ABSTRACT
BACKGROUND
There are few rigorous studies to confirm or refute the commonly cited concern that control of blood pressure to lower thresholds may result in an increased risk of falls and fractures.
OBJECTIVE
To compare falls and fractures in participants with type 2 diabetes in the intensive (targeting a systolic blood pressure of < 120 mmHg) and standard (targeting a systolic blood pressure of < 140 mmHg) blood pressure control arms of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) randomized trial (N = 4,733).
PARTICIPANTS
A subset of 3,099 participants self-reported annually on the occurrence of falls and non-spine fractures. Fractures were centrally adjudicated.
MAIN MEASURES
The incidence of falls in the two treatment groups was compared using a random-effects negative binomial model, and fracture risk was compared using Cox proportional hazards models.
KEY RESULTS
At enrollment in both groups, the mean age was 62 years, 44 % were women, 25 % were Black, and mean blood pressure was 138/75 mmHg. During follow-up, all classes of medications, particularly thiazide diuretics, were more commonly prescribed in the intensive group. After 1 year of follow-up, the mean systolic blood pressure was 133 ± 15 mmHg in the standard group and 119 ± 14 mmHg in the intensive group. The adjusted rate of falls did not differ in the intensive and standard groups (62.2/100 person-years vs. 74.1/100 person-years, RR = 0.84, 95 % CI 0.54–1.29, p = 0.43). The risk of non-spine fractures was nonsignificantly lower in the intensive than in the standard blood pressure group (HR 0.79, 95 % CI 0.62–1.01, p = 0.06).
CONCLUSIONS
We conclude that intensive antihypertensive treatment that lowered mean systolic blood pressure to below 120 mmHg was not associated with an increased risk of falls or non-spine fractures in patients age 40 to 79 years with type 2 diabetes.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2961-3) contains supplementary material, which is available to authorized users.
KEY WORDS: type 2 diabetes mellitus, hypertension, falls, fractures
Despite evidence from clinical trials that treatment of hypertension prevents cardiovascular events,1–3 physicians and patients commonly express concern that tight control of blood pressure (BP) may place patients at risk for hypotension, falls and fractures.4,5 Data to support this notion are exceedingly sparse, because few trials have achieved substantial differences in blood pressure (BP) between comparison groups or have collected data on falls or fractures. In the Systolic Hypertension in the Elderly Program (SHEP), systolic BP (SBP) fell from 170 to 144 mmHg in the active treatment group and to 155 mmHg in the placebo group. About one-third of participants in both groups reported feelings of unsteadiness or imbalance, and more active treatment group participants (12.8 %) than placebo group participants (10.6 %) reported faintness on standing.6,7 However, falls and fractures did not differ significantly between the treatment groups in SHEP.7 A similar BP reduction was achieved in the Hypertension in the Very Elderly Trial (HYVET) in patients age 80 years and older, with very few reported adverse effects. An analysis of fractures as a secondary outcome in HYVET reported a nonsignificant decrease with treatment; falls were not reported.8
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, participants with type 2 diabetes were randomly assigned to intensive BP therapy, targeting an SBP of < 120 mmHg, or to standard therapy, targeting an SBP of < 140 mmHg. Although there was a significantly lower risk for stroke in the intensive group compared to the standard group, the intensive group experienced more hypotension and adverse events attributed to BP medications.9 In this paper, we compare the rates of falls and fractures in the intensive and standard groups of the ACCORD BP trial, which achieved lower SBP in the intensive group than either SHEP or HYVET.
METHODS
Study Design and Participants
The ACCORD trial was designed to test the effects of intensive treatment of glycemia, blood pressure, and lipids on cardiovascular disease (CVD) outcomes among people with diabetes. Details regarding the general design of ACCORD are provided elsewhere.10 Briefly, ACCORD was a multicenter, randomized controlled clinical trial with a double factorial design that enrolled 10,251 middle-aged and older participants with type 2 diabetes at high risk for CVD events from 77 clinical centers (Supplemental Figure 1 available online). The study protocol was approved by an institutional review board or ethics committee at all clinical centers and all participants provided written informed consent.
All participants were enrolled in the glycemia trial, and randomized to intensive glycemia control (goal HbA1c < 6.0 %) or standard glycemia control (goal HbA1c 7.0–7.9 %). A subset of 4,733 of these subjects who had systolic BP 130 to 179 mmHg and met eligibility criteria for the ACCORD BP Trial were also randomized a second time to either intensive BP control (goal SBP < 120 mmHg, N = 2,362), or standard BP control (goal SBP < 140 mmHg, N = 2,371). Randomization in both the glycemia and BP trials was stratified by clinical center. The ACCORD BP Trial was designed to test the hypothesis that a treatment strategy of more intensive SBP control would reduce the incidence of a composite outcome of death from CVD, or nonfatal myocardial infarction or stroke.11 Neither patients nor investigators were blinded to treatment assignment. The principal results of the BP trial have been published, and demonstrated that intensive BP control did not reduce the main pre-specified composite cardiovascular outcome, although there was a benefit on the stroke component of the composite outcome.9
The ACCORD BP trial was a study of a treatment strategy to achieve specific SBP goals, rather than an evaluation of any specific drug regimen. However, all the antihypertensive regimens were to include drug classes that had been shown to result in a reduction in CVD events among participants with diabetes [thiazide diuretic, beta-blocker, calcium channel blocker (CCB), angiotensin converting-enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB)]. All of these antihypertensive drugs classes and many combination medications were provided by the study. For intensive participants, a combination of a thiazide diuretic and either an ACE inhibitor or a beta-blocker was recommended as initial therapy. Details of the adjustment of medication doses, and antihypertensive drug regimens have been published.11 BP was measured by trained technicians using an appropriate sized cuff and an automated device (Omron 907). It was recorded as the average of three measurements after 5 min rest with the participant seated in a chair.
