2337/diacare.23.9.1339. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality. Prevention Quality Indicators Version 4.4: Rate of Lower-Extremity Amputation Among Patients With Diabetes. [cited 2013 July 24];2012 Mar; [Internet]. Available from: http://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V44/TechSpecs/PQI%2016%20Rate%20of%20Lower-Extremity%20Amputation%20Diabetes.pdf.
- 7.Peek M, Ferguson M, Bergeron N, Maltby D, Chin M. Integrated Community-Healthcare Diabetes Interventions to Reduce Disparities. Curr Diab Rep. 2014;14(3):1–9. doi: 10.1007/s11892-013-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinlay J, Piccolo R, Marceau L. An additional cause of health care disparities: the variable clinical decisions of primary care doctors. Journal of Evaluation in Clinical Practice. 2013;19(4):664–673. doi: 10.1111/jep.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leese GP, Stang D, Pearson DW. A national approach to diabetes foot risk stratification and foot care. Scott Med J. 2011 Aug;56(3):151–155. doi: 10.1258/smj.2011.011113. [DOI] [PubMed] [Google Scholar]
- 10.Venermo M, Manderbacka K, Ikonen T, Keskimaki I, Winell K, Sund R. Amputations and socioeconomic position among persons with diabetes mellitus, a population-based register study. BMJ Open. 2013;3(4) doi: 10.1136/bmjopen-2012-002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis AJ, Lee W-AA. Spatial patterns of diabetes related health problems for vulnerable populations in Los Angeles. International Journal of Health Geographics. 2010;9(1):43. doi: 10.1186/1476-072X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis AB, Kothari C, Paul R, Connors E. Using GIS and Secondary Data to Target Diabetes-Related Public Health Efforts. Public Health Reports. 2013 May-Jun;128(3):212–220. doi: 10.1177/003335491312800311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coberley CR, Puckrein GA, Dobbs AC, McGinnis MA, Coberley SS, Shurney DW. Effectiveness of disease management programs on improving diabetes care for individuals in health-disparate areas. Disease Management. 2007;10(3):147–155. doi: 10.1089/dis.2007.641. [DOI] [PubMed] [Google Scholar]
- 14.Wachtel MS. Family poverty accounts for differences in lower-extremity amputation rates of minorities 50 years old or more with diabetes. J Natl Med Assoc. 2005 Mar;97(3):334–338. [PMC free article] [PubMed] [Google Scholar]
- 15.Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. Journal of vascular surgery. 2007 Jan;45(1):55–59. doi: 10.1016/j.jvs.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. Journal of vascular surgery. 2011 Feb;53(2):330–339. e1. doi: 10.1016/j.jvs.2010.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson HJ, Nightingale P, Pathak R, Jayatunga AP. The influence of socio-economic deprivation on rates of major lower limb amputation secondary to peripheral arterial disease. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2010 Jul;40(1):76–80. doi: 10.1016/j.ejvs.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Census Bureau. Geography Reference: ZIP Code Tabulation Areas (ZCTAs) [cited 2013 June];2013 [Internet]. Available from: https://www.census.gov/geo/reference/zctas.html.
- 19.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 20.Patient Discharge Data. Sacramento, CA: State of California, Office of Statewide Health Planning and Development; 2009. [Google Scholar]
- 21.Emergency Department and Ambulatory Surgery Data. Sacramento, CA: State of California, Office of Statewide Health Planning and Development; 2009. [Google Scholar]
- 22.Mendez-Luck CA, Yu H, Meng YY, Jhawar M, Wallace SP. Estimating health conditions for small areas: asthma symptom prevalence for state legislative districts. Health Serv Res. 2007 Dec;42(6 Pt 2):2389–2409. doi: 10.1111/j.1475-6773.2007.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Meng YY, Mendez-Luck CA, Jhawar M, Wallace SP. Small-area estimation of health insurance coverage for California legislative districts. Am J Public Health. 2007 Apr;97(4):731–737. doi: 10.2105/AJPH.2005.077743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.California Health Interview Survey. CHIS 2009 Small Area Estimates. Los Angeles, CA: UCLA Center for Health Policy Research; 2012. Aug, [Google Scholar]
- 25.U.S. Census Bureau. 2009 American Community Survey California. 2011 [Google Scholar]
- 26.The Dartmouth Atlas of Health Care. PCSA Downloads: Zip code to ZCTA 2009. [cited 2012 Aug 1 (no longer available as of April 2014)];2013 [Internet]. Available from: http://www.dartmouthatlas.org/tools/downloads.aspx?tab=37.
- 27.Missouri Census Data Center. MABLE/Geocorr12: Geographic Correspondence Engine, Version 1.1. [cited 2012 Aug 1];2012 [Internet]. Available from: http://mcdc.missouri.edu/websas/geocorr12.html.
- 28.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Annals of internal medicine. 2014 Apr 15;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.California Health Interview Survey. CHIS 2009 Adult Public Use File. Los Angeles, CA: UCLA Center for Health Policy Research; 2009. [Google Scholar]
- 30.Ko F, Vitale S, Chou CF, Cotch MF, Saaddine J, Friedman DS. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999–2002 and 2005–2008. Jama. 2012 Dec 12;308(22):2361–2368. doi: 10.1001/jama.2012.85685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan S, Billimek J, Sorkin D, Ngo-Metzger Q, Greenfield S. Reducing Racial/Ethnic Disparities in Diabetes: The Coached Care (R2D2C2) Project. J Gen Intern Med. 2013 Oct;28(10):1340–1349. doi: 10.1007/s11606-013-2452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner RM, Goldman L, Dudley R. COmparison of change in quality of care between safety-net and non–safety-net hospitals. Jama. 2008;299(18):2180–2187. doi: 10.1001/jama.299.18.2180. [DOI] [PubMed] [Google Scholar]
- 33.Walker R, Smalls B, Campbell J, Strom Williams J, Egede L. Impact of social determinants of health on outcomes for type 2 diabetes: a systematic review. Endocrine. 2014 Feb;:1–20. doi: 10.1007/s12020-014-0195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bindman AB, Grumbach K, Osmond D, Komaromy M, Vranizan K, Lurie N, et al. Preventable hospitalizations and access to health care. Jama. 1995 Jul 26;274(4):305–311. [PubMed] [Google Scholar]
- 35.Regenbogen SE, Gawande AA, Lipsitz SR, Greenberg CC, Jha AK. Do differences in hospital and surgeon quality explain racial disparities in lower-extremity vascular amputations? Annals of surgery. 2009 Sep;250(3):424–431. doi: 10.1097/SLA.0b013e3181b41d53. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Press Release: CDC report finds large decline in lower-limb amputations among U.S. adults with diagnosed diabetes. [cited 2013 29 Apr];2012 Jan 24; [Internet]. Available from: http://www.cdc.gov/media/releases/2012/p0124_lower_limb.html.
- 37.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014 Apr 17;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 38.Bodenheimer T, Pham HH. Primary Care: Current Problems And Proposed Solutions. [2010 May 1];Health Affairs (Millwood) 2010 29(5):799–805. doi: 10.1377/hlthaff.2010.0026. [DOI] [PubMed] [Google Scholar]
- 39.Baicker K, Finkelstein A. The effects of Medicaid coverage--learning from the Oregon experiment. N Engl J Med. 2011 Aug 25;365(8):683–685. doi: 10.1056/NEJMp1108222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baicker K, Taubman SL, Allen HL, Bernstein M, Gruber JH, Newhouse JP, et al. The Oregon Experiment — Effects of Medicaid on Clinical Outcomes. New England Journal of Medicine. 2013;368(18):1713–1722. doi: 10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.