Abstract
Objective(s):
Hypothermia and decompressive craniectomy (DC) have been shown to be neuroprotective. This study was designed to evaluate neuroprotective effects of delayed singular or combination of DC and local hypothermia on stroke.
Materials and Methods:
Cerebral ischemia was induced in 48 Wistar rats assigned to 4 groups: control, decompressive craniectomy (DC), local hypothermia (LH), combination of hypothermia and craniectomy (HC). Infarct size and BBB disruption were measured 48 hr after ischemia insult. Neurological deficits were assessed at 24 and 48 hr after stroke by using sticky tape test, hanging-wire test and Bederson’s scoring system. BBB disruption was measured by Evans blue dye leakage.
Results:
Although infarct size was significantly reduced in LH, DC and HC groups (P<0.001), combination therapy was more neuroprotective compared to craniectomy alone (P<0.01). BBB disruption was significantly reduced in DC (P< 0.05) and LH and HC (P< 0.01).While sticky tape test (P<0.05 at 24 hr; P<0.001 at 48 hr) and hanging-wire test (P<0.05) showed better behavioral performance only in HC, Bederson test showed improved behavioral functions of both LH (P<0.05 at 24 hr and P<0.01 at 48 hr) and HC animals (P<0.01). Neurological deficits were also decreased in LH (P<0.05) or HC (P<0.05 at 24 hr; P<0.01 at 48 hr) groups compared to the DC group at the same time.
Conclusion:
Based on our data, although both delayed local hypothermia and craniectomy are protective after stoke, combination therapy of them is more neuroprotective than given alone.
Keywords: Decompressive craniectomy, Local hypothermia, Stroke
Introduction
Ischemic stroke is the third leading cause of death and the first leading cause of disability in adults around the globe. Stroke is accompanied by a robust inflammatory response, glutamate mediated excitotoxicity, release of reactive oxygen species and the initiation of apoptosis(1). Malignant middle cerebral artery infarction is the most common cause of death during the first week after an ischemic stroke (2). It is defined as a brain infarction affecting most or all of the MCA territory, accompanied within days by a massive brain edema causing increased intracranial pressure (ICP), clinical deterioration and transtentorial herniation. The therapeutic window of acute ischemic stroke for thrombolysis drug usage is short and has side effects including higher risk of intracranial hemorrhage (ICH) (3). Because of this and other complications, use of thrombolysis drug like recombinant tissue plasminogen activator (rt-PA) is limited.
Some experimental research and clinical works have reported the neuroprotective effect of mild hypothermia (MH) and moderate hypothermia (4-6). There are some mechanisms that proven the neuroprotective effect of MH. Stabilization of the blood brain barrier, down regulation of cerebral metabolism, decrease of excitatory transmitter release and decrease generation of free radicals are of some neuroprotective mechanisms of hypothermia (7, 8). Several excellent articles have reviewed the protective effects of hypothermia as function of onset time, duration, and depth of hypothermia, as well as its underlying protective mechanisms (9-11). Systemic hypothermia is used in most of adult patients. This procedure is induced by different methods including: surface-cooling blankets and adhesive pads, the use of an endovascular cooling system and sometimes cold intravenous fluids. Prolonged systemic hypothermia however has some side effects including myocardial arrhythmia, dehydration and blood hypercoagulability (12).
There are some experimental and clinical studies indicate that decompressive craniectomy (DC) significantly reduced mortality and improved clinical outcomes (13-16). As an alternative therapy, surgical decompression techniques (large hemicraniectomy with durotomy) have been proposed to relieve the high intracranial pressure (17-19). On the other hand, combination therapies of hypothermia with some strategies and drugs have been evaluated. Combined general hypothermia and DC yield significant additional benefit including infarct size and neurological scores compared with singular effect of them (20). The combination of MH and minocycline had a small but not significantly additional effect over the single treatments after focal cerebral ischemia in the rat (21).
Although it has been shown that selective brain hypothermia was neuroprotective in experimental stroke (22) but , to our knowledge, there are no data available indicating therapeutic effect of direct local cerebral hypothermia with craniectomy in acute ischemic stroke. Therefore, we investigated whether a combination of DC with the local hypothermia, may provide more neuroprotection during the permanent MCAO (middle cerebral artery occlusion) model of stroke in rats.
Material and Methods
Surgical preparation, animals and experimental groups
Experimental protocols were approved by animal ethic committee of Rafsanjan University of Medical Sciences. Rats had free access to food and water during the experiments.
