Abstract
Objective(s):
The aim of this study was to evaluate distribution and changes of glycoconjugates of retinal photoreceptors during both pre- and postnatal development.
Materials and Methods:
Tissue sections from days 15 to 20 of Wistar rat embryos and 1 to 12 postnatal days of rat newborns including developing eye were prepared for lectinhistochemistry technique. Horseradish peroxidase (HRP)-labeled lectins including Vicia villosa (VVA), peanut agglutinin (PNA), Maclura pomifera (MPA) and wheat germ agglutinin (WGA-ІІ) were used. Alcian blue (pH 2.5) was used for counterstaining.
Results:
Interphotoreceptor matrix (IPM) plays a crucial role in photoreceptors differentiation and acts as a mediator in interactions between photoreceptors and retinal pigment epithelium (RPE). Specific cell surface glycoconjugates secreted from cone cells could help us to distinguish these cells from rod photoreceptors. Our results for the first time revealed the strong reaction of cone photoreceptors with the cone-specific lectin (PNA) at postnatal day 12 (P12). Postnatal day 12 can be determined as the final differentiation of cone photoreceptors.
Conclusion:
According to our findings, we suggest that the generation of the eye photoreceptors begins from pre- natal period and their final differentiations will continue to postnatal period. Glycoconjugates including (β-D-Gal [1-3]-D-GalNac) and (β-D-Gal) terminal sugars play a critical role in the pre- and postnatal development and differentiation of retinal photoreceptors.
Keywords: Development, Lectin histochemistry, Photoreceptors, Retina
Introduction
Development of the vertebrate multilayered retina is dependent on the interactions between surface ectoderm, neuroepithelium, and mesenchy-mal cells from embryonic mesoderm (1, 2). Retina is one of the best structural models for developmental and histochmical studies of the nervous system (3).
In retina, light is absorbed by retinal neurons, transmitted to photoreceptors and then dissemin-ated over several cell layers where it is moderated by lateral connections by means of horizontal and amacrine cells. Finally the visual object is transmitted to fibers that make the optic nerve and leave the eye toward the brain for advance processing (4, 5).
During the eye development, progressive process of retina differentiation starts with neural retina domain inside the optic vesicle neuroepithelium (6, 7). All cells in the retina generally develop from retinal neuroepithelial tissue (8, 9).
Retinal photoreceptor layer accompanied with retinal pigment epithelium (RPE) constitute the outmost part of retina. There are generally two categories of retinal photoreceptors: rods that function only in faint light and respond to single light, cones that function in bright light (operate color vision and support high resolution of visual images) (10). Observations and comparison between human and laboratory animals (mouse) showed that photoreceptors and their subtypes form the largest number of retinal cells (3, 12).
Pre- and postnatal studies on mouse showed that cells across the retina are interfering and overlapping with each other, so that four types of the retinal cells including ganglionic, amacrine, horizontal and cone photoreceptors are generated prenatally. It is important to note that the most abundant retinal cells, rod photoreceptors, are created both pre- and postnatally, with a maximum level of genesis at the first day of birth in the mouse newborns. In other words, cons are the first type of photoreceptor cells that undergo the terminal mitosis to begin their subsequent differentiations, while rods are created later and their differentiations will continue to postnatal period (8, 13, 14).
Variety of epithelial tissue provides different organ- specific purposes in the human body. One of the unique samples of this tissue is the uncommon simple cuboidal epithelium in the eye called the RPE. It is characterized by the high polarity cells containing lysosome-like organelle in order to biosynthesis and packing of the melanin pigment.
RPE is located between choriocapillaris of the choroid layer and photoreceptor cells of the retina.
The embryonic differentiation stages of the RPE demonstrate its exceptional correlation and close association with photoreceptors. The RPE firmly interacts with photoreceptors and it is critical for the survival and appropriate function of these cells. For instance, due to loss of direct blood supply in photo-receptors region, RPE regulates the water, glucose, retinol, fatty acids, and essential ions transportation between neural retina and the choroid layer. It protects photoreceptors against free radicals and provides the renewal conditions by regular daily phagocytosis of photoreceptors outer segment. Additionally, it has been shown that cone cells differentiation requires the RPE presence (15-17).