Outcomes
Data on falls and fractures were collected as part of an ancillary study called ACCORD-BONE, and these outcomes in the ACCORD glycemia trial have been published.12 Five of the seven clinical center networks agreed to participate in ACCORD-BONE, including 54 of 77 clinical sites and 3,099 BP trial participants (1,534 in the intensive and 1,565 in the standard BP control groups, Supplemental Figure 1, available online). Beginning in January 2006, participants were asked at their annual visits about the occurrence of any falls in the previous 12 months. Falling was defined as having “fallen or landed on the floor or ground, or fallen and hit an object like a table or stair.”13 Participants who reported any fall were asked the number of times they fell in the previous 12 months. Participants were also asked about the occurrence of non-spine fractures at annual visits. In 2006, they were asked if they had suffered any fractures since randomization, and after 2006, about fractures since their last annual visit. The mean follow-up time for falls was 3.5 years, and the mean follow-up time for fractures was 4.9 years.
Reported fracture events were centrally adjudicated, based on radiology reports, at the University of California, San Francisco, with the adjudicators blinded to treatment assignment. A sample of the adjudication results was reviewed by a panel of experts at two time-points during the trial. Pathological fractures, confirmed as occurring secondary to neoplasm, necrosis, or sepsis, or periprosthetic fractures, were excluded (N = 6). Only confirmed non-spine fractures were included in these analyses as the primary outcome. Hip, proximal humerus, distal forearm, ankle and foot fractures were analyzed individually as secondary outcomes.
Other Variables
Information on age, sex, race, ethnicity, diabetes duration, diabetes and antihypertensive treatment, history of smoking, and history of CVD were collected at baseline using standardized questionnaires. Height, weight, BP, and visual acuity were measured according to a standardized protocol by trained technicians. The presence of peripheral neuropathy at baseline was determined from a foot exam based on the Michigan Neuropathy Screening Instrument. Hgb A1c and creatinine were measured in a central laboratory, and estimated glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation.14 The number and classes of antihypertensive medications and BP were assessed at visits occurring at 4-month intervals following enrollment.
Statistical Analysis
The incidence of all falls, including repeated falling, was compared using a random-effects negative binomial model for repeated measures of the number of falls in each reporting period, with robust standard errors to account for clustering by participant, and including the log of the length of the reporting period as a so-called offset. The proportion of participants who fell at least once or twice per year was compared using logistic regression, also using robust standard errors. Fracture risk was compared using Cox proportional hazards models. All models were adjusted for randomized treatment assignment in the glycemia trial, history of CVD at baseline, and study year for the falls analysis. Cox regression models were also examined after controlling for baseline use of estrogen in women and use of thiazide diuretics as a time dependent covariate based on reports from the annual exams. We tested for interaction by age, sex, race/ethnicity, diabetes medication treatment at baseline, and comorbidities at baseline (CVD, neuropathy, visual impairment.)
RESULTS
The mean age of the participants included in this analysis was 62 years, 44 % were women, 25 % were Black, and 2 % were Hispanic (Table 1). About 88 % were treated with antihypertensive drugs, 22 % were treated with a thiazolidinedione and 39 % were treated with insulin at baseline. Baseline characteristics were generally comparable between intensive and standard groups, and mean BP in both groups was about 138/75 mmHg. Data on use of osteoporosis medications was not collected at baseline; however, only 4.7 % of intensive participants and 3.6 % of standard group participants (p = 0.14) ever reported use of an osteoporosis medication after 1 January 2007.
Table 1.
