Abstract
Objective(s):
Experimental allergic encephalomyelitis (EAE) is an autoimmune disease validated as animal model of multiple sclerosis (MS). Administration of genistein, a phytoestrogenic component of soy, to mice at the onset of EAE is known to attenuate the clinical signs of the disease. The potential effects of genistein on established EAE is less studied. In the current study, we aimed to compare the effects of genistein administration on EAE severity in early and late phases of the disease.
Materials and Methods:
The C57BL/6 mice were induced with EAE, using MOG 35-55 and gavaged with genistein (300 mg/kg) either after the appearance of the first clinical sign or 30 days post disease induction for ten days. 24 hr after the last gavage, mice were sacrificed. Brains and spleens were removed for assessing lymphocyte proliferation, cell cytotoxicity, and cytokine profile. Spinal cords were dissected to assess the amount of demyelination using Luxol fast blue/cresyl violet staining.
Results:
Administering mice with genistein, after the establishment of EAE, did not reverse the clinical signs of disease. However, treating with genistein at the onset of disease alleviated the clinical signs by reducing neuronal demyelination. Genistein suppressed the production of IFN-γ and enhanced IL-10 secretion in splenocyte and brain. Genistein also reduced IL-12 and TNF-α secretion in splenocytes, suppressed the proliferation of T-cells, and reduced the cell cytotoxicity.
Conclusion:
Genistein oral therapy might only reduce EAE severity if started in early phases of the disease.
Keywords: Experimental allergic, encephalomyelitis (EAE), Genistein, Immunomodulation, Interferon-gamma, Multiple sclerosis
Introduction
Multiple Sclerosis (MS) is a complex autoimmune disease identified as the most common debilitating neurological disease in young adults (1). Both genetic and environmental factors contribute to MS pathology, including inflammation, demyelination, and/or axonal transaction. Experimental allergic encephalomyelitis (EAE) is an inducible T-cell mediated autoimmune disease, validated as a relevant model of MS (2, 3). CD4+ T-cells are considered essential in the initial stages of CNS inflammation; however, both CD8+ T-cells and microglia also play curtailing roles. During the inflammation process, cytokines such as IFN-γ, TNF- α, IL-12, and IL-17 are involved in many of the key pathological features of MS and EAE.
A distinct female predominance exists for a variety of human autoimmune disorders including MS (4). However, estrogens have also shown to be suppressive in autoimmune diseases mediated by T cells. The Estrogen treatment of MS patients seems to be feasible; however, it might have serious side effects. Phytoestrogens are plant derived compounds that have a stradiol-like structure exerting estrogenic and anti-estrogenic effects (5). The most widely distributed phytoestrogens are isoflavones. Genistein is the major bioactive isoflavone found in soy bean. Previous studies have demonstrated the anti-inflammatory and anti-oxidative effects of genistein (6, 7). A few recent studies were designed to investigate the effect of genistein on EAE course. The injection of genistein to ovariectomized mice was reported to decrease EAE severity (8). Also, 7-O-tetradecanoyl-genistein oral therapy was reported to ameliorate EAE signs (9). However, little has been elucidated regarding the effects of genistein on the severity of EAE after the establishment of disease. We designed this study to evaluate the potential therapeutic effect of genistein on established EAE.
Materials and Methods
Animals
In this study, EAE was induced in 8–12 week female C57Bl/6 mice (22.4±0.37 g; Pasteur Institute of Iran). Each group contained 6 animals. The animals were housed in Shefa Neuroscience Research Center and kept at approximately 21°C, on 12 hr light-dark cycle, with access to food and water ad libitum. All the protocols were approved by Neurological Research Center Committee, Tehran University of Medical Sciences.