Inter-photoreceptor matrix (IPM) has a crucial role in the bidirectional correlation between RPE and photoreceptors. Recent investigations showed that sulphated and unsulphated chondroitin are the main IPM constituents (18). Several histochemical studies have proven that the IPM contains special proteins and glycoproteins that cause both cone and rod photoreceptors differentiation and their association with the RPE (18, 19).
At the primary eye development that photorece-ptor outer segment has not been organized yet, the RPE functions as a source of developmental messages and growth factors such as glycoproteins for photoreceptors induction (20).
Lectins are specific carbohydrate-binding proteins that bind polysaccharides and glycoconjugates. They are broadly used in research fields and therapeutic proceedings for the purpose of glycoconjugates detection. Results from several previous histochemical studies on the vertebrate species such as human demonstrated developmental changes of the retina (12, 17).
Various special aspects of photoreceptors and RPE have been evaluated in previous histochemical investigations (21-23). For instance, RPE revealed an intense reaction to lens culinaris (LCA) , wisteria floribunda (WFA) , wheat germ agglutinin (WGA) and Ulex europeus I (UEA1) lectins. This intense reaction is suggested to be through the terminal sugars of this layer such as α-mannose (α-Man), α-glucose (α-Glc), galactose/n-acetylgalactosamine (α-Gal/GalNAc), β-GalNAc, α-fucosidase (α-Fuc), and neuraminic acid (NeuA) (21). In addition, the outer and inner segments of the photoreceptors were stained separately by succinylated wheat germ agglutinin (S-WGA), Griffonia simplicifolia-II (GS-II), UEA-II, UEA-I, Lotus tetragonolobus (LTA), and Maackia amurensis (MAA) lectins, so it can be said that in this region is glycoconjugates rich in α-Fuc and NeuAc(α2,3) Gal residues. This study demonstrated that lectins could be used as markers for specific components of the rat retina (21).
In another study it is shown that two terminal sugars Gal and GalNAc are expressed at the surface of RPE and photoreceptor cells (22).
Another study on cat and monkey species, peanut agglutinin (PNA) and WGA lectins were used in order to compare IPM domains. In the monkey retina, PNA was bound to both cone outer segments and the cone matrix sheath, and WGA specifically bound to the IPM rod cells. In the cat, PNA labeled the region of IPM between the outer borders cone matrix sheath and rod cells matrix and WGA detected cone sheath matrix along with both rod and cone outer segments. According to these results it can be suggested that each photoreceptor has an own-specific extra cellular matrix (ECM)-rich microenvironment (23). In another histochemical study on adults by using the PNA it was suggested that the IPM may contain glycoconjugates that are involved in surveillance and organization of the RPE-photo-receptor complex (24). In this study, we used lectin histochemistry to evaluate the expression pattern of different glycoconjugates in the photoreceptors and RPE and compare the results with other studies. It is also attempted to show the photoreceptors association with RPE and their differentiation during pre- and postnatal. Finally, determination of the exact time of retinal cone cells differentiation was evaluated as our principal goals.
Materials and Methods
Embryos and newborns tissue preparation
For this study, 20 female and 10 male Wistar rats (with an average weight of 250 to 300 g) were obtained from the Faculty of Medicine, Animal Lab, Mashhad University of Medical Sciences. According to NIH instruction all animals were kept under standard environmental conditions including temperature of about 24°C, photoperiod with 12 hr light and 12 hr darkness and adequate access to food and water. In order to mate, for each male two females were placed in specific mating cages.
After 24 hr and ensuring of pregnancy via observation of vaginal plug and using vaginal smear method, pregnant females were separated from males and the day 0 of pregnancy (E0) was determined.
