Abstract
Background
We compared cardiac electrophysiological indicators and regional expression levels of cardiac hyperpolarization-activated cyclic nucleotide-gated (HCN) channels between adult and aged dogs to identify possible mechanisms of age-related atrial fibrillation.
Material/Methods
Corrected sinus node recovery time (SNRTc) and effective refractory period (ERP) of the atrium and pulmonary veins were measured in 10 adult (3–6 years old) and 10 aged dogs (>9 years old). Expression levels of HCN2 and HCN4 channel mRNAs and proteins were measured in the sinoatrial node, atrium, and pulmonary veins by real-time PCR and Western blotting.
Results
Aged dogs exhibited a higher induction rate of atrial fibrillation (AF) in response to electrical stimulation, longer AF duration after induction, longer SNRTc, longer right atrial effective refractory period (AERP), shorter left AERP, and increased AERP dispersion compared to adults. Expression levels of HCN2 and HCN4 channel mRNAs and proteins were lower in the sinoatrial node but higher in the atrium and pulmonary veins of aged dogs.
Conclusions
Changes in atrial electrophysiological indicators in aged dogs revealed sinoatrial node dysfunction. There was a reversal in the local tissue distribution of HCN2 and HCN4 channel mRNA and protein, a decrease in sinoatrial node expression, and increase in atrial and pulmonary vein expression with age. Changes in atrial electrophysiological characteristics and regional HCN channel expression patterns were associated with the onset and maintenance of age-related atrial fibrillation.
MeSH Keywords: Aging, Atrial Fibrillation, Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels
Background
Atrial fibrillation (AF) is the most common persistent arrhythmia. Many factors such as atrial fibrosis, inflammation, and electrical remolding are involved in AF pathogenesis [1,2]. Older age is an independent risk factor for AF [3], with approximately 70% of AF patients between 65 and 85 years old [4]. Both human patients and research animals show degenerative changes of the sinoatrial node with age [5]. If activities of latent pacemakers such as atrial myocardium and pulmonary vein muscle sleeves (PVMSs) are enhanced with age, it will cause local automaticity or increased triggered activity, providing conditions for the occurrence of AF [6,7].
The cardiac hyperpolarization-activated cation current (or “funny current”, If) is a depolarizing mixed sodium/potassium conductance activated by both membrane hyperpolarization and intracellular cAMP. If is a major determinant of diastolic depolarization following repolarization of action potentials in pacemaker cells, such as those of the sinoatrial node [8]. Despite the critical importance of If in cardiac rhythmicity, the molecular structure of the If channels was only recently elucidated. If is mediated by a family of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels consisting of 4 homologous isoforms (HCN1–HCN4). These isoforms are differentially expressed across cardiac tissue types and species. The HCN3 channel is mainly expressed in central tissues, whereas HCN1, HCN2, and HCN4 are abundantly expressed in heart tissues [9]. It was previously thought that HCN channels were located mainly to the cardiac conduction system, particularly the sinoatrial node, but it is now known that HCN channels are also expressed in the atria, ventricles, and pulmonary veins [10]. Under certain pathological conditions, the expression of HCN channels decreases in the sinoatrial node but increases in other regions such as the atrium, which is a change in expression pattern that can trigger ectopic tachyarrhythmia [11,12].
We hypothesized that aging, which is associated with a dramatic increase in AF susceptibility, would also lead to changes in HCN channel distribution in the sinoatrial node, atrium, and pulmonary veins. To test this hypothesis, we compared the induction rate of AF between adult and aged dogs with sinus rhythm, as well as the effective refractory period, corrected sinus-node recovery time (SNRT C), and the mRNA and protein expression levels of HCN2 and HCN4 channels in the sinoatrial node, atria, and pulmonary veins.
Material and Methods
Experimental methods
Ethics description
All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health [13]. All animal studies were approved by the Animal Use and Care Committee of the First Teaching Hospital, Xinjiang Medical University (Urumqi, China).
Animal selection and grouping
Twenty beagles weighing 12.11±2.15 kg were divided into an adult group (3.65±1.24 years old, range: 3–6 years) and an aged group (11.36±2.54 years old, all > 9 years old). The ages of the dogs were estimated by a veterinarian based on standard measures for age, including dentition, coat, eyes, and musculoskeletal and conformational descriptors.