Baseline Characteristics of ACCORD-BONE Participants in the ACCORD Blood Pressure Trial (N = 3,099) Intensive and Standard Treatment Groups
| Intensive* (N = 1,534) | Standard* (N = 1,565) | p value | |
|---|---|---|---|
| Mean age (yr) | 62.2 ± 6.4 | 62.4 ± 6.7 | 0.56 |
| Female | 660 (43.0) | 695 (44.4) | 0.45 |
| Median duration of diabetes (yr) | 10.0 ± 10.0 | 10.0 ± 11.0 | 0.12 |
| Mean Body mass index (kg/m2) | 32.5 ± 5.5 | 32.4 ± 5.2 | 0.46 |
| Previous cardiovascular event | 525 (34.2) | 521 (33.3) | 0.59 |
| Race/ethnicity | |||
| White | 1,040 (67.8) | 1,014 (64.8) | 0.08 |
| Black | 375 (24.5) | 403 (25.8) | 0.41 |
| Other | 119 (7.8) | 148 (9.5) | 0.10 |
| Medications | |||
| Any antihypertensive | 1,351 (88.1) | 1,374 (87.8) | 0.83 |
| ACE Inhibitor | 844 (55.0) | 816 (52.1) | 0.11 |
| Thiazide diuretic | 423 (27.6) | 467 (29.8) | 0.16 |
| Insulin | 590 (38.5) | 637 (40.7) | 0.21 |
| Metformin | 866 (56.4) | 901 (57.6) | 0.54 |
| Any sulfonylurea | 753 (49.1) | 730 (46.6) | 0.18 |
| Any thiazolidinedione | 331 (21.6) | 336 (21.5) | 0.97 |
| Estrogen (women only) | 82/578 (12.4) | 59/636 (8.5) | 0.02 |
| Statin | 1,013 (66.0) | 1,056 (67.5) | 0.40 |
| Blood pressure (mm Hg) | |||
| Mean systolic | 138.0 ± 16.2 | 138.6 ± 15.9 | 0.32 |
| Mean diastolic | 75.3 ± 10.7 | 75.5 ± 10.3 | 0.81 |
| Glycated hemoglobin (%) | |||
| Mean | 8.4 ± 1.1 | 8.3 ± 1.0 | 0.08 |
| Median | 8.2 ± 1.3 | 8.1 ± 1.2 | 0.06 |
| Mean serum creatinine (mg/dl) | 0.90 ± 0.23 | 0.91 ± 0.23 | 0.57 |
*Cell values are n (%), means ± standard deviations or medians ± interquartile ranges
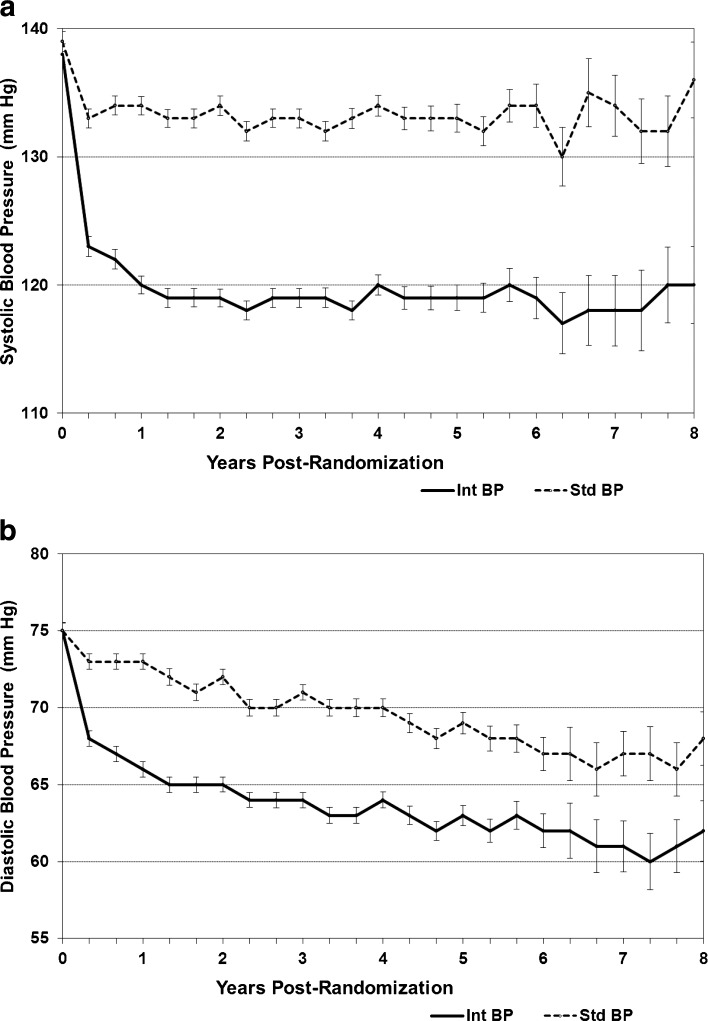
Systolic and diastolic BP (DBP) fell in both groups, separating by 4 months after randomization (Fig. 1). SBP stabilized by 1 year of follow-up in both groups, and the mean ± standard deviation SBP thereafter was 133 ± 15 mmHg in the standard group and 119 ± 14 mmHg in the intensive group. DBP continued to fall throughout the study in both groups, but maintained a mean ± standard deviation difference of 6 ± 10 mmHg.
Figure 1.
a Systolic blood pressure (SBP) of ACCORD-BONE participants in the intensive and standard treatment groups of the ACCORD Blood Pressure trial. b Diastolic blood pressure (DBP) of ACCORD-BONE participants in the intensive and standard treatment groups of the ACCORD Blood Pressure trial.