Induction of EAE
The mice were immunized with a 1:1 ratio of MOG 35-55 (Alexis, Switzerland) dissolved in Complete Freund’s Adjuvant (CFA) containing 0.4 mg of mycobactererium tuberculosis (Sigma-Aldrich, USA). For this purpose, 300 µg of MOG was dissolved in 100 µl Phosphate buffer saline (PBS) 10% (Sigma-Aldrich, USA) and mixed with equal volume of CFA. On day 0, the MOG-CFA emulsion was subcutaneously injected into four sites of the upper flanks (200 µl / mouse). The additional immune adjuvant, pertussis toxin (PTX), (Sigma-Aldrich, USA) was injected intraperitoneally (400 ng/ mouse) on day 0 and again 48 hr later.
Genistein administration
Genistein was dissolved in dimethyl sulfoxide (DMSO) 11% (Sigma Aldrich, USA). Genistein (300 mg/kg) or DMSO, as vehicle, was administered daily by oral gavage (n=6 mice/group). In the first set of the experiments, the mice received genistein or DMSO after the appearance of the first clinical sign for 10 days. The treatment did not begin until each individual mouse began to develop signs of EAE, and thus initiation of compound administration occurred over a period of days. In the second set of experiments, the mice were gavaged with genistein (300 mg/kg) or DMSO, from day 30 post induction (p.i.) for 10 days. The day after the last treatment, the mice in all groups were sacrificed for further evaluation.
Clinical evaluation of EAE
The clinical score of each mouse was assessed daily for 10 days. The following grading scheme was used to clinically score the animals: 0, no clinical signs; 0.5, hook tail; 1, flaccid/floppy tail; 2, walking deficits; 2.5, unilateral hind limb paralysis 3, bilateral hind limb paralysis; 3.5, paraplegia with forelimb weakness; 4, quadriplegia and 5, moribund. In the first set of experiments, the assessing of daily clinical score of each mouse began on day 0 and continued for 10 days after the appearance of the first clinical sign (score 1). In the second set of experiments, daily clinical score of each mouse was recorded from day 0 to 40. Three clinical parameters were used to compare the course of disease. Mean daily clinical score: average of the sum of the daily clinical scores of the mice in each group. Disease onset: average of the first day of the appearance of clinical signs (score 0.5) of the mice in each group. Maximum disease score: average of the highest scores of the mice in each group (10).
Histological evaluation
To assess the percent of demyelination, the mice were sacrificed with deep anesthesia one day after the last administration of drugs. The spinal cords of the animals were removed. Prior to sectioning, the spinal cords were put in paraformaldehyde 4% for overnight. Fixed spinal cords were processed in tissue processor (Payroll Process) for dehydration and cleaned with xylol prior to embedding in paraffin. Embedded spinal cords were serially sectioned. Axial spinal cord sections (8 microns) were cut on the rotary cryostat. Dorsal of lumbar sections were assessed for the presence of demyelination. A series of 4–5 cuts were pasted on one slide using albumin glue.
The samples were stained with Luxol fast blue-LFB (Merck, Germany)/ Cresyl violet (BDH, England). Briefly, spinal cord sections were deparaffinized and transformed through a graded serial of ethanol to 1% solution of LFB in 0.05% acetic acid and 96% ethanol. Sections were left in LFB solution in 56°C overnight, then rinsed in 95% ethyl alcohol and distilled water prior to differentiating in 70% ethyl alcohol. After differentiation in the lithium carbonate solution, sections were placed in distilled water again and counterstained with cresyl violet. Myelin stained blue and neurons appeared in pink and violet. The amount of demyelination was determined by assessing at least 5 sections for each mouse using infinity software (version 4.6).
Brain cytokine assay
One day after the last treatment, the mice were sacrificed and their brains were removed. For assessing the concentration of cytokines in CNS, 100 mg of frontal and temporal lobes of each mouse was homogenized in 10 ml of an extracting solution containing 50 µM tris (Sigma-Aldrich, USA), 2 mM EDTA (Merck, Germany), 0.1 M NaCl (Sigma-Aldrich, USA), 1 mM dithiotheritol (Merck, Germany), 200 µM phenylmethylsulfonyl fluoride (Merck, Germany), 1 µg/ml chymostatin (Sigma-Aldrich, USA), and 1 µg/ml trypsin inhibitor (Sigma-Aldrich, USA).