Male Wistar rats were anesthetized with halothane 1% to 1.2% under spontaneous respiration in an air-oxygen mixture. Rectal temperature was maintained between 37°C and 37.5°C with a thermostat-controlled heating pad. The right femoral artery was cannulated and physiological parameters including rectal temperature, mean blood pressure, pH, pO2, and pCO2, were monitored.
Forty eight male Wistar rats (weighing 220 to 300 g) were allocated to 4 treatment groups of 12 animals each (8 for infarct volume and neurological outcomes and 4 for Evans blue) as following: control, decompressive craniectomy (DC), local hypothermia (LH), hypothermia combined with craniectomy (HC). The duration of the surgery did not exceed 30 min in any case. The LH and DC were induced 6 hr after MCAO. All animals were euthanized at 48 hr after MCAO.
Induction of MCAO
Focal cerebral ischemia was induced using a modified intraluminal suture method as described previously (23). In brief, the bifurcation of right common carotid artery was exposed through a midline incision. The internal carotid artery and external carotid artery were distally dissected, free from the surrounding tissues under an operating microscope. After ligation of the external and common carotid arteries, a 3–0 silicon rubber-coated monofilament was gently inserted through the common carotid artery into the internal carotid artery to 18-20 mm beyond the carotid bifurcation at the base of the middle cerebral artery. Then, the distal end of monofilament was tied up and the wound was closed using the surgical suture. The cerebral blood flow (CBF) in parietal cortex was continuously measured with laser doppler flowmetry (Moor Instrument, England). The filament was advanced until laser doppler flowmetry indicated adequate MCA occlusion by a sharp decrease in ipsilateral blood flow. The rat was considered as a successful occlusion when its CBF reduced at least to 30% of initial flow.
Induction of hypothermia and decompressive craniectomy
Local hypothermia was induced 6 hours after MCAO. An incision on scalp skin was made and the parietal and temporal bone was exposed entirely. Then the scalp was kept cold for 15 min by pouring 4°C normal saline (two drops per second) over it. The temperature was measured by contralateral temporalis muscle probe. Contralateral temporalis muscle temperature has been reported to approximate closely to the intraparenchymal brain temperature (24). Hypothermia in HC group was induced after craniectomy at 6 hour after MCAO. DC was performed in animals by creating a bone flap (10 × 5 mm) in the temporal and parietal bone with the use of a dental drill. Additional bone was removed under microscopic control with the use of microscissors (14, 20). The Dura matter was then opened in a cruciate incision. No cortical resection of infracted brain was attempted. At the end of the procedure, the temporalis muscle and skin flap were adapted and sutured in place.
Measurement of infarct volume
At 48 hr after stroke, rats were decapitated; brains removed and cut in to 2 mm coronal sections using a rat brain matrix. Infarct volume was quantified in sections stained with 2% 2,3,5-triphenyltetrazolium chloride using image J analyzer (NIH Image, version 1.61) (25). The total volume of each hemisphere and infarction was determined by integration of distance of the 6 sections. Infarctions were adjusted to the size of contralateral hemisphere by applying the following formula: Infarct volume = (volume of left hemisphere- (volume of right hemisphere-measured infarct volume))/volume of left hemisphere (26). For these analyses, the experimenter was blinded to the treatment assignations.
Behavioral testing
Rats were trained on the adhesive removal test of sensorimotor function (27), daily for 3 days before stroke. Animals were then tested before surgery and at 24 and 48 hr post-stroke. Time taken to touch and remove each stimulus from the limb was recorded during 3 trials and averaged. Behavioral tests were carried out by an observer blind to the experimental groups.
Neurological deficits were recorded at 24 and 48 hr after stroke and determined with a modified 6-point scoring system of Bederson and coworkers (28) as follows: 0, no observable deficit; 1, forelimb flexion; 2, forelimb flexion plus decreased resistance to lateral push; 3, unidirectional circling; 4, unidirectional circling plus decreased level of consciousness; and 5, death. Endurance in the wire-hanging test was measured on a horizontal steel wire (1 mm) stretched horizontally 50 cm above a foam pad. For the pole test, the rat was placed head upward on the top of a vertical wooden rough-surfaced pole (diameter 1 cm, height 50 cm). Each rat was habituated on the day before testing then allowed to descend 5 times on a single session. The total time needed to turn completely head downward (“time-to-turn”) and the time until the animal reached the floor with its 4 paws (“time-to-come-down”) was recorded. Results were expressed as the mean of the 5 trials (29).