Surgery
Animals were anesthetized with 3% pentobarbital sodium (30 mg/kg) and ventilated with atmospheric air using a positive-pressure respirator. Before surgery, echocardiography was used to exclude structural heart defects such as valvular heart disease and congenital heart disease. Surgery was conducted under continuous ECG and dynamic monitoring of blood pressure via a right femoral artery pressure sensor. A median sternotomy was performed to expose the pericardium. Mapping electrodes were sutured on the left and right auricle and pulmonary veins. Ten mapping electrodes (Biosense-Webster, Diamond Bar, CA) were delivered through the jugular veins into the coronary sinus via a sheath. Two mapping electrodes (Biosense-Webster) were delivered through femoral veins into the high right atrium. The mapping electrodes were connected to a multi-lead multi-channel electrophysiological recorder (Lead-7000, Sichuan Jinjiang Electronic Science and Technology Co., Ltd.).
Induction of atrial fibrillation (AF) and measurement of effective refractory period (ERP) and the corrected sinus-node recovery time (SNRTc)
Sinoatrial node function was evaluated by SNRTc. Recovery of sinoatrial node rhythmicity was observed following stimulation at double the diastolic threshold using a 250 ms S1S1 programmed pacing cycle to the high right atrium for 30 s. SNRTc was defined as the time between the first sinus A wave and the second A wave subtracted by the time between the last stimulation-induced atrium A wave and the first sinus A wave (intrinsic pacing). The AF induction rate was defined as follows. A 120-ms S1S1 programmed stimulus train was applied to the high right atrium for 60 s at double the diastolic threshold. After stimulation, a spontaneous AF lasting more than 1 s was considered to be successful induction. Ten induction stimuli were administered for each dog at 5-min intervals. The AF duration was the duration of spontaneous AF after termination of programmed stimulation. A 300-ms programmed stimulation S1S2 at double the diastolic threshold was adopted for measuring ERP in each region. The ERP was defined as the longest S1S2 interval that could not be effectively captured. The dispersion of ERP (ERPd) was defined as the difference between the longest and shortest ERP.
Tissue preparation of the sinoatrial node
The sinoatrial node was identified in tissue sections by the absence of connexin 43 expression [14].
RNA extraction and cDNA synthesis
Total RNA was extract from atria, the sinoatrial node, and pulmonary veins using an RNA extraction kit (Tian Gen). Purity and concentration were evaluated by the absorbance ratio (OD260/OD280) using a UV spectrophotometer. An OD260/OD280 between 1.8 and 2.0 was required for use in PCR. Primary cDNAs were prepared using a reverse transcription kit (Fermentas) from 0.1 ng to 5 μg sample RNA in a 12-μL reaction volume containing oligo (dT) 18 primer. After uniform mixing and centrifugation, the reaction mixture was incubated in a water bath at 65°C for 5 min and then immediately cooled on ice. Then, 4 μL reaction buffer, 1 μL RNase inhibitor, 2 μL of 10 mM dNTP mix, and 1 μL M-MuLV reverse transcriptase (200 U/μL) were added and the new solution mixed, centrifuged, incubated in water bath at 42°C for 60 min, and then heated to 70°C for 5 min. The synthesized cDNA was stored at −20°C until use.
The Premier 5.0 program was used to design primers according to HCN2 and HCN4 sequences in GenBank (Table 1).
Table 1.
Gene primer sequences for HCN2 and HCN4.
| Gene | Sequence | Length (bp) |
|---|---|---|
| GAPDH | 5′-TGTCCCCACCCCCAATGTATC-3′ | 100 |
| 5′-CTCCGATGCCTGCTTCACTACCTT-3′ | ||
| HCN2 | 5′-ATCGTGGAGAAGGGCATTGAC-3′ | 134 |
| 5′-TCCCACTGGTGGATGTAGCG-3′ | ||
| HCN4 | 5′-GAGTCAACAAATTATCCCAGAG-3′ | 168 |
| 5′-GAATGATGATTAGGTTTCCAAC-3′ |
Real-time PCR
Real-time PCR analysis was performed with sample cDNA dilutions of 10, 102, 103, 104, 105, and 106 to ensure that the amplification efficiency of primers was between 0.9 and 1.1. Real-time PCR analysis of primers was conducted using 0.25, 0.5, 0.75, 1, 1.25, or 1.5 μL of a 10-μM primer stock and the primer concentration with higher amplification efficiency was chosen for quantitative measures of HCN expression. The RT-PCR reaction system included 10 μL SYBR Premix (2×) (TianGen), 0.6 μL of upstream and downstream primer (from 10 μM stocks), and 1 μL of the cDNA sample. The reaction volume was adjusted to 20 μL with double-distilled water. Reactions were conducted using a Bio-Rad real-time PCR amplifier with thermocycle settings: denaturing at 95ºC for 15 min, followed by 40 amplification cycles of 95ºC for 10 s (denaturing), 60ºC for 10 s (annealing), and 72ºC for 20 s (extension). Fluorescence signals were recording at the end of each extension cycle. At the end of the reaction, a 95–65ºC melting curve was acquired and analyzed.