Afterwards, the homogenate was sonicated for 60 sec at 40000 hz on ice. Brain homogenate was centrifuged (10000*10 min at 4°C). The supernatant was analyzed for IFN-γ, TNF-α, IL-12 (as the proinflammatory cytokines), and IL-10 (as an anti-inflammatory cytokine) concentrations using sandwich-based enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, USA) by following the manufacturer’s instruction. All tests were performed in triplicate for each mouse.
Spleen cytokine assay
The mice were sacrificed 24 hr after the last gavage and their splenocytes were isolated. Mononuclear cells from spleens of immunized mice (3 mice/ group) were prepared and incubated with 1.5 ml of RPMI-1640 at a concentration of 2×105 cells/well in 24-well plate (Nunc, Denmark) for two days. The RPMI was supplemented with 10% FCS, 1% L-glutamine, 1% HEPES, 0.1% 2ME, and 0.1% penicillin/streptomycin and pulsed with 10 µg/ml genistein. The cell supernatants were collected. The amount of cytokines in cell supernatants was assessed using sandwich-based ELISA kits (eBioscience, USA) performed according to the manufacturer’s protocol. All tests were performed in triplicate for each mouse (11).
Lymphocyte proliferation assay (LPA)
24 hr after the last oral administration, single cell suspension of mononuclear cells was obtained from immunized mice and used for lymphocyte proliferation assay. Briefly, the suspension of isolated spleen cells was treated with lyses buffer [0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA, (pH 7.2)] in order to clear red blood cells. 2x105 cells/well were cultured in 96-well flat-bottom culture plates (Nunc, Denmark). The preparations were cultured with RPMI-1640 supplemented with 10% fetal calf serum, 1% L-glutamine, 1% HEPES, 0.1% 2ME, and 0.1% penicillin/streptomycin and were incubated in the presence of 10 µg/ml genistein. T cell mitogen phytohemagglutinin- PHA (Sigma chemicals, Australia) was used as positive control at the concentration of 5 µg/ml. After 3 days, MTT (3-(4, 5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (Sigma chemicals, Australia) in concentration of 5 µg/ml was added per well and incubated for 5 hr at 37°C in 5% CO2. DMSO (dimethyl sulfoxide) (100 µl) was added to dissolve the produced formazan crystals.
The plates were read at 540 nm, and the results were expressed as stimulation index (SI). The SI was determined as follows: OD values of stimulated cells (Cs) minus relative cell numbers of unstimulated cells (Cu) divided by relative OD values of unstimulated cells.
SI = (Cs - Cu)/Cu
All tests were performed in triplicate for each mouse.
Cell cytotoxicity assay
In order to assess cell cytotoxicity, lactate dehydrogenase (LDH) level was determined in splenocytes. 24 hr after the final administration, the mice were sacrificed and their splenocytes were isolated. For each sample obtained from an individual mouse, the single cell suspension of mononuclear cells (used as the effecter cells) was co-cultured in RPMI 1640 medium with washed target cells EL4 at various effector-to-target cells for 4 hr in phenol red-free RPMI 1640 containing FCS 3%. For preparation of the target cells, EL-4 cells were stimulated with genistein antigen.
After centrifugation, the supernatants (50 µl/well) were transferred to the 96-well flat-bottom plates, and lysis of target cells was determined by measuring LDH release using Cytotoxicity Detection Kit (LDH) according to the procedures stated by the manufacturer (Takara Company, Japan). Several controls were used for the cytotoxicity assay.
“High control” was the total LDH released from target cells, and all EL4 cells were lysed by a medium containing 1% Triton X-100. “Low control” was the natural release of LDH from target cells, which was obtained by adding EL4 cells only to the assay medium. “T-cell control” was used to measure the natural release of LDH from T cells obtained by adding different ratios of T cells only in the assay medium. The assay for all samples, including the controls, was performed in triplicates (11).