At the 12-month visit, the most commonly prescribed antihypertensive medications were ACE inhibitors, followed by thiazide diuretics and beta-blockers (Fig. 2). All classes of medications were more commonly prescribed in the intensive group than in the standard group at the 12-month visit, but the largest difference was in thiazide diuretic use (76 % in the intensive group and 40 % in the standard group.) In the intensive group the mean number of antihypertensive medications prescribed was 3.3, compared with 1.9 in the standard group.
Figure 2.
Proportion of participants treated with various antihypertensive drug classes at the 12-month visit in intensive and standard treatment groups of the ACCORD Blood Pressure Trial (ARB angiotensin receptor blocker, CCB calcium channel blocker).
Overall, the raw rate of falls was 69.8/100 person-years during 3.5 years of follow-up. In the intensive group the adjusted rate was slightly lower than in the standard group (62.2/100 person-years vs. 74.1/100 person-years), but the difference was not statistically significant (RR = 0.84, 95 % CI 0.54–1.29, p = 0.43). In any given year, about 21 % of the participants had at least one fall (Fig. 3) and 8 % had two or more falls. The proportion with a fall did not differ between the intensive (20 %) and standard groups (21 %, OR 0.94, 95 % CI 0.84–1.05, p = 0.27) There was no heterogeneity in the rate of falls between treatment groups over time or by age, sex, ethnicity, baseline diabetes treatments, or comorbidities (all p for interaction > 0.05).
Figure 3.
Proportion of participants who fell in previous year at each visit, by blood pressure treatment group.
Overall, 270 participants had at least one confirmed non-spine fracture (Table 2, Supplemental Figure 2, available online). Of these, there were 17 hip, 63 ankle, 29 foot, 34 proximal humerus, and 25 distal forearm fractures. The risk of all non-spine fractures combined was nonsignificantly lower in the intensive compared to the standard BP group (HR 0.79, 95 % CI 0.62–1.01, p = 0.06). With additional adjustment for baseline estrogen use and thiazide diuretic use during follow-up, the risk of fractures was not materially changed (Table 2). The point estimate of the fracture risk for various sites was in general nonsignificantly lower in the intensive compared to the standard BP group. Foot fractures were significantly lower in the intensive group in the adjusted models (models 2 and 3).
Table 2.
Site-Specific Fracture Risk in the Intensive and Standard Treatment Groups of the ACCORD Blood Pressure Trial
| Fracture site | Intensive | Standard | Model 1‡ | Model 2§ | Model 3‖ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N * | Rate† | N * | Rate† | HR | 95 % CI | p value | HR | 95 % CI | p value | HR | 95 % CI | p value | |
| All non-spine | 116 | 15.41 | 154 | 19.73 | 0.79 | (0.62, 1.01) | 0.06 | 0.79 | (0.62, 1.01) | 0.06 | 0.81 | (0.63, 1.05) | 0.11 |
| Hip | 5 | 0.64 | 12 | 1.48 | 0.44 | (0.16, 1.26) | 0.13 | 0.45 | (0.16, 1.29) | 0.14 | 0.52 | (0.18, 1.51) | 0.23 |
| Ankle | 25 | 3.23 | 38 | 4.71 | 0.70 | (0.42, 1.16) | 0.17 | 0.70 | (0.42, 1.17) | 0.17 | 0.70 | (0.41, 1.18) | 0.18 |
| Foot | 9 | 1.16 | 20 | 2.47 | 0.47 | (0.21, 1.03) | 0.06 | 0.45 | (0.21, 0.99) | 0.048 | 0.44 | (0.19, 0.99) | 0.048 |
| Proximal humerus | 15 | 1.93 | 19 | 2.34 | 0.84 | (0.43, 1.66) | 0.62 | 0.84 | (0.43, 1.66) | 0.62 | 0.78 | (0.38, 1.57) | 0.48 |
| Distal forearm | 12 | 1.55 | 13 | 1.60 | 1.00 | (0.46, 2.20) | 0.99 | 1.01 | (0.46, 2.22) | 0.98 | 1.18 | (0.52, 2.64) | 0.69 |
*Number of participants with at least one fracture that occurred after randomization and before the close of the ACCORD trial (June 2009)
†Rate of first fracture at specific site per 1,000 person-years
‡Model 1 is adjusted for glycemia treatment assignment and baseline history of CVD
§Model 2 is adjusted for glycemia treatment assignment, baseline history of CVD, and baseline estrogen use
‖Model 3 is adjusted for glycemia treatment assignment, baseline history of CVD, baseline estrogen use and use of thiazide-like diuretics at baseline and annually thereafter
DISCUSSION
These results in patients with diabetes from the subset of ACCORD-BONE participants in the ACCORD BP Trial show that intensive antihypertensive treatment that lowered mean systolic BP to below 120 mmHg was not associated with an increased risk of falls or non-spine fractures. We did not identify a difference between treatment groups in the rate of falls in any vulnerable subgroup, or find any evidence that the treatment effect on fall risk varied over time.