Western blotting
Western and IP lysis buffer (Beyotime) was used to lyse tissue and extract total protein. Lysate total protein concentrations were assayed with a BSA kit (Beyotime). Lysates were mixed with gel loading buffer, boiled, and separated by 12% polyacrylamide gel electrophoresis (20 μg protein per lane). Separated proteins were electrically transferred onto the nitrocellulose (NC) membranes. Membranes were blocked in buffer containing 5% skimmed milk powder overnight at 4ºC and then immunolabeled with a primary antibody [HCN2 (Bioss): 1:500; HCN4 (Bioss): 1:250; GAPDH (Santa Cruz, Santa Cruz, CA, USA): 1:500] at room temperature (RT) for 2 h. Membranes were washed 3 times with 1×TBST (10 min/wash), incubated in a corresponding secondary antibody solution at RT for 1 h, and washed 3 times with 1×TBST (10 min/wash). Immunolabeling was visualized by ECL chemiluminescent substrate (Millipore) and images acquired by the ChemiDoc-It HR 410 imaging system (Upland, CA).
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). All values are expressed as the mean ±SD. Comparisons between the 2 groups were made using the Student’s t test. P<0.05 was considered to indicate a statistically significant difference.
Results
Electrophysiological indicators
The overall atrial fibrillation (AF) induction rate in aged dogs was 85% compared to 0% in adult dogs. The AF duration in old dogs was 60.21±12.22 s (range, 1–1148 s). The corrected sinus-node recovery time was significantly longer in aged dogs (126.80±14.61 vs. 94.20±30.84; P<0.05). In aged dogs, the effective refractory period (ERP) of the left auricle was shorter than in adult dogs (94.50±4.38 ms vs. 104.5±7.25 ms), as was the ERP of the distal coronary sinus (100.00±4.08 ms vs. 108.05±5.30 ms), although these differences did not reach significance. In contrast, older dogs exhibited prolonged right auricle and proximal coronary sinus ERPs compared to adult dogs (130.00±4.08 ms vs. 120.00±9.72 ms and 129.50±7.25 ms vs. 114.50±10.40 ms), but again the differences were not significant. However, dispersion of the atrial ERP was significantly greater in older dogs (36.00±7.38 ms vs. 21.50±10.01 ms; P<0.05). Neither ERP nor ERP dispersion in the 4 pulmonary veins differed between old and adult dogs (superior pulmonary vein ERP: 109.50±4.38 vs. 108.00±6.33; left inferior pulmonary vein ERP: 112.00±7.89 vs. 106.50±6.69; right superior pulmonary vein ERP: 108.50±6.26 vs. 108.00±7.89; right inferior pulmonary vein ERP: 112.50±7.91 vs. 109.50±5.50; overall ERP dispersion: 14.00±7.75 vs. 12.50±4.86) (Figure 1).
Figure 1.
(A, B) Atrial and pulmonary vein effective refractory periods (AERPs, PVERPs), atrial and pulmonary vein ERP dispersions (AERPd, PVERPd), and corrected sinus-node recovery times (AERPd) were measured in adult dogs (3–6 years old) and aged dogs (>9 years). * P<0.05, aged group vs. adult group; # P>0.05, aged group in contrast to adult group. LA: left auricle, CSd: distal coronary sinus, RA: right auricle, CSp: proximal coronary sinus, LSPV: left superior pulmonary vein, LIPV: left inferior pulmonary vein, RSPV: right superior pulmonary vein, RIPV: right inferior pulmonary vein.