The LDH-mediated conversion of the tetrazolium salt into red formazan product was measured at 490 nm after incubation at room temperature for 30 min. The percentage of specific cytolysis was determined by the following formula: 
Statistical analysis
The results represented at least two independent variables and were presented as mean± SEM. For analyzing the effect of treatment on clinical score, cytokine profile, lymphocyte proliferation, and cell cytotoxicity one-way analysis of variance was used. All analysis was followed by the Scheff’s intra group comparison. The differences were considered significant at P< 0.05. Data were analyzed with SPSS 17.
Results
Clinical signs of EAE
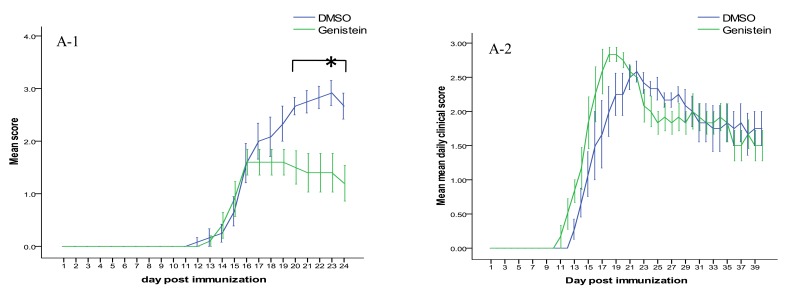
In the first set of the experiments, female c57BL/6 mice were gavaged with genistein or DMSO after the appearance of the flaccid tail (score 1) for 10 days. 12 to 16 days following immunization, the mice developed the clinical signs of EAE. No significant differences in the mean onset time were observed between studied groups. The mean daily clinical score of genistein treated animals were almost always lower than controls. The difference became significant between days 20 and 24 (Figure 1). As presented in Table 1, genistein also significantly reduced the maximum clinical score (P<0.05).
Figure 1.
Effect of oral administration of 300 mg/kg genistein or DMSO on clinical course of experimental autoimmune encephalomyelitis . (A-1) Genistein-treated animals (in early phase of disease) manifested lower clinical scores between days 20 and 24. A-2. In mice receiving treatment after the establishment of disease, clinical scores were not significantly different in comparison with DMSO
Table 1.
Effect of oral administration of 300 mg/kg genistein or DMSO on clinical signs of experimental autoimmune encephalomyelitis
| Incidence† | Onset (day)‡ | Maximum clinical score‡ | ||
|---|---|---|---|---|
| Treatment began at the onset of disease | DMSO | 6/6 | 14.5±0.56 | 2.8±0.21 |
| Genistein | 5/6 | 14.8±0.37 | 1.6±0.25 ** | |
| Treatment began after 30 days p.i. | DMSO | 6/6 | 14.7±0.52 | 2.7±0.17 |
| Genistein | 6/6 | 12.8±0.57 | 2.8±0.26 | |
Gavaging with genistein (300 mg/kg) at the onset of clinical signs significantly reduced maximum disease score.
Ratio,
mean± SEM,
P< 0.05,
P< 0.005 compared to DMSO-gavaged group post induction
In the second set of the experiments, genistein or DMSO was administered to the mice from Day +30 to Day +40 post induction. Genistein administration at the late phase of disease did not have a significant effect on the mean daily and the mean maximum clinical scores.
Demyelination of the spinal cord
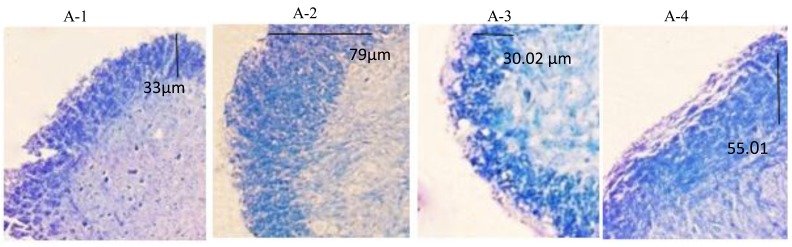
To compare the rate of demyelination among the studied groups, the spinal cords were removed one day after the last gavage. Luxol Fast blue/Cresyl violet staining was used to determine the amount of demyelination in the dorsal of lumbar sections. As apparent in Figure 2 (A-1 and A-2), in the first set of experiments, demyelination was obvious in the spinal cord of the DMSO-treated group, while it was notably reduced in genistein-treated mice. Unlike the first experiments, gavaging with genistein at the late phase of disease did not notably affect the extent of demyelination (Figure 2, B-1 and B-2).