Determination of BBB permeability
BBB permeability was determined using Evans blue dye )EB) extravasation technique, as described earlier (30, 31). Briefly, a catheter was placed into the jugular vein through which 4 ml/kg of EB solution (2% Evans blue in normal saline, Sigma, Germany) was infused into the animal during 5 min period. Then, the animal was allowed to completely recover for at least 30 min. After killing the animal with an overdose of sodium thiopental and opening the chest, a catheter was placed into the left ventricle to infuse 300 ml of warm normal saline (37°C) for 15 min in order to washout the remains of EB from general circulation. Following decapitation, the brain was gently excised, its cerebellum and olfactory bulb were separated, and the rest was divided into the right and left hemispheres with a brain matrix. Each hemisphere was carefully weighed and absorption was measured by a spectrophotometer (UV7500, Spectro Lab, England) at 620 nm.
Statistical analysis
Data are expressed as mean ± S.E.M. Physiological parameters and behavioral tests were compared by two-way ANOVA and individual differences determined by Tukey’s test. Infarct volume and BBB permeability were compared by one-way ANOVA followed by Tukey’s test. A value of P < 0.05 was considered to be significant.
Results
There were no statistically significant differences between groups for intra operative physiological indices (Table 1).
Table 1.
Physiological parameters of rats male wister in different group
| Control | DC | LH | HC | |
|---|---|---|---|---|
| Glucose (mg/dl) | 223±13 | 208±26 | 218±20 | 219 ± 21 |
| pH | 7.389± 0.016 | 7.377 ± 0.005 | 7.403± 0.021 | 7.378±0.008 |
| pO2 (mmHg) | 176 ± 9 | 174 ± 7 | 175 ± 6 | 169 ± 9 |
| pCO2 (mmHg) | 35.5 ± 1.5 | 33.5 ± 1.7 | 33.9 ± 1.3 | 34.0 ± 1.6 |
| *MAP (mmHg) | 92 ± 4 | 93 ± 3 | 94 ± 3 | 92 ± 4 |
| Heart rate (beats/min.) | 369 ± 36 | 379 ± 29 | 381 ± 33 | 359 ± 28 |
Data are presented as mean±SEM. n = 4 in each group. Differences between the groups were not significant (P>0.05).
MAP, mean arterial pressure
Infarct volume
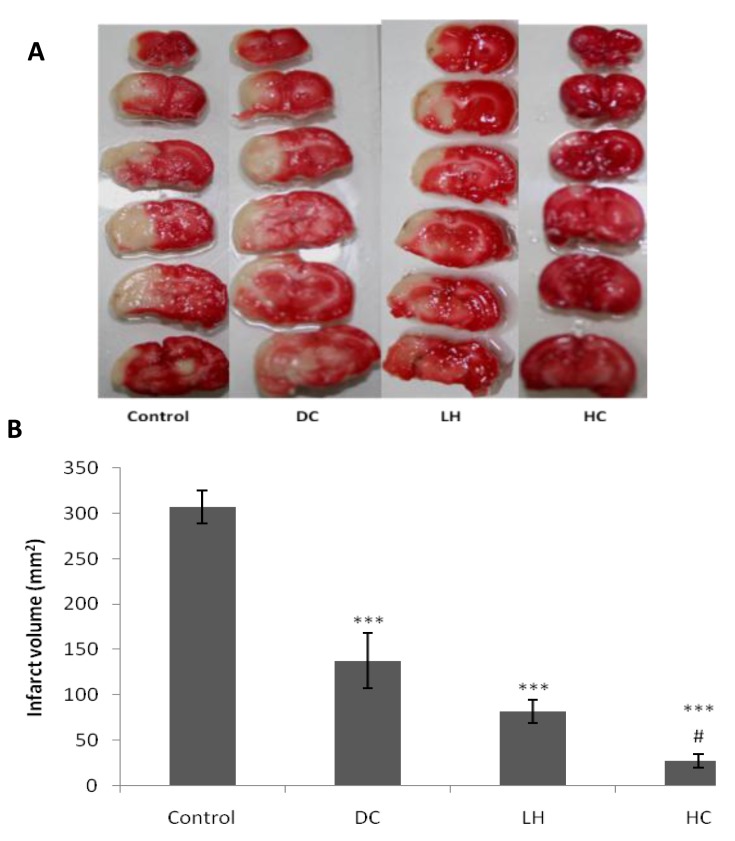
Infarct volumes were calculated using TTC-Stained brain sections. Infarct volume in control group was 307.34 ± 18.09 mm3 and the difference between control and hypothermia (82.05 ± 12.86 mm3), craniectomy (137.88 ± 30.38 mm3) and HC (27.40 ± 17.61 mm3) groups were statistically significant (P < 0.001; Figure 1). Combination therapy with LH and DC were more neuroprotective than DC alone (P<0.01; Figure 1).