Expression levels of HCN2 and HCN4 channel mRNAs and proteins
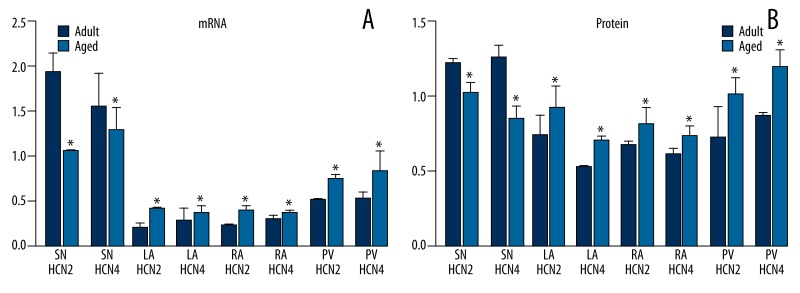
Expression levels of HCN2 and HCN4 channel mRNAs and proteins were significantly lower in the sinoatrial node of aged dogs compared to adults (P<0.05). In contrast, expression levels of HCN2 and HCN4 mRNAs and proteins were significantly higher in the pulmonary veins and atria of aged dogs (P<0.05) (Figure 2).
Figure 2.
(A, B) Expression levels of HCN2 and HCN4 channel mRNAs and proteins in adult and aged dogs. * P<0.05, aged group vs. adult group. SN: sinoatrial node, LA: left atrium, RA: right atrium, PV: pulmonary vein.
Discussion
Age-related changes in cardiac electrophysiology
Action potential duration (APD) is prolonged with age in the rabbit right atrium [15], dog right atrium [16], and dog left atrium [17], suggesting age-related changes in cardiac ion channel expression patterns. We also observed significant age-related changes in the electrophysiological properties of the sinoatrial node, atrium, and pulmonary veins, including prolonged right atrial effective refractory period (AERP), shorter left atrial ERP, and increased AERP dispersion (AERPd). Moreover, the aged dog heart was more susceptible to induced atrial fibrillation (AF). These changes were associated with altered regional expression of HCN2 and HCN4, which are non-specific cation channels critical for cardiac pacemaking.
Previous clinical observations have revealed increased prevalence of sinus node dysfunction (SND) in the elderly [5], which is often accompanied by AF [18]. Impaired sinoatrial node function could change the electrophysiology of the atrium and increase AF susceptibility. Li et al. [19] showed increased AERPd and AF induction in dogs with SND. In our study, aged dogs exhibited prolonged corrected sinus-node recovery time (SNRTc), consistent with sinoatrial node dysfunction. We suggest that these electrophysiological changes in the atria and increased AF propensity are related not only to structural remodeling, such as the enlargement of the atrium, or to myocardial apoptosis and atrial tissue fibrosis [18], but also to changes in HCN expression patterns.
Involvement of HCN channels in ectopic tachyarrhythmia
Hyperpolarization-activated cyclic nucleotide-gated channels mediate phase 4 action potential depolarization. The HCN-mediated ionic current If is important for rhythm control and impulse propagation through the sinoatrial node [21]. However, HCN channels are also expressed in multiple regions of the heart besides the sinoatrial node [22–24]. The known role of these channels in rhythmicity suggests that channel dysregulation may be involved in arrhythmias. That is, various pathological factors and (or) aging may lead to abnormal HCN channel expression, which could in turn cause ectopic tachyarrhythmia. Kuwahara et al. [25] found that neuron repressor element silencing transcription factor (REST) is an important regulator of the fetal cardiac gene expression program. In the progressive cardiomyopathy mouse model expressing a dominant-negative mutant REST (dnNRSF-TG), HCN2 and HCN4 were highly expressed in the ventricles. All dnNRSF-TG mice died of sudden arrhythmia at 8 weeks of age, and overexpression of HCN2 and HCN4 in the ventricle increased susceptibility to β-adrenergic-induced ventricular arrhythmias, an effect blocked by an HCN antagonist [26]. In studies on atrial arrhythmia, Zorn-Pauly et al. [24] found that increased If in the atrial myocardium conferred atrial myocytes with features of pacemaker cells, increasing the local atrial automaticity and predisposing to atrial arrhythmia. Li et al. [27] compared aged dogs with AF to those with sinus rhythm and found significantly higher If density and mRNA expression levels of HCN4 channels in pulmonary vein muscle sleeves (PVMSs) of the AF group compared to the sinus rhythm group. Thus, enhanced If in pulmonary veins may also increase susceptibility to AF. Stillitano et al. [28] compared right atrial tissues collected from coronary artery bypass grafting patients with either persistent AF or sinus rhythm. In contrast to patients with sinus rhythm, AF patients exhibited significantly elevated HCN4 channel protein expression in the right atrium, again implicating upregulation of atrial If and concomitant ectopic rhythmicity in the pathogenesis of human AF.
Age-related changes in regional HCN channel expression
Increased HCN channel expression in atrium, pulmonary veins, and ventricle could enhance automaticity and trigger local pacemaker activity in the myocardium. Under pathological conditions, there is often an inverse relationship between HCN channel expression levels between the sinoatrial node and other myocardial tissues. Zicha et al. [29] found that in dog models with heart failure, the expression of HCN2 and HCN4 channels in the sinoatrial node was downregulated while HCN4 channel expression was upregulated in atrial tissues. In accord with the current study, this result suggests that the increased incidences of both sick sinus syndrome and AF in patients with heart failure may be related to reversal in the normal regional expression levels of HCN channels between sinoatrial node and atrium. Furthermore, Yeh et al. [30] reported lower HCN channel expression in the sinoatrial node and higher atrial channel expression in a dog model of atrial tachycardia. Studies by Stephen and Yung showed lower expression of HCN channels in the sinoatrial node with increased expression in the atrium, ventricle, and pulmonary veins of dogs with HF and atrial tachycardia. Thus, this reversal in expression can induce both SND and ectopic myocardial automaticity. These studies provide compelling evidence that a reversal in HCN channel expression levels between sinoatrial node cells and other cardiac myocytes can contribute to the pathogenesis of ectopic tachyarrhythmia.
Huang et al. [31] reported a reduction in HCN2 and HCN4 channel expression in sinoatrial node cells with age. In the current study, we also found that aged dogs expressed lower levels of HCN2 and HCN4 channel mRNA and protein in the sinoatrial node with concomitant increased expression in the atrium and pulmonary veins. These changes in the regional expression of HCN channels may contribute to the onset and maintenance of age-related AF.
Conclusions
Changes in atrial electrophysiological indicators in aged dogs revealed sinoatrial node dysfunction. There was a reversal in the local tissue distribution of HCN2 and HCN4 channel mRNA and protein, with a decrease in sinoatrial node expression and increase in atrial and pulmonary vein expression with age. Changes in atrial electrophysiological characteristics and regional HCN channel expression patterns were associated with the onset and maintenance of age-related atrial fibrillation.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (no. 81260069)
References
- 1.Sonmez O, Ertem FU, Vatankulu MA, et al. Novel fibro-inflammation markers in assessing left atrial remodeling in non-valvular atrial fibrillation. Med Sci Monit. 2014;20:463–70. doi: 10.12659/MSM.890635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin T, Acar E, Celikyurt U, et al. Relation of hs-CRP and BNP levels with the atrial spontaneous echo contrast and thrombi in permanent atrial fibrillation patients with different etiologies. Med Sci Monit. 2012;18(2):CR78–87. doi: 10.12659/MSM.882461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen LY, Shen WK. Epidemiology of atrial fibrillation: a current perspective. Heart Rhythm. 2007;4(5):S1–S6. doi: 10.1016/j.hrthm.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8(9):651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 5.Semelka M, Gera J, Usman S. Sick sinus syndrome: a review. Am Fam Physician. 2013;87(10):691–96. [PubMed] [Google Scholar]
- 6.Zorn-Pauya K, Schaffer P, Pelzmann B, et al. If in left human atrium: a potential contributor to atrial ectopy. Cardiovasc Res. 2004;64:250–59. doi: 10.1016/j.cardiores.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Chen YJ, Chen SA, Chen YC, et al. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation. 2001;104(23):2849–54. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- 8.DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res. 2006;53:399–406. doi: 10.1016/j.phrs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Santoro B, Liu DT, Yao H, et al. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–29. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 10.Zorn-Pauly K, Schaffer P, Pelzmann B, et al. If in left human atrium: a potential contributor to atrial ectopy. Cardiovasc Res. 2004;64(2):250–59. doi: 10.1016/j.cardiores.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Zicha S, Fernandez-Velasco M, Lonardo G, et al. Sinus node dysfunction and hyperpolarization-activated (HCN)channel subunit remodeling in a canine heart failure model. Cardiovasc Res. 2005;66:472–81. doi: 10.1016/j.cardiores.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Yeh YH, Burstein B, Qi XY, et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia. Circulation. 2009;119:1576–85. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- 13.Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society Physiologist. 1996;39:199, 208–211. [PubMed] [Google Scholar]
- 14.ten Velde I, de Jonge B, Verheijck EE, et al. Spatial distribution of connexin43, the major cardiac gap junction protein, visualizes the cellular network forimpulse propagation from sinoatrial node to atrium. Circ Res. 1995;76(5):802–11. doi: 10.1161/01.res.76.5.802. [DOI] [PubMed] [Google Scholar]
- 15.Toda N. Age-related changes in the transmembrane potential of isolated rabbit sino-atrial. Cardiovasc Res. 1980;14(1):58–63. doi: 10.1093/cvr/14.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Dun W, Boyden PA. Aged atria: electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol. 2009;25(1):9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anyukhovsky EP, Sosunov EA, Chandra P, et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66(2):353–63. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Gomes JA, Kang PS, Matheson M, et al. Coexistence of sick sinus rhythm and atrial flutter-fibrillation. Circulation. 1981;63(1):80–86. doi: 10.1161/01.cir.63.1.80. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Liu E, Liu T, et al. Atrial electrical remodeling in a canine model of sinus node dysfunction. Int J Cardiol. 2011;146(1):32–36. doi: 10.1016/j.ijcard.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54(2):230–46. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 21.Viscomi C, Altomare C, Bucchi A, et al. C terminus-mediated control of voltage and cAMP gating of hyperpolarization-activated cyclic nucleotide-gated channels. J Bio Chem. 2001;276:29930–34. doi: 10.1074/jbc.M103971200. [DOI] [PubMed] [Google Scholar]
- 22.Porciatti F, Pelzmann B, Cerbai E, et al. The pacemaker current I(f) in single human atrial myocytes and the effect of beta-adrenoceptor and A1-adenosine receptor stimulation. Br J Pharmacol. 1997;122(5):963–69. doi: 10.1038/sj.bjp.0701473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zorn-Pauly K, Schaffer P, Pelzmann B, et al. If in left human atrium: a potential contributor to atrial ectopy. Cardiovasc Res. 2004;64(2):250–59. doi: 10.1016/j.cardiores.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Zorn-Pauly K, Schaffer P, Pelzmann B. If in left human atrium: a potential contributor to atrial ectopy. Cardiovasc Res. 2004;64:250–59. doi: 10.1016/j.cardiores.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Kuwahara K, Saito Y, Ogawa E, et al. The neuron-restrictive silencer element-neuron-restrictive silencer factor system regulates basal and endothelin 1-inducible atrial natriuretic peptide gene expression in ventricular myocytes. Mol Cell Biol. 2001;21(6):2085–97. doi: 10.1128/MCB.21.6.2085-2097.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuwabara Y, Kuwahara K, Takano M, et al. Increased expression of HCN channels in the ventricular myocardium contributes to enhanced arrhythmicity in mouse failing hearts. J Am Heart Assoc. 2013;2(3):e000150. doi: 10.1161/JAHA.113.000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JY, Wang HJ, Xu B, et al. Hyperpolarization activated cation current (I(f)) in cardiac myocytes from pulmonary vein sleeves in the canine with atrial fibrillation. J Geriatr Cardiol. 2012;9(4):366–74. doi: 10.3724/SP.J.1263.2012.04161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stillitano F, Lonardo G, Giunti G, et al. Chronic atrial fibrillation alters the functional properties of if in the human atrium. J Cardiovasc Electrophysiol. 2013;24(12):1391–400. doi: 10.1111/jce.12212. [DOI] [PubMed] [Google Scholar]
- 29.Zicha S, Fernandez-Velasco M, Lonardo G, et al. Sinus node dysfunction and hyperpolarization-activated (HCN)channel subunit remodeling in a canine heart failure model. Cardiovasc Res. 2005;66:472–81. doi: 10.1016/j.cardiores.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Yeh YH, Burstein B, Qi XY, et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia. Circulation. 2009;119:1576–85. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Yang P, Du Y, et al. Age-related down-regulation of HCN channels in rat sinoatrial node. Basic Res Cardiol. 2007;102(5):429–35. doi: 10.1007/s00395-007-0660-5. [DOI] [PubMed] [Google Scholar]