Our possible trend toward lower fracture risk is consistent with trends seen in HYVET, which examined treatment with indapamide (with or without perindopril) compared with matching placebos in hypertensive people age 80 years and older.15 Both treatment groups in HYVET had BP 173/91 mmHg at baseline; at 2 years of follow-up, the active treatment group’s mean BP was 145/78 mmHg, 15/6 mmHg lower than the placebo group. The trial was stopped early for beneficial effects of treatment on stroke, total mortality, cardiovascular events and heart failure. The risk of incident fracture, a secondary endpoint, was 31 % lower in the active treatment group (HR 0.69, 95 % CI 0.46–1.05, p = 0.09).8 Following adjustment for age, sex, and previous use of beta blockers, the HR for fractures was 0.58 (95 % CI 0.33–1.00, p = 0.0498). The most common fracture types were femur and forearm, but specific treatment effects on fracture sub-types were not reported.
There are several possible mechanisms that could account for the reduced fracture risk observed in both ACCORD-BONE and HYVET. One potential mechanism is a thiazide-induced increase in passive reabsorption of calcium in the proximal renal tubules and resulting decreased excretion of calcium.16,17 This physiologic effect of thiazides has been linked with decreased bone loss in randomized trials18,19 and decreased fracture risk in observational studies.20–25 In ACCORD (and also in ACCORD-BONE), there was more thiazide use in the intensive treatment group (Fig. 2), and in HYVET, the active treatment group received indapamide, a thiazide-like diuretic that has been shown to have similar effects on calcium excretion as other thiazides.26–30 However, thiazide diuretic use in the standard BP control group was common and adjustment for thiazide diuretic use during the study had a minimal effect on the observed hazard ratios for fracture. Therefore, it is worth considering whether factors other than the greater thiazide diuretic use in the intensive BP control group could have contributed to the 22 % reduction in the relative rate of fracture.
Other antihypertensive drugs may also have played a role in the reduced risk of fracture in the intensive BP treatment group in ACCORD-BONE. Several observational studies have suggested that treatment with beta blockers may reduce the incidence of fractures,21,31–34 although other studies have not found this association.25,35–37 Other reports have linked treatment with CCB, ARB, or ACE inhibitors with a decreased risk of fractures.20,34 It is not possible to distinguish effects of medication treatment and BP lowering in either HYVET or ACCORD-BONE, although some studies have suggested that hypertension itself is a risk factor for bone mineral loss and fractures,38,39 and therefore that lowering BP with a variety of drugs may contribute to decreasing fracture risk.40 In contrast to these long-term studies, two recent observational studies found a short-term increased risk of hip fracture in the period immediately following initiation of antihypertensive or diuretic therapy in elderly patients.41,42
The central strength of this study is its design as a randomized trial comparing intensive and standard BP treatment. Our null finding in ACCORD regarding an effect of intensive BP treatment on falls runs counter to a commonly accepted belief that intensive BP control will increase the risk of falls and fractures. However, the evidence for this belief is limited. Only one previous trial comparing BP interventions, the SHEP trial, considered the outcome of falls, and this randomized trial found no difference across BP treatment assignment.7 We now provide a second randomized trial showing that falls are not increased, even with the much lower BP levels achieved in the ACCORD intensive BP group.
A substantial proportion of ACCORD BP trial participants reported dizziness on standing following randomization, but the proportion did not differ between the intensive and standard groups (44 % vs. 40 %, p = 0.36).9 Orthostatic hypotension is also common in the elderly, with many population estimates of prevalence in excess of 25 % in people age 60 years and older,43–46 although some studies report a lower prevalence.47,48 In addition to age, hypertension and diabetes are both risk factors for orthostatic hypotension.46,49,50 However, neither orthostatic hypotension nor antihypertensive medications have been consistently associated with falls in observational studies, many of which had important limitations.5,51–57 Our results and review of the literature suggest a need to carefully reconsider current thinking about associations of antihypertensive treatment and BP lowering with risk for falls and fractures.
Given their risk factors for falls, it is somewhat surprising that the rate of falls and fractures in the ACCORD BP trial population was similar to that reported for non-diabetic populations. Most published data on falls is in people age 65 years and older. In this age group, about one-third report at least one fall per year.58 One published study provided data on younger ages and reported at least one fall in the previous 2 years in 18.5 % of people age 20–45, 21.3 % of people age 46–64, and 34.8 % of people age 65 and older.59 At baseline in the ACCORD BP trial, 66 % were age 40–64 and 34 % were 65 years and older. The rate of non-spine fractures in Olmsted County among adults age 55–59 was 15.16 (per 1,000 person-years) in men and 25.97 in women, similar to the 19.17 fracture incidence in the ACCORD BP Trial.60 These data suggest that there was sufficient room for more falls and fractures to occur if intensive BP control increased the risk.