During pregnancy days 15-20, pregnant rats at different stages were killed under deep anesthesia by chloroform. Their embryos were carefully separated from the placenta and embryonic membranes by surgery and immediately they were kept in formalin fixator solution for about 24 hr. Because of their large size, the head of 15-20 days embryos were cut for better infiltration of fixator and also better dehydration at the next step. Newborns at postnatal ages of one day to two weeks (P1 to P12), were processed according to above- mentioned method.
After the fixation, routine histology procedures were performed to obtain paraffin embedded blocks. Prepared paraffin blocks were cut into transverse 5 µm-thick serial sections by a rotary microtome. All abnormal samples were excluded from the study.
Lectin histochemistry
All lectins (Table 1), conjugated with horseradish peroxidase enzyme (HRP) were purchased from Sigma company. Vicia villosa (VVA) for GalNac terminal sugar was used only for composite positive control sections and correspondingly to differentiate our selected areas from other structures of the eyeball.
Table 1.
Tested lectins and their terminal sugar specificities
| Tested lectins | Abbreviation | Carbohydrate-binding specificity |
|---|---|---|
| Peanut agglutinin | PNA | β-D-Gal[1–3]-D-GalNac |
| Maclura pomifera agglutinin | MPA | β-D-Gal |
| Wheat germ agglutinin | WGA-ІІ | GluNac[1–4]Glu |
| Vicia villosa Agglutinin | VVA | GalNac |
Gal: Galactose, GalNac: N-acetylgalactosamine, GulNac: N-acetylglucosamine
Lectins (10 µg/ ml) were diluted in 0.1 M phosphate buffered saline (PBS) pH 6.8 containing 0.02 g magnesium chloride (MgCl2), 0.02 g manganese chloride (MnCl2) and 0.05 g sodium chloride. After standard discharge of 5 µm-thick tissue sections followed by elimination of mercuric chloride pigments, they were incubated with 0.01% H2O2 in methanol for 5-10 min in order to block endogenous peroxidase activity.
At least three sections were stained from each developmental stage with each lectin and one section was exposed to HRP, H2O2 and diaminobenzidine (DAB) as a negative control sample (without using any lectin). In addition, one composite section from each lectin was used as a positive control sample.
The sections were incubated with lectins for 2 hr in a dark wet chamber and placed for 10 min in a DAB solution (0.03 g DAB and 200 µl H2O2 per 100 ml buffer). To stop the DAB reaction, all sections were washed in water for 10 min and then counterstained with a 1% solution of Alcian blue at pH 2.5 for 1 min.
Finally the sections were dehydrated in increa-sing graded ethanol series, cleared in xylene, and mounted on glass slides. In order to detect staining intensity, the reactions of the tested lectins were observed by three examiners separately and blindly with Olympus AH-2 microscopy and then photogra-phed by Bx51/Olympus light microscope. Similar with previous comparable studies lectins reactions were graded using Likert spectrum method based on the reactions intensity with specific lectins (27).
Results
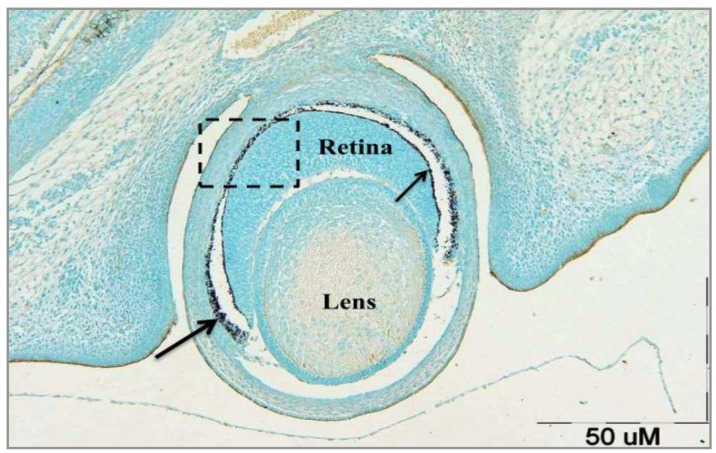
Figure 1 shows a tissue section of eyeball with all essential layers of the eye (sclera, choroid and retina) at 15-day of embryonic stage (E15) incubated with VVA, GalNac terminal sugar-specific lectin. According to Figure 1, no reaction of retinal cells to VVA can be observed. Significant association is demonstrated between both RPE and photoreceptors layer in the presence of IPM. It seems that after finishing interactions between RPE and photorecep-tors, IPM gradually gets smaller or maybe disappears and subsequently pigmented layer is limited to the outer segment of photoreceptors layer (Figure 1).