Figure 1.
Effect of decompressive craniectomy (DC) and local hypothermia (LH) alone or in combination (HC) on Infarct volume. Infarct volume was calculated by TTC scanned sections (A) at 48 hr after stroke and expressed as mean ± SEM (B) *** P< 0.001 in compare with control group. # P< 0.01 is in comparison with craniectomy group
Neurological tests
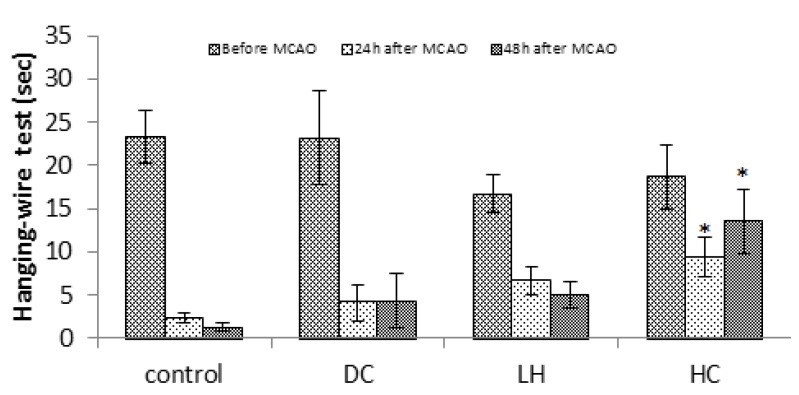
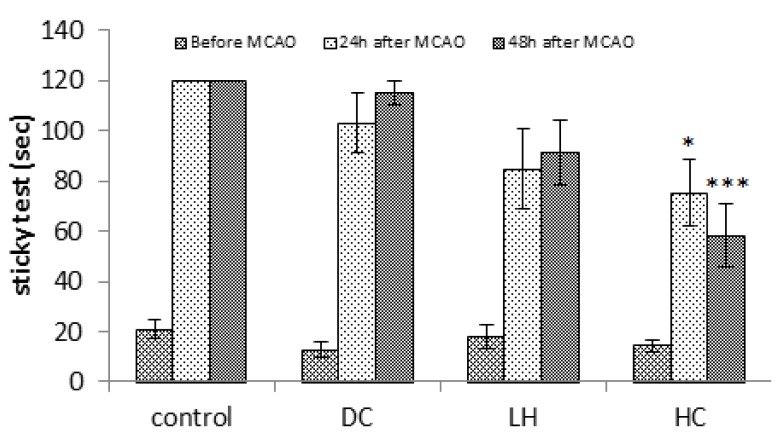
Data of hanging test showed that only HC significantly increased endurance time at 24 or 48 hr after ischemia (P<0.01), but neither LH nor DC showed any significant difference (Figure 2). While compared to the control group, sticky tape test (P<0.05 at 24 hr; P<0.001 at 48 hr; Figure 3) showed better behavioral performance only in HC, Bederson test showed improved behavioral functions of both LH (P<0.05 at 24 hr and P<0.01 at 48 hr) and HC animals (P<0.01). Neurological deficits were also decreased in LH (P<0.05) or HC (P<0.05 at 24 hr; P<0.01 at 48 hr) groups compared to the DC group at the same times (Figure 4).
Figure 2.
Effect of decompressive craniectomy (DC) and local hypothermia (LH) alone or in combination (HC) on hanging-wire test in rats. Data are expressed as mean ± SEM. N= 8 in each group. * P< 0.05 compared with control group
Figure 3.
Effect of decompressive craniectomy (DC) and local hypothermia (LH) alone or in combination (HC) on sticky neurological test in rats. Data are expressed as mean ± SEM. N= 8 in each group. *P< 0.05 and *** P< 0.001 compared to the control group
Figure 4.
Effect of decompressive craniectomy (DC) and local hypothermia (LH) alone or in combination (HC) on neurological deficits at 24 and 48 hr after stroke in rats. Data are expressed as mean ± SEM. N= 8 in each group. * P<0.05 compared to the control group. ** P< 0.01 compared to the control group. # P< 0.05 compared to the DC group. ## P< 0.01 compared to the DC group
Blood brain barrier disruption
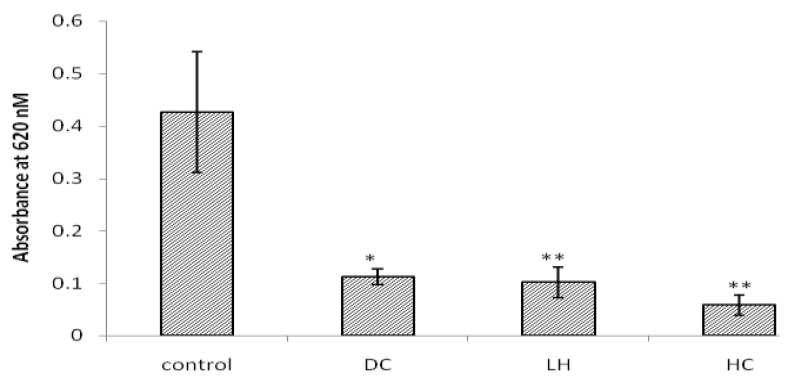
EB penetration through BBB measured using absorption value in spectrophotometry. Absorption values in control, DC, LH and HC were 0.42 ± 0.11, 0.11 ± 0.01, 0.10 ± 0.02 and 0.05 ± 0.01, respectively. Compared to the control group, DC (P<0.05) LH and HC (P<0.01) significantly decreased absorption (Figure 5). Although mean absorption were more decreased in combination therapy, there were no significant differences between it and the other groups.
Figure 5.
Effect of decompressive craniectomy (DC) and local hypothermia (LH) alone or in combination (HC) on BBB disruption. Data are expressed as mean ± SEM. N= 4 in each group. Spectrophotometry absorption in 620 nm reveals blood brain barrier disruption and Evans blue penetration. * P< 0.05 in compare with control group. ** P< 0.01 in compare with control group
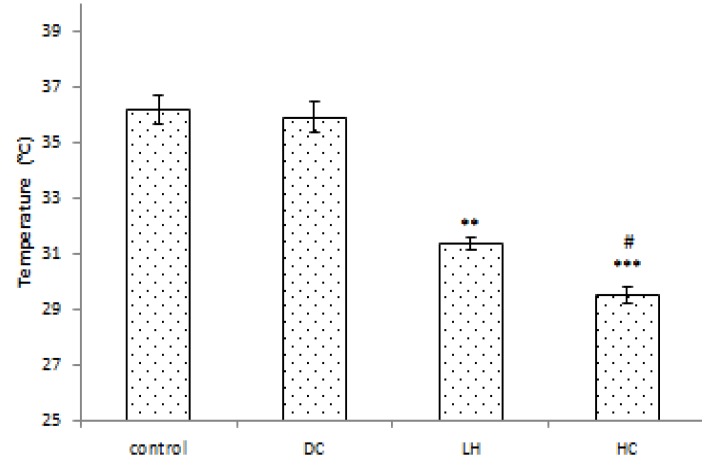
Contralateral temporal muscle temperature
Contralateral temporal muscle temperatures were measured by a temporal probe. As expected, there was no significant difference in brain temperature between the control and DC groups. In both LH (P<0.01) and HC (P<0.001) groups, brain temperature decreased compared to the control group. Compared to the DC group, LH and HC also significantly decreased the brain temperature (P<0.01; Figure 6).
Figure 6.
Effect of craniectomy and hypothermia on brain temperature after local hypothermia. Contralateral temporal muscle temperature was measured as approximate closely temperature to the intraparenchymal brain temperature. Data are expressed as mean ± SEM. N= 5 in each group. ** P< 0.01 compared to the control and DC groups. *** P< 0.001 compared to the control and DC groups. # P< 0.05 compared to the hypothermia group
Discussion
To the best of our knowledge, there are no available data for the effect of directly local brain hypothermia through craniectomy on stroke in rodents. Thus, the novelty of the current study is that we investigated delayed effect of direct brain hypothermia, by opening the scalp, on permanent focal cerebral ischemia in rat. Our data showed that this method is more effective in cerebral ischemia therapies. We observed that both late decompressive craniectomy and local hypothermia decrease infarct volume but combination of them has more neuroprotective effects. Some studies showed that early decompressive craniectomy could significantly decrease infarct volume and mortality rates (24, 32), that were parallel with our study. Many investigators have reported that general hypothermia decreases infarct volume in different models of stroke in rat (4, 8). On the other hand, neuroprotective effect of focal brain hypothermia through scalp has been reported (33) and mentioned it as a effectual, simple, cost-effective and safe hypothermia method in rats.