Figure 2.
Demyelination of the lumbar spinal cords in mice induced with experimental autoimmune encephalomyelitis and treated with genistein (300 mg/kg) or DMSO. LFB/Cresyl violet staining was applied to assess spinal cord demyelination. A-1 and A-2 demonstrate demyelination in the mice receiving DMSO (A-1) or genistein (A-2) from the onset of disease for 10 days. A-3 and A-4 are the spinal cord sections of the mice that received DMSO (A-3) or genistein (A-4) from day 30 post induction for 10 days. Blue areas represent myelin
Cytokine patterns
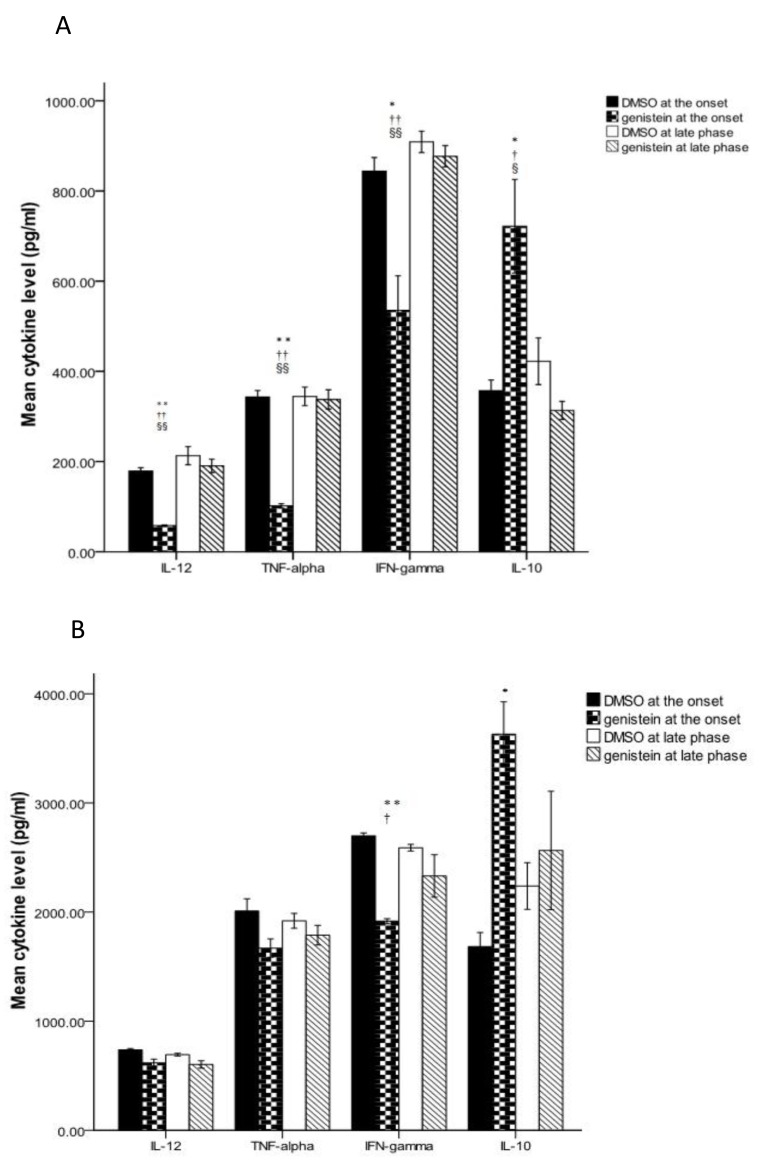
A day after the last gavage, the animals were sacrificed. Spleen cell supernatants were examined for the amount of IFN-γ, TNF-α, IL-10, and IL-12.