Figure 1.
Transverse section of developing rat eyeball at E15 incubated with VVA lectin (specific for GalNac terminal sugar). Pigmented layer, photoreceptor layer, and interactions between these two are evident. RPE and thin pigmented layer on photoreceptors are approaching to each other to make a single pigmented layer (shaded area). Since this section belongs to to a few days before the birth, IPM still exists between RPE (large arrow) and photoreceptors layer (small arrow). Almost no part of the eye reacted to VVA. Instead, the counter stain (Alcian blue) is the dominant color of the slide (Control slide). Scale bar = 50 µm
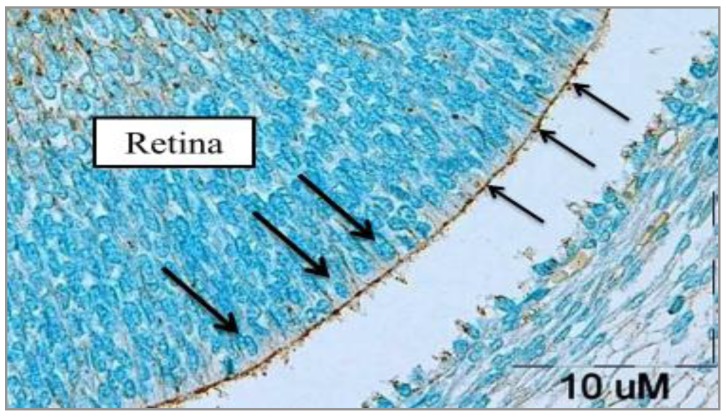
Results obtained from the photomicrographs of sections related to the late 15th and early 16th embryonic days (E15 and E16, respectively) incubated with MPA (β-DGal terminal sugar-specific lectin), revealed weak reaction of photoreceptors to the MPA (Figure 2). In addition, there was no differentiation in photoreceptor cells and no morphologically difference between photoreceptors and other cells through the retina.
Figure 2.
Transverse section photomicrograph of E16 of rat retina incubated with MPA lectin (specific for β-D-Gal terminal sugar). The photoreceptors cell surface reacted weakly (+) to MPA. Thin arrows show the thin border of pigmented epithelium on photoreceptors and thick arrows indicate the photoreceptor precursor cells in the late E15 and early E16. Through the retinal cells, nerve fibers are detected and some inner retinal cells such as ganglionic cells reacted moderately (++) to MPA. )dark brown spots). Scale bar = 10 µm
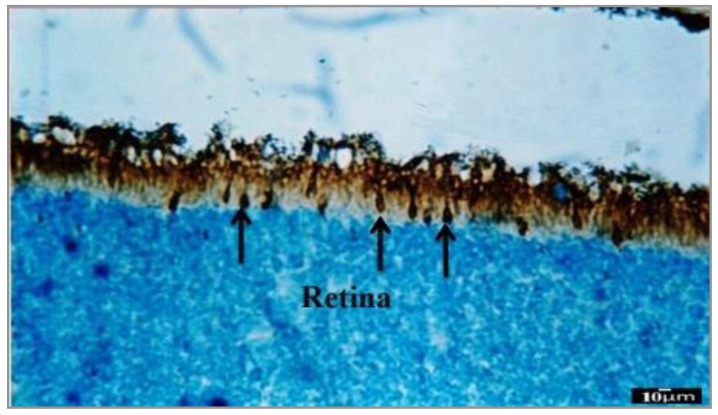
MPA reacted intensely with 10-day postnatal (P10) developing photoreceptors especially the cone cells; intense interaction between pigmented layer and photoreceptor layer is clearly obvious in Figure 3.