Several limitations of this analysis should also be kept in mind. First, questions about falls were only assessed annually starting in 2006 and were based on self-report. Second, the ACCORD BP trial was not blinded, so it is possible that participants’ reports of falls may have been influenced by their knowledge of their treatment assignment. However, given the prevailing perception that treatment to lower BP is a risk factor for falls, one would expect that the direction of bias in self-reported falls would result in a higher rate of falls in the intensive group. Third, because the study was not originally designed to examine fracture risk, we lack data on some important baseline variables, including a history of falls and fractures, and complete data on medications to treat osteoporosis. However, the trial design with randomization stratified by clinical center should have resulted in equal distribution of these variables in both groups. Fourth, the ACCORD BP trial design tested a strategy to achieve BP targets, thus limiting our ability to draw conclusions about effects of specific antihypertensive treatments. Fifth, the ACCORD trial was designed to examine the long-term effects of the interventions and was not able to examine short-term effects of antihypertensive treatment on falls and fractures. Given that the biggest fall in SBP in the intensive group took place in the first several months of the study, this might be the period of highest risk. Finally, the ACCORD-BONE population had a mean age of 62 years and excluded patients age 80 and older, so our data on falls may not apply to an older age group. However, our results are consistent with the HYVET fracture results in the very elderly.
Lowering BP using the medication algorithm in ACCORD compared with standard treatment did not result in an increased rate of falls or fractures, and in fact showed possible trends towards fewer fractures in the intensively treated patients. Intensive lowering of systolic BP in ACCORD did not affect the primary outcome of the trial and is not likely to be adopted as a treatment guideline for patients with type 2 diabetes.9 However, the data reported here call into question whether the commonly cited concern about the long-term risk of falls and fractures should hinder the adoption of evidence-based guidelines for blood pressure treatment in older people and those with diabetes. Additional analyses in ACCORD and studies in other populations are needed to explore other mechanisms by which BP treatment may influence risk of falls and fractures, including effects on orthostatic hypotension and orthostatic dizziness.
Electronic supplementary material
(DOCX 282 kb)
(DOCX 45 kb)
Acknowledgments
Sources of Funding
The ACCORD Trial was funded by the National Heart, Lung and Blood Institute, and ACCORD-BONE was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK069514, Ann Schwartz, PI), Trial Registration: ClinicalTrials.gov Identifier: NCT00000620.
Financial Disclosure
Ann Schwartz has research funding from GLAXOSMITHKLINE (GSK). Margaret Tiktin consults for NovoNordisk.
Conflicts of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Collins R, Peto R, MacMahon S, Hebert P, Fiebach N, Eberlein K, Godwin J, Qizilbash N, Taylor J, Hennekens C. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-Z. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/S0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 3.Czernichow S, Zanchetti A, Turnbull F, Barzi F, Ninomiya T, Kengne AP, Lambers Heerspink HJ, Perkovic V, Huxley R, Arima H, Patel A, Chalmers J, Woodward M, MacMahon S, Neal B. The effects of blood pressure reduction and of different blood pressure-lowering regimens on major cardiovascular events according to baseline blood pressure: meta-analysis of randomized trials. J Hypertens. 2011;29(1):4–16. doi: 10.1097/HJH.0b013e32834000be. [DOI] [PubMed] [Google Scholar]
- 4.Stokes GS. Management of hypertension in the elderly patient. Clin Interv Aging. 2009;4:379–389. doi: 10.2147/CIA.S5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riefkohl EZ, Bieber HL, Burlingame MB, Lowenthal DT. Medications and falls in the elderly: a review of the evidence and practical considerations. Pharm Ther. 2003;28(11):724–733. [Google Scholar]
- 6.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265(24):3255–3264. doi: 10.1001/jama.1991.03460240051027. [DOI] [PubMed] [Google Scholar]
- 7.Curb J, Applegate W, Vogt T, Pressel S, Lee M, Hoffmeier M, Schron R, Bearden D, Huber M, Moye L, Systolic Hypertension in the Elderly Program Cooperative Group Antihypertensive therapy and falls and fractures in the systolic hypertension in the elderly program. J Am Geriatr Soc. 1993;41:SA15. [Google Scholar]
- 8.Peters R, Beckett N, Burch L, de Vernejoul MC, Liu L, Duggan J, Swift C, Gil-Extremera B, Fletcher A, Bulpitt C. The effect of treatment based on a diuretic (indapamide) +/− ACE inhibitor (perindopril) on fractures in the Hypertension in the Very Elderly Trial (HYVET) Age Ageing. 2010;39(5):609–616. doi: 10.1093/ageing/afq071. [DOI] [PubMed] [Google Scholar]