Since neurological evaluations have more clinical importance in stroke patients, we used batteries of neurological tests in the current study. Quantitive neurological evaluations including sticky tape or hanging-wire tests showed that only combination of both craniectomy and local hypothermia improved behavioral functions. On the other hand, Bederson’s neurological deficits test showed that local hypothermia alone or its combination with craniectomy is neuroprotective. Only a few studies have examined the influence of hypothermia on delayed reperfusion after ischemic injury. In parallel with our findings, it has been suggested that general hypothermia also improved neurological performances in temporary middle cerebral artery occlusion (5). More interestingly, when applied up to 6 hr after ischemia insult, general hypothermia has decreased neurological deficits after stroke (34).
Furthermore, we observed that both craniectomy and local hypothermia prevent BBB disruption and although combination of them was more effective, this difference was not significant. It has been recently reported that combination of general hypothermia and minocycline may also prevent BBB disruption through matrix metalloproteinase (MMP) inhibition in focal cerebral ischemia (21).
Different neuroprotective mechanisms for hypothermia have been postulated including: stabilization of the blood brain barrier, down regulation of cerebral metabolism, elimination of excitatory transmitter release and reduction of free radical generation (7, 8, 35). It has been suggested that the attenuation of oxidative DNA damage and DNA damage triggered pro-death signaling events can be an important mechanism for neuroprotective effect of mild hypothermia in cerebral ischemia (36). Protection of basal lamina, reduction in infarct volume and hemorrhage, and reduction in proteolytic enzymes are some of other neuroprotective mechanisms of hypothermia (37). Reduction of neuronal inflammation was also mentioned as a main contributor in neuroprotective effects of post-ischemic mild hypothermia (38). Despite of these advantages of general hypothermia, some adverse effects have been limited its using such as myocardial arrhythmia, hypotension, blood hypercoagulability, and hemodynamic instability (12, 39).
A comparison between different methods of hypothermia including intracarotid cold saline infusion, ice cap, and systemic cooling was investigated showing that the former was more effective than other methods (40). Hence, these findings suggest that methods like intracarotid cold saline infusion and our current method of directly exposed brain cooling are strategies that not only reveal more neuroprotective effects of hypothermia but also prevent the side effects of general hypothermia.
Combinations of hypothermia with other methods have been evaluated in some studies. One study reported that combination therapy of hypothermia with MgSO4 reduced infarct volumes at 2 hr and 4 hr after permanent MCAO but there was no therapeutic effect at 6 hr or in hypothermia and MgSO4 groups singularly (41). Another study showed that combination of citicoline with hypothermia suppressed apoptotic processes that are more effective than using each of them lonely (42). Jieyong et al showed that the combination between craniectomy and mild hypothermia reduce the infarction size (43). Doerfler et al (2001) showed that combination of decompressive craniectomy at 4 hr after MCAO with mild hypothermia have diminished the infarct volume and improved neurological outcomes (20). We increased the therapeutic window of cerebral ischemia to 6 hr by directly local cerebral hypothermia through combination of craniectomy and using of cold saline.
In contrast with the above, we used direct local cerebral hypothermia for 15 min in our experiment. The brain temperature was decreased to 31.5°C in local hypothermia group and 28.5°C in combination therapy group, while body temperature was maintained at normal range by warm pad (36.5-37°C). This temperature is lesser than the mild hypothermia which used in most studies (33°C) (20, 39). Therefore, we decreased the complication of general hypothermia whereas we increased the depth of hypothermia. Our study has some limitations. The end point of our study was 48 hr because the animals were become sick and they loosed weight. This end point time was not enough for more evaluation of delayed outcomes.
Conclusion
Our findings reveal that although late decompressive craniectomy and local hypothermia decrease infarct volume, delayed directly brain hypothermia, by opening the scalp, is more neuroprotective in stroke therapy. Conversely, only combination of craniectomy and local hypothermia could improve behavioral functions. Further studies are needed to evaluate the best starting time of hypothermia, durations as well as its exact neuroprotective mechanisms.
Acknowledgment
This study was supported by the financial aid of Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
References
- 1.Allahtavakoli M, Shabanzadeh AP, Sadr SS, Parviz M, Djahanguiri B. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, reduces infarction volume and neurological deficits in an embolic model of stroke. Clin Exp Pharmacol Physiol. 2006;33:1052–1058. doi: 10.1111/j.1440-1681.2006.04486.x. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 3.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1588. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Hamann GF, Burggraf D, Martens HK, Liebetrau M, Jager G, Wunderlich N, et al. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke. 2004;35:764–769. doi: 10.1161/01.STR.0000116866.60794.21. [DOI] [PubMed] [Google Scholar]
- 5.Kollmar R, Blank T, Han JL, Georgiadis D, Schwab S. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 2007;38:1585–1589. doi: 10.1161/STROKEAHA.106.475897. [DOI] [PubMed] [Google Scholar]
- 6.Saini M, Saqqur M, Kamruzzaman A, Lees KR, Shuaib A. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke. 2009;40:3051–3059. doi: 10.1161/STROKEAHA.109.556134. [DOI] [PubMed] [Google Scholar]
- 7.Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997;14:171–201. doi: 10.1007/BF02740655. [DOI] [PubMed] [Google Scholar]
- 8.Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461–2466. doi: 10.1161/01.str.29.12.2461. [DOI] [PubMed] [Google Scholar]
- 9.Lyden TMHaPD. Hypothermia after acute ischemic stroke. J Neurotrauma. 2009;26:387–391. doi: 10.1089/neu.2008.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Worp HB, Macleod MRM, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials. J Cereb Blood Flow Metab. 2010;30:1079–1093. doi: 10.1038/jcbfm.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmen MAYa TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke. 2010;41:S72–S74. doi: 10.1161/STROKEAHA.110.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1994;14:620–627. doi: 10.1038/jcbfm.1994.77. [DOI] [PubMed] [Google Scholar]
- 13.Doerfler A, Forsting M, Reith W, Staff C, Heiland S, Schabitz WR, et al. Decompressive craniectomy in a rat model of "malignant" cerebral hemispheric stroke: experimental support for an aggressive therapeutic approach. J Neurosurg. 1996;85:853–859. doi: 10.3171/jns.1996.85.5.0853. [DOI] [PubMed] [Google Scholar]
- 14.Forsting M, Reith W, Schabitz WR, Heiland S, von Kummer R, Hacke W, et al. Decompressive craniectomy for cerebral infarction. An experimental study in rats. Stroke. 1995;26:259–264. doi: 10.1161/01.str.26.2.259. [DOI] [PubMed] [Google Scholar]
- 15.Flechsenhar J, Woitzik J, Zweckberger K, Amiri H, Hacke W, Juttler E. Hemicraniectomy in the management of space-occupying ischemic stroke. J Clin Neurosci. 2013;20:6–12. doi: 10.1016/j.jocn.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Els T, Oehm E, Voigt S, Klisch J, Hetzel A, Kassubek J. Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovasc Dis. 2006;21:79–85. doi: 10.1159/000090007. [DOI] [PubMed] [Google Scholar]
- 17.Delashaw JB, Broaddus WC, Kassell NF, Haley EC, Pendleton GA, Vollmer DG, et al. Treatment of right hemispheric cerebral infarction by hemicraniectomy. Stroke. 1990;21:874–881. doi: 10.1161/01.str.21.6.874. [DOI] [PubMed] [Google Scholar]
- 18.Foerch C, Lang JM, Krause J, Raabe A, Sitzer M, Seifert V, et al. Functional impairment, disability, and quality of life outcome after decompressive hemicraniectomy in malignant middle cerebral artery infarction. J Neurosurg. 2004;101:248–254. doi: 10.3171/jns.2004.101.2.0248. [DOI] [PubMed] [Google Scholar]
- 19.Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–2517. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- 20.Doerfler A, Schwab S, Hoffmann TT, Engelhorn T, Forsting M. Combination of decompressive craniectomy and mild hypothermia ameliorates infarction volume after permanent focal ischemia in rats. Stroke. 2001;32:2675–2681. doi: 10.1161/hs1101.098369. [DOI] [PubMed] [Google Scholar]
- 21.Nagel S, Su Y, Horstmann S, Heiland S, Gardner H, Koziol J, et al. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008;1188:198–206. doi: 10.1016/j.brainres.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz AE, Finck AD, Stone JG, Connolly ES, Edwards NM, Mongero L. Delayed selective cerebral hypothermia decreases infarct volume after reperfused stroke in baboons. J Neurosurg Anesthesiol. 2011;23:124–130. doi: 10.1097/ANA.0b013e3181fa75ca. [DOI] [PubMed] [Google Scholar]
- 23.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Onesti ST, Baker CJ, Sun PP, Solomon RA. Transient hypothermia reduces focal ischemic brain injury in the rat. Neurosurgery. 1991;29:369–373. doi: 10.1097/00006123-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Allahtavakoli RM, Arababadi NK, Shamsizadeh A, Javanmardi K. Delayed post ischemic treatment with Rosiglitazone attenuates infarct volume, neurological deficits and neutrophilia after embolic stroke in rat. Brain Res. 2009;1271:121–127. doi: 10.1016/j.brainres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 26.Rezazadeh H, Hoseini Kahnuee M, Roohbakhsh A, Shamsizadeh A, Rahmani MR, Bidaki R, et al. Neuroprotective consequences of postconditioning on embolic model of cerebral ischemia in rat. Iran J Basic Med Sci. 2013;16:144–149. [PMC free article] [PubMed] [Google Scholar]
- 27.Whishaw IQ, Kolb B. The Behavior of the Laboratory Rat. 1st. New York: Oxford University Press; 2005. [Google Scholar]
- 28.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 29.Prinz V, Laufs U, Gertz K, Kronenberg G, Balkaya M, Leithner C, et al. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39:433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- 30.Awad AS. Role of AT1 receptors in permeability of the blood-brain barrier in diabetic hypertensive rats. Vascul Pharmacol. 2006;45:141–147. doi: 10.1016/j.vph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Mohammadi MT, Shid-Moosavi SM, Dehghani GA. Contribution of nitric oxide synthase (NOS) in blood-brain barrier disruption during acute focal cerebral ischemia in normal rat. Pathophysiology. 2012;19:13–20. doi: 10.1016/j.pathophys.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Cho DY, Chen TC, Lee HC. Ultra-early decompressive craniectomy for malignant middle cerebral artery infarction. Surg Neurol. 2003;60:227–232. doi: 10.1016/s0090-3019(03)00266-0. [DOI] [PubMed] [Google Scholar]
- 33.Clark DL, Colbourne F. A simple method to induce focal brain hypothermia in rats. J Cereb Blood Flow Metab. 2007;27:115–122. doi: 10.1038/sj.jcbfm.9600327. [DOI] [PubMed] [Google Scholar]
- 34.Kollmar R, Schabitz WR, Heiland S, Georgiadis D, Schellinger PD, Bardutzky J, et al. Neuroprotective effect of delayed moderate hypothermia after focal cerebral ischemia: an MRI study. Stroke. 2002;33:1899–1904. doi: 10.1161/01.str.0000019603.29818.9c. [DOI] [PubMed] [Google Scholar]
- 35.Baker CJ, Fiore AJ, Frazzini VI, Choudhri TF, Zubay GP, Solomon RA. Intraischemic hypothermia decreases the release of glutamate in the cores of permanent focal cerebral infarcts. Neurosurgery. 1995;36:994–1001. doi: 10.1227/00006123-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Ji X, Luo Y, Ling F, Stetler RA, Lan J, Cao G, et al. Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: a mechanism for neuroprotection. Front Biosci. 2007;12:1737–1747. doi: 10.2741/2185. [DOI] [PubMed] [Google Scholar]
- 37.Burk J, Burggraf D, Vosko M, Dichgans M, Hamann GF. Protection of cerebral microvasculature after moderate hypothermia following experimental focal cerebral ischemia in mice. Brain Res. 2008;1226:248–255. doi: 10.1016/j.brainres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Ohta H, Terao Y, Shintani Y, Kiyota Y. Therapeutic time window of post-ischemic mild hypothermia and the gene expression associated with the neuroprotection in rat focal cerebral ischemia. Neurosci Res. 2007;57:424–433. doi: 10.1016/j.neures.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Goto Y, Kassell NF, Hiramatsu K, Soleau SW, Lee KS. Effects of intraischemic hypothermia on cerebral damage in a model of reversible focal ischemia. Neurosurgery. 1993;32:980–984. doi: 10.1227/00006123-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Ji Y, Wu Y, Ji Z, Song W, Xu S, Wang Y, et al. [Comparison of neuroprotective effects of hypothermia induced by different methods in rats with early cerebral ischemia] Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:89–92. [PubMed] [Google Scholar]
- 41.Campbell K, Meloni BP, Knuckey NW. Combined magnesium and mild hypothermia (35 degrees C) treatment reduces infarct volumes after permanent middle cerebral artery occlusion in the rat at 2 and 4, but not 6 h. Brain Res. 2008;1230:258–264. doi: 10.1016/j.brainres.2008.06.110. [DOI] [PubMed] [Google Scholar]
- 42.Sahin S, Alkan T, Temel SG, Tureyen K, Tolunay S, Korfali E. Effects of citicoline used alone and in combination with mild hypothermia on apoptosis induced by focal cerebral ischemia in rats. J Clin Neurosci. 2010;17:227–231. doi: 10.1016/j.jocn.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Jieyong B, Zhong W, Shiming Z, Dai Z, Kato Y, Kanno T, et al. Decompressive craniectomy and mild hypothermia reduces infarction size and counterregulates Bax and Bcl-2 expression after permanent focal ischemia in rats. Neurosurg Rev. 2006;29:168–172. doi: 10.1007/s10143-005-0010-8. [DOI] [PubMed] [Google Scholar]