Figure 3.
Transverse section photomicrograph of P10 incubated with MPA lectin (specific for GalNac terminal sugar). Cone photoreceptors have reacted strongly (+++) to MPA and they are gradually approaching to the final differentiation (arrows). Pigmented epithelium reacted intensely (+++) to MPA, while inner retinal cells which are seen in the different situations did not reveal any reaction to MPA. Scale bar=10 µm
Present study showed that although PNA is a cone-specific lectin, MPA identifies both types of photoreceptor cells. However, because of the conical shape of most of the cells in Figure 3, it can be expected that the MPA lectin is more willing to identify the cone photoreceptors.
Our results showed that the expression, secretion, and distribution of β-D-Gal-containing glycoconju-gates on the photoreceptors cells surface were at the highest level in E15 and E16 and 10-day of postnatal.
Figure 4 illustrates developing eye transverse section prepared from day 12 of postnatal (P12) incubated with WGA-II lectin (Specific for GluNac [1–4] Glu terminal sugar). As it is obvious in Figure 4, photoreceptors did not show any reaction to WGA-II lectin like other retinal cells. Cone and rod cells are not distinguishable from each other with using of WGA-II lectin, only the apical region of photorecep-tors near the pigmented layer is partly characterized. There is a space between the pigmented and photoreceptor layers, so we strongly believe that pigmented layer plays a fundamental role in ultimate photoreceptors differentiations.
Figure 4.
Transverse section photomicrograph of P12 incubated with WGA-II lectin (Specific for GluNac [1–4] Glu terminal sugar). No retinal cell including photoreceptors showed any reaction to this lectin. Pigmented layer can be observed out of photoreceptors area (thick arrows). Apical region of undifferentiated photoreceptors (thin arrows) can be partly distinguished from other retinal cells. Scale bar = 10 µm
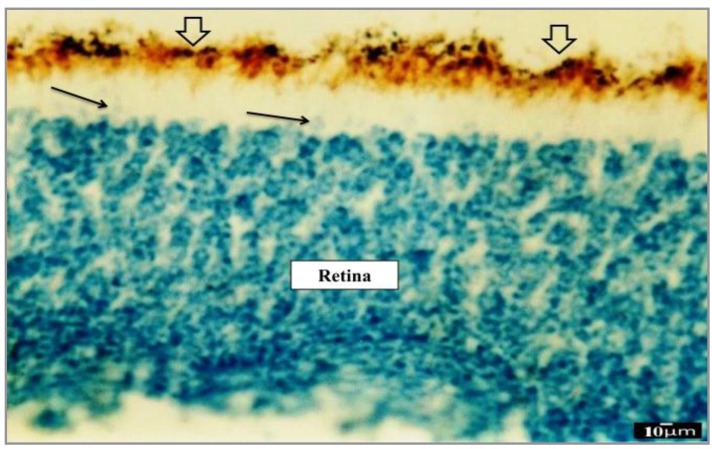
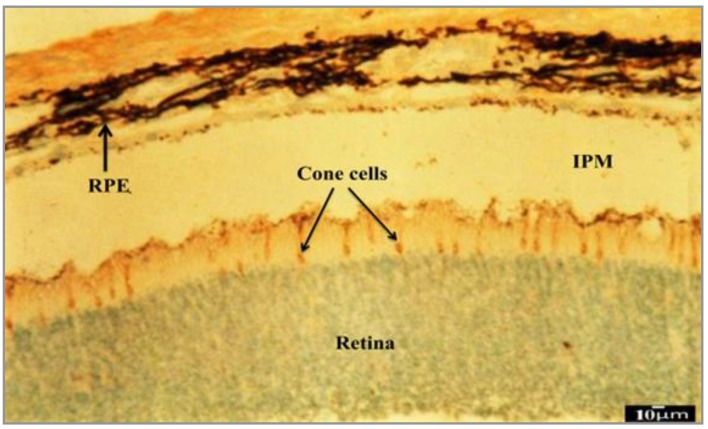
Evaluating sections incubated with PNA lectin clearly showed that the cone progenitor cells started final differentiations during P12 eye development (Figure 5). On the other hand, our results confirmed that PNA is a high specific lectin for terminal sugars secreted on the cell surface of the cone cells such as β-D-Gal [1–3]-D-GalNac. So β-D-Gal [1–3]-D-GalNac-containing glycoconjugates play an important role in retinal cone photoreceptors postnatal development.
Figure 5.
Photomicrograph of P12 transverse section incubated with PNA cone-specific lectin (that has high specificity for β-D-Gal [1–3]-D-GalNac terminal sugar). Cone photoreceptors have reacted strongly (+++) to PNA. So P12 is the date of ultimate differentiation of cone cells. Rod photoreceptors and other retinal cells similarly did not show any reaction to PNA. RPE will establish outermost portion of the retina. Another separated thin pigmented layer located on the surface region of photoreceptors is coming to RPE during postnatal development. IPM is an extracellular matrix-rich space between the RPE and photoreceptors and it is the place of RPE effect on photoreceptors final differentiation. Scale bar=10 µm
Discussion
Cell to cell and cell to ECM interactions provide a rich source of vital information of various developmental events such as cell differentiation, cell migration, cell fate determination, morphogenesis, and even cell death (28, 29). Like many previous researchers, we used lectin histochemistry to study the cell surface, ECM glycoconjugates terminal sugars distribution, and changes in fetal and neonatal development of the rat retina. Among various retinal neurons and supporting cells, photoreceptors and RPE were candidates for our study due to their reputation in retinal intercellular interactions (30).
On the other hand, differentiation and morphology phenomena were selected to be evaluated because of their importance in pre- and postnatal retinal photoreceptors development.
Lectins used in this study were identified as β-D-Gal, β-D-Gal [1–3]-D-GalNac, and GluNac [1–4] Glu-containing cells in the retina. The present study showed that the difference between cell surface components in different cells regarding the presence or absence of these compounds could be a reflection of cell morphological changes during morphogenesis and developmental stages. Since lectins are specific substances, we used them to evaluate the time of differentiation of photoreceptors from other retinal cells.
Our results about the photoreceptors reactions to different lectins from fetal to neonatal develop-mental stages showed that VVA could be used to determine the initial interactions between RPE and photoreceptor cells during embryonic morphogen-esis stage.
Different results were obtained by evaluating MPA in embryonic and postnatal period including weak reaction of cell surface of prenatal undifferentiated (E15-E16) photoreceptors to MPA and intense reaction of postnatal photoreceptors. According to these results, MPA lectin can be suggested as an appropriate detector of distribution and changes of cell surface glycoconjugates terminal sugars during postnatal photoreceptors differen-tiation. In addition, our findings showed that β-D-Gal has an important role in the development of retinal photoreceptors.
Based on our results, WGA-II lectin cannot distinguish photoreceptors differentiation in pre- and postnatal developmental stages; nevertheless it can relatively show their close association with RPE. According to other studies, there is a strict associate-on between photoreceptor layer and RPE that can lead to complete photoreceptors development (15, 16).
The results of our study expressed that cone photoreceptors are highly reactive to PNA. Although some previous studies indicated PNA as a cone-specific lectin, development of cone cells and the time of their surface reactivity with PNA are undecided in these studies (31, 32).
In present study, for the first time, it was found that the cone photoreceptors reacted strongly to the PNA lectin at P12 developmental stage; before this time pigmented layers and photoreceptors did not show any reaction to this lectin. So the day 12 of postnatal development can be proposed as the approximate final differentiation of the cone cells.
On the other hand, by using the specific lectins, it can be suggested that the development of retinal photoreceptors especially cone cells continues for long time, in post natal period. In this regard, our stu-dy confirmed the results of previous investigations based on postnatal development of the visual system. However, unlike our study, the results of some previ-ous investigations showed that both photoreceptors are generated prenatally in human (13, 14).
In our opinion, IPM is one of the best tissues for studying the role of ECM in development, because the most significant intercellular interactions occur in this area of the eyeball (24, 33). As can be seen obviously in the photos of this research, this developmental factors and signals-rich space provide an environment for bilateral connection between photoreceptors and RPE. According to these images, after whole interactions between the photoreceptor precursor cells and RPE in the last stages of postnatal development, it seems that the IPM becomes smaller gradually and its role in the development process becomes less and the photoreceptors complete their final differentiations.
According to our pilot study, we decided to select postnatal days. In other words, we obtained the time of cone cells morphogenesis and their critical period of formation and differentiation. According to our results, cone photoreceptors started their differentiation at the first days of postnatal until P12. It can be probably proposed that the newborns have low vision accuracy and color vision. In addition, our findings showed that the conclusive differentiation of rod and cone photore-sceptors does not begin at the same time.
Conclusion
The time of expression, distribution, and changes of surface glycoconjugates terminal sugars of retinal cells during eye development are regulated and glycoconjugates with β-D-Gal [1–3]-D-GalNac and β-D-Gal terminal sugars play a critical role in the development and ultimate differentiations of retinal cells specially the photoreceptors. Future advanced molecular experiments in addition to histochemical studies can facilitate the determination of several eye malformations origin.
Table 2.
Summary of the photoreceptors tissue sections reactions with tested lectins in different embryonic and postnatal stages
| Lectins | E15 | E16 | E17 | E18 | E19 | E20 | P2 | P4 | P6 | P8 | P10 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPA | + | + | - | - | - | - | - | - | - | - | +++ | - |
| WGII | - | - | - | - | - | - | - | - | - | - | - | - |
| PNA | - | - | - | - | - | - | - | - | - | - | - | +++ |
| VVA | - | - | - | - | - | - | - | - | - | - | - | - |
Negative (-), Weak (+), Moderate (++), Severe (+++). MPA: Maclura pomifera; WGII: wheat germ agglutinin; PNA: peanut agglutinin; VVA: Vicia villosa
Acknowledgment
Presented data in this article is from the MSc student thesis results and research protocol (911216) which was supported financially by the Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran. In addition, the authors would like to thank Mrs Fatemeh Motejaded for her excellent technical assistance.
References
- 1.Araki M. Regeneration of the amphibian retina: role of tissue interaction and related signaling molecules on RPE transdifferentiation. Dev Growth Differ. 2007;49:109–120. doi: 10.1111/j.1440-169X.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 2.Hartenstein V, Reh TA. Drosophila eye development. Vol. 37. Springer; 2002. Homologies between vertebrate and invertebrate eyes; pp. 219–255. [DOI] [PubMed] [Google Scholar]
- 3.Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls JG, Martin AR, Wallace BG, Fuchs PA. From neuron to brain. Sunderland MA: Sinauer Associates; 2001. [Google Scholar]
- 5.Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development. 1998;125:869–877. doi: 10.1242/dev.125.5.869. [DOI] [PubMed] [Google Scholar]
- 7.Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 9.Hinds JW, Hinds PL. Differentiation of photoreceptors and horizontal cells in the embryonic mouse retina: an electron microscopic, serial section analysis. J Comp Neurol. 1979;187:495–511. doi: 10.1002/cne.901870303. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs GH, Williams GA, Fenwick JA. Influence of cone pigment coexpression on spectral sensitivity and color vision in the mouse. Vis Res. 2004;44:1615–1622. doi: 10.1016/j.visres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Lin B, Masland RH, Strettoi E. Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res. 2009;88:589–599. doi: 10.1016/j.exer.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- 14.Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- 15.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 16.Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier—implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010. pp. 119–129. [DOI] [PMC free article] [PubMed]
- 17.Aramant RB, Seiler MJ. Transplanted sheets of human retina and retinal pigment epithelium develop normally in nude rats. Exp Eye Res. 2002;75:115–125. doi: 10.1006/exer.2002.2001. [DOI] [PubMed] [Google Scholar]
- 18.Hauck SM, Schoeffmann S, Deeg CA, Gloeckner CJ, Swiatek de Lange M, Ueffing M. Proteomic analysis of the porcine interphotoreceptor matrix. Proteomics. 2005;5:3623–3636. doi: 10.1002/pmic.200401223. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus HS, Sly WS, Kyle JW, Hageman GS. Photoreceptor degeneration and altered distribution of interphotoreceptor matrix proteoglycans in the mucopolysaccharidosis VII mouse. Exp Eye Res. 1993;56:531–541. doi: 10.1006/exer.1993.1067. [DOI] [PubMed] [Google Scholar]
- 20.Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho E, Choi H, Chan FL. Expression pattern of glycoconjugates in rat retina as analysed by lectin histochemistry. Histochem J. 2003;34 doi: 10.1023/a:1026032005521. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza YB, Jones CJ, Bonshek RE. Comparison of lectin binding of drusen, RPE, Bruch’s membrane, and photoreceptors. Mol Vis. 2009;15:906. [PMC free article] [PubMed] [Google Scholar]
- 23.Fariss RN, Anderson DH, Fisher SK. Comparison of photoreceptor-specific matrix domains in the cat and monkey retinas. Exp Eye Res. 1990;51:473–485. doi: 10.1016/0014-4835(90)90160-v. [DOI] [PubMed] [Google Scholar]
- 24.Garlipp MA, Gonzalez-Fernandez F. Cone photo-receptor and Müller cell pericellular matrices are binding domains for Interphotoreceptor Retinoid-Binding Protein (IRBP) Exp Eye Res. 2013;13:192–202. doi: 10.1016/j.exer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Rouge P, Culerrier R, Granier C, Rance F, Barre A. Characterization of IgE-binding epitopes of peanut (Arachis hypogaea) PNA lectin allergen cross-reacting with other structurally related legume lectins. Mol Immunol. 2010;47:2359–2366. doi: 10.1016/j.molimm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Fazel A, Thompson R, Sumida H, Schulte B. Lectin histochemistry of the embryonic heart: expression of terminal and penultimate galactose residues in developing rats and chicks. Am J Anatomy. 1989;184:85–94. doi: 10.1002/aja.1001840110. [DOI] [PubMed] [Google Scholar]
- 27.Ebrahimzadeh BA, Hassanzadeh TMM, Nikravesh MR, Fazel AR. Lectin histochemical study of vasculogenesis during rat pituitary morphogenesis. Iran J Basic Med Sci. 2011;14:35–41. [Google Scholar]
- 28.White JM. ADAMs: modulators of cell–cell and cell–matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Chen SS, Fitzgerald W, Zimmerberg J, Kleinman HK, Margolis L. Cell-Cell and cell-extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553–561. doi: 10.1634/stemcells.2006-0419. [DOI] [PubMed] [Google Scholar]
- 30.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology. 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa M, Fujiwara T, Yoshitomi T. Temperature-dependent ultrastructural changes in the cone interphotoreceptor matrix. Japan J Ophthalmol. 2009;53:536–540. doi: 10.1007/s10384-009-0700-9. [DOI] [PubMed] [Google Scholar]
- 32.Cronin T, Raffelsberger W, Lee-Rivera I, Jaillard C, Niepon M-L, Kinzel B, et al. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010;17:1199–1210. doi: 10.1038/cdd.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausman RE. Ocular extracellular matrices in development. Prog Retin Eye Res. 2007;26:162–188. doi: 10.1016/j.preteyeres.2006.11.001. [DOI] [PubMed] [Google Scholar]