- 9.The ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Jr, Grimm RH, Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Cushman WC, Grimm RH, Jr, Cutler JA, Evans GW, Capes S, Corson MA, Sadler LS, Alderman MH, Peterson K, Bertoni A, Basile JN. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12A):44i–55. doi: 10.1016/j.amjcard.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz AV, Margolis KL, Sellmeyer DE, Vittinghoff E, Ambrosius WT, Bonds DE, Josse RG, Schnall AM, Simmons DL, Hue TF, Palermo L, Hamilton BP, Green JB, Atkinson HH, O’Connor PJ, Force RW, Bauer DC. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35(7):1525–1531. doi: 10.2337/dc11-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson MJ, Andres RO, Isaacs B. The prevention of falls in later life. A report of the Kellogg International Work Group on the Prevention of Falls by the Elderly. Dan Med Bull. 1987;34(Suppl 4):1–24. [PubMed] [Google Scholar]
- 14.National Kidney Foundation Kidney Disease Outcomes Quality Initiative K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–246. [PubMed] [Google Scholar]
- 15.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 16.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115(6):1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholz D, Schwille PO, Sigel A. Double-blind study with thiazide in recurrent calcium lithiasis. J Urol. 1982;128(5):903–907. doi: 10.1016/s0022-5347(17)53269-3. [DOI] [PubMed] [Google Scholar]
- 18.LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;133(7):516–526. doi: 10.7326/0003-4819-133-7-200010030-00010. [DOI] [PubMed] [Google Scholar]
- 19.Reid IR, Ames RW, Orr-Walker BJ, Clearwater JM, Horne AM, Evans MC, Murray MA, McNeil AR, Gamble GD. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med. 2000;109(5):362–370. doi: 10.1016/S0002-9343(00)00510-6. [DOI] [PubMed] [Google Scholar]
- 20.Solomon DH, Mogun H, Garneau K, Fischer MA. Risk of fractures in older adults using antihypertensive medications. J Bone Miner Res. 2011;26(7):1561–1567. doi: 10.1002/jbmr.356. [DOI] [PubMed] [Google Scholar]
- 21.Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of beta-blockers and risk of fractures. JAMA. 2004;292(11):1326–1332. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- 22.Wiens M, Etminan M, Gill SS, Takkouche B. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med. 2006;260(4):350–362. doi: 10.1111/j.1365-2796.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones G, Nguyen T, Sambrook PN, Eisman JA. Thiazide diuretics and fractures: can meta-analysis help? J Bone Miner Res. 1995;10(1):106–111. doi: 10.1002/jbmr.5650100115. [DOI] [PubMed] [Google Scholar]
- 24.Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst Rev. 2011;10 doi: 10.1002/14651858.CD005185.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Rejnmark L, Vestergaard P, Mosekilde L. Reduced fracture risk in users of thiazide diuretics. Calcif Tissue Int. 2005;76(3):167–175. doi: 10.1007/s00223-004-0084-2. [DOI] [PubMed] [Google Scholar]
- 26.Lemieux G. Treatment of idiopathic hypercalciuria with indapamide. CMAJ. 1986;135(2):119–121. [PMC free article] [PubMed] [Google Scholar]
- 27.Borghi L, Elia G, Trapassi MR, Melloni E, Amato F, Barbarese F, Novarini A. Acute effect of indapamide on urine calcium excretion in nephrolithiasis and human essential hypertension. Pharmacology. 1988;36(5):348–355. doi: 10.1159/000138405. [DOI] [PubMed] [Google Scholar]
- 28.Ceylan K, Topal C, Erkoc R, Sayarlioglu H, Can S, Yilmaz Y, Dogan E, Algun E, Gonulalan H. Effect of indapamide on urinary calcium excretion in patients with and without urinary stone disease. Ann Pharmacother. 2005;39(6):1034–1038. doi: 10.1345/aph.1E544. [DOI] [PubMed] [Google Scholar]
- 29.Borghi L, Meschi T, Guerra A, Novarini A. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol. 1993;22(Suppl 6):S78–S86. doi: 10.1097/00005344-199312050-00014. [DOI] [PubMed] [Google Scholar]
- 30.Lalande A, Roux S, Denne MA, Stanley ER, Schiavi P, Guez D, De Vernejoul MC. Indapamide, a thiazide-like diuretic, decreases bone resorption in vitro. J Bone Miner Res. 2001;16(2):361–370. doi: 10.1359/jbmr.2001.16.2.361. [DOI] [PubMed] [Google Scholar]
- 31.Meisinger C, Heier M, Lang O, Doring A. Beta-blocker use and risk of fractures in men and women from the general population: the MONICA/KORA Augsburg cohort study. Osteoporos Int. 2007;18(9):1189–1195. doi: 10.1007/s00198-007-0354-8. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, Benhamou CL. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone. 2007;40(5):1209–1216. doi: 10.1016/j.bone.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19(1):19–24. doi: 10.1359/jbmr.0301214. [DOI] [PubMed] [Google Scholar]
- 34.Rejnmark L, Vestergaard P, Mosekilde L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case–control study. J Hypertens. 2006;24(3):581–589. doi: 10.1097/01.hjh.0000203845.26690.cb. [DOI] [PubMed] [Google Scholar]
- 35.de Vries F, Pouwels S, Bracke M, Leufkens HG, Cooper C, Lammers JW, van Staa TP. Use of beta-2 agonists and risk of hip/femur fracture: a population-based case–control study. Pharmacoepidemiol Drug Saf. 2007;16(6):612–619. doi: 10.1002/pds.1318. [DOI] [PubMed] [Google Scholar]