In the first set of experiments, as presented in Figure 3-A, genistein reduced the splenic production of IL-12, TNF-α and IFN-γ. It further increased IL-10 level. In the second set of experiments, treating with genistein did not significantly affect cytokine levels in spleen (Figure 3-A).
Figure 3.
Cytokine levels of brain and splenocytes in the mice induced with experimental autoimmune encephalomyelitis and receiving genistein (300 mg/kg) and DMSO either after the appearance of the first clinical sign or in late phase of disease. The TNF-α, IFN-γ, IL-12, and IL-10 levels were assessed in splenocytes (A) and the brain (B). All data expressed as the mean± SEM. ** P<0.005 and * P<0.05 compared to DMSO administration at the onset of disease, †P<0.05and ††P<0.005 compared to DMSO at late phase, § P<0.05 and §§ P<0.005 compared to genistein at late phase
We further assessed the pattern of cytokine secretion in brains of the treated animals. Compared to DMSO (Figure 3-B), gavaging with genistein at the onset of clinical signs of the disease reduced the production of IFN-γ and raised the secretion of IL-10 significantly.
Proliferative response of T cells
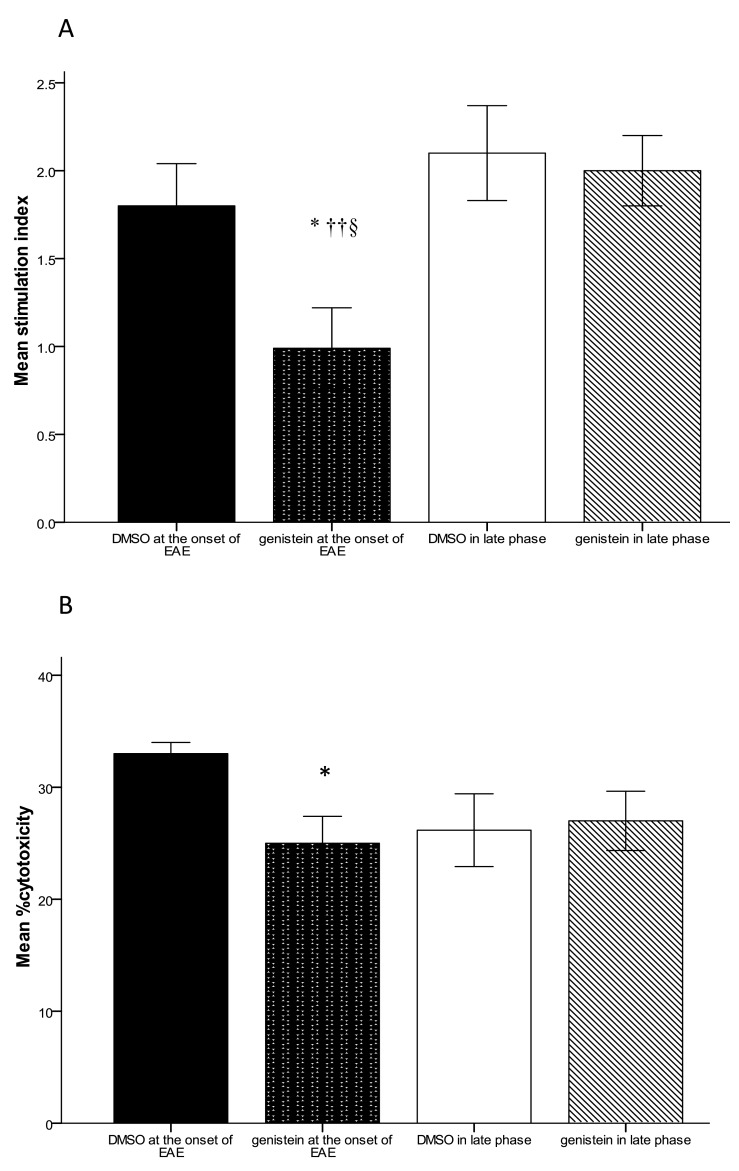
We evaluated LPA using MTT methods. For this purpose, twenty-four hr after the last gavage, the animals (3 mice/ group) were sacrificed and the spleen cells were harvested. The lymphocytes were stimulated in vitro by 10 µg/ml genistein. As presented in Figure 4-A, in mice gavaged with genistein after the onset of clinical signs, the proliferative response of T cells was suppressed, compared to vehicle control (P≤0.05).