- 36.Levasseur R, Dargent-Molina P, Sabatier JP, Marcelli C, Breart G. Beta-blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l’Osteoporose prospective study. J Am Geriatr Soc. 2005;53(3):550–552. doi: 10.1111/j.1532-5415.2005.53178_7.x. [DOI] [PubMed] [Google Scholar]
- 37.Reid IR, Gamble GD, Grey AB, Black DM, Ensrud KE, Browner WS, Bauer DC. Beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res. 2005;20(4):613–618. doi: 10.1359/JBMR.041202. [DOI] [PubMed] [Google Scholar]
- 38.Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354(9183):971–975. doi: 10.1016/S0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- 39.McCarron DA, Pingree PA, Rubin RJ, Gaucher SM, Molitch M, Krutzik S. Enhanced parathyroid function in essential hypertension: a homeostatic response to a urinary calcium leak. Hypertension. 1980;2(2):162–168. doi: 10.1161/01.HYP.2.2.162. [DOI] [PubMed] [Google Scholar]
- 40.Vestergaard P, Rejnmark L, Mosekilde L. Hypertension is a risk factor for fractures. Calcif Tissue Int. 2009;84(2):103–111. doi: 10.1007/s00223-008-9198-2. [DOI] [PubMed] [Google Scholar]
- 41.Berry SD, Zhu Y, Choi H, Kiel DP, Zhang Y. Diuretic initiation and the acute risk of hip fracture. Osteoporos Int. 2013;24(2):689–695. doi: 10.1007/s00198-012-2053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch Intern Med. 2012;19:1–6. doi: 10.1001/2013.jamainternmed.469. [DOI] [PubMed] [Google Scholar]
- 43.Hiitola P, Enlund H, Kettunen R, Sulkava R, Hartikainen S. Postural changes in blood pressure and the prevalence of orthostatic hypotension among home-dwelling elderly aged 75 years or older. J Hum Hypertens. 2009;23(1):33–39. doi: 10.1038/jhh.2008.81. [DOI] [PubMed] [Google Scholar]
- 44.Wu JS, Yang YC, Lu FH, Wu CH, Chang CJ. Population-based study on the prevalence and correlates of orthostatic hypotension/hypertension and orthostatic dizziness. Hypertens Res. 2008;31(5):897–904. doi: 10.1291/hypres.31.897. [DOI] [PubMed] [Google Scholar]
- 45.Verwoert GC, Mattace-Raso FU, Hofman A, Heeringa J, Stricker BH, Breteler MM, Witteman JC. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56(10):1816–1820. doi: 10.1111/j.1532-5415.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 46.Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing. 2010;39(1):51–56. doi: 10.1093/ageing/afp192. [DOI] [PubMed] [Google Scholar]
- 47.Masaki KH, Schatz IJ, Burchfiel CM, Sharp DS, Chiu D, Foley D, Curb JD. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98(21):2290–2295. doi: 10.1161/01.CIR.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 48.Luukinen H, Koski K, Laippala P, Airaksinen KE. Orthostatic hypotension and the risk of myocardial infarction in the home-dwelling elderly. J Intern Med. 2004;255(4):486–493. doi: 10.1111/j.1365-2796.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- 49.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project) Eur Heart J. 2010;31(1):85–91. doi: 10.1093/eurheartj/ehp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu JS, Yang YC, Lu FH, Wu CH, Wang RH, Chang CJ. Population-based study on the prevalence and risk factors of orthostatic hypotension in subjects with pre-diabetes and diabetes. Diabetes Care. 2009;32(1):69–74. doi: 10.2337/dc08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maurer MS, Cohen S, Cheng H. The degree and timing of orthostatic blood pressure changes in relation to falls in nursing home residents. J Am Med Dir Assoc. 2004;5(4):233–238. doi: 10.1016/S1525-8610(04)70128-0. [DOI] [PubMed] [Google Scholar]
- 52.Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108(2):106–111. doi: 10.1016/S0002-9343(99)00425-8. [DOI] [PubMed] [Google Scholar]
- 53.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59(3):383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gribbin J, Hubbard R, Gladman J, Smith C, Lewis S. Risk of falls associated with antihypertensive medication: self-controlled case series. Pharmacoepidemiol Drug Saf. 2011;20(8):879–884. doi: 10.1002/pds.2176. [DOI] [PubMed] [Google Scholar]
- 55.Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP, Zhou B, Lin H. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174(4):588–595. doi: 10.1001/jamainternmed.2013.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47(1):40–50. doi: 10.1111/j.1532-5415.1999.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 57.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, Marra CA. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 58.Tinetti ME, Speechley M. Prevention of falls among the elderly. N Engl J Med. 1989;320(16):1055–1059. doi: 10.1056/NEJM198904203201606. [DOI] [PubMed] [Google Scholar]
- 59.Talbot LA, Musiol RJ, Witham EK, Metter EJ. Falls in young, middle-aged and older community dwelling adults: perceived cause, environmental factors and injury. BMC Public Health. 2005;5:86. doi: 10.1186/1471-2458-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9(1):29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 282 kb)
(DOCX 45 kb)