Figure 4.
Lymphocyte proliferation and cell cytotoxicity assay in mice induced with experimental autoimmune encephalomyelitis and receiving genistein (300 mg/kg) and DMSO either after the appearance of first clinical sign or 30 days post induction, A) Lymphocyte proliferation evaluation using MTT assay. B) Cell cytotoxicity assays using quantitative measurement of LDH release. Data were collected from LDH results at various E/T ratios (E/T=25:1, 50:1, 100:1). The most significant differences between all these groups were at 50:1 E/T ratios. All data expressed as the mean± SEM, * P<0.05 compared to DMSO administration at the onset of disease, ††P<0.005 compared to DMSO at late phase, § P<0.05 compared to genistein at late phase
Lymphocyte proliferative responses were not significantly different in mice receiving genistein from 30 days p.i. compared to the control group (Figure 4-A).
Cell cytotoxicity
In order to assess the effect of genistein on the lactate dehydrogenase release, three mice were used from each group. 24 hr after the final treatment, single cell suspensions of mononuclear cells (effecter cells) were co-cultured with pulsed EL-4 cells (target cell).
In the first experiment, as shown in Figure 4, lymphocytes of immunized mice with genistein (25±2.4%) suppressed a specific cytolytic activity at an E/T ratio of 50:1 significantly, compared to DMSO group (33.01±1.03%). However, lymphocytes from mice gavaged with genistein after the establishment of the disease did not show any significant difference compared to the DMSO group (Figure 4-B).
Discussion
Flavonoids including genistein were reported to have beneficial effects in EAE. Evidences demonstrated that genistein has a variety of molecular targets, including enzymes, cytokines, cell proliferation, gens regulating, transcription factors, and apoptosis. Each of which can potentiate genistein to protect against and improve signs of EAE (12). In previous studies mice were administered with genistein at the onset of disease (8–9). Whether genistein can modify EAE feature in progressed EAE is still unknown.
Our results indicate that unlike in early phase, the administration of genistein in the late phase of EAE is not effective in controlling clinical signs. The observed difference may be due to the fact that in the progressive phase of EAE, inflammation and demyelination continue to worsen (13). In progressed disease, genistein was unable to modify cytokine pattern, lymphocyte proliferation, and the percent of cytotoxicity. Each of which might make genistein effective in ameliorating EAE severity at the early phase of disease.
Gavaging with genistein in the early phase of EAE reduced the stimulation index of lymphocytes which mainly represents memory CD4+ T-cell proliferation. Oral genistein in early phase also decreased IFN-γ secretion and the percent of cytotoxicity which mostly manifests the CD8+ activity. EAE is mainly characterized by infiltration of myelin-auto reactive CD4+ and CD8+ T-cells into the central nervous system (14). CD8+ T-cells were detectable in MS plaques and reported to mediate inflammation in EAE and MS. They activated to express IFN-γ (15). Among the many cytokines implicated in the pathogenesis of MS and EAE, IFN-γ has the principal regulatory role, directing chemokine production, involvement in T cell proliferation and subsequently affecting the onset and progression of the disease (16). IFN-γ secreting auto reactive T-cells are elevated in blood and cerebrospinal fluid of MS patients, in comparison with controls (17). The administration of IFN-γ to MS patients leads to the exacerbation of the disease (18). In accordance with the results of the current study, the only two other studies on the effect of genistein administration to EAE-induced mice in early phase of disease, reported a deduction in brain (8, 9) and spleen (8) levels of IFN-γ.
In the present study, gavaging with genistein at the onset but not in late phase of disease also enhanced the production of IL-10 in brain and splenocytes. IL-10 is a critical cytokine in down-regulating Th1 responses in MS/EAE. Furthermore, in remission phase of EAE, the expression of IL-10 mRNA rises in CNS and IL-10 deficit mice are not able to recover from the disease (19, 20). Studies of IL-10 in relapsing- remitting MS (RRMS) patients showed a reduction of this cytokine level before attacks (21). In addition, endogenous IL-10 suppresses IFN-γ production in RRMS patients (22). De Paula et al (8) and Castro et al (9) reported a significant increase of IL-10 secretion in brain and splenocyte of genistein-injected mice, which is in agreement with our results.
Moreover, our results clarified that treating mice with genistein immediately after the onset of clinical signs significantly inhibits IL-12 production in spleen. Comparable effect on IL-12 level was not observed when genistein was administered to mice with progressed EAE. One of the crucial pro-inflammatory cytokines altered in EAE/MS is IL-12 , an increase in secretion of which results in augmentation of the demyelination. Moreover, inhibiting IL-12 secretion or signaling involves the prevention of pathological and clinical signs of EAE (23). The IL-12 level of peripheral blood mononuclear cells was elevated in RRMS and Secondary Progressive MS patients in comparison with controls. IL-12 p40 also showed a relationship with the development of active lesions in MRI (24). In current study, unlike De Paula et al results (8), genistein did not affect the brain IL-12 level significantly. The observed difference might be due to the difference in the length of the treatment between De Paula and our study. We gavaged mice for 10 days after the appearance of first clinical signs, but in De Paula et al study mice were injected with genistein from day +14 to day +21. The peak inflammatory response is usually reached by day 18–20. Unlike current study, in mentioned previous study mice were sacrificed at the peak of clinical score.
Conclusion
The oral administration of genistein at the onset of clinical signs, but not in late phase of EAE, alleviates the severity of the disease by regulating cytokine production, lymphocyte proliferation, and CD8+ cytotoxicity. Human studies on the potential effect of oral genistein in early phase of MS are proposed.
Acknowledgment
This study was supported by Iranian Center of Neurological Disease, Tehran University of Medical Sciences. The authors would like to thank the staff of Shefa Neurosciences Research Center. We also thanked Mrs. Bita Pourmand (Research Development Center of Sina Hospital) for linguistic editing of the article.
References
- 1.Togha M, Karvigh SA, Nabavi M, Moghadam NB, Harirchian MH, Sahraian MA, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Mult Scler. 2010;16:848–854. doi: 10.1177/1352458510369147. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida , Kimura A, Fukaya T, Sekiya T, Morita R, Shichita T, et al. Low dose CP-690,550 (tofacitinib), a pan-JAK inhibitor, accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation. Biochem Biophys Res Commun. 2012;418:234–240. doi: 10.1016/j.bbrc.2011.12.156. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 4.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 5.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 6.Khodaparast A, Sayyah M, Sardari S. Anticonvulsant activity of hydroalcoholic extract and aqueous fraction of ebenus stellata in Mice. Iran J Basic Med Sci. 2012;15:811–819. [PMC free article] [PubMed] [Google Scholar]
- 7.Fatehi hasanabad Z, Jafarzadeh M, Fatehi M. The effects of genistein, a tyrosine kinase inhibitor, on acute and diabetic mice chronic inflammation in iranian journal of basic medical sciences. Iran J Basic Med Sci. 2004;7:23–27. [Google Scholar]
- 8.De Paula ML, Rodrigues DH, Teixeira HC, Barsante MM, Souza MA, Ferreira AP. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2008;8:1291–1297. doi: 10.1016/j.intimp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Castro SB, Junior CO, Alves CC, Dias AT, Alves LL, Mazzoccoli L, et al. Immunomodulatory effects and improved prognosis of experimental autoimmune encephalomyelitis after O-tetradecanoyl-genistein treatment. Int Immunopharmacol. 2012;12:465–470. doi: 10.1016/j.intimp.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Kafami L, Raza M, Razavi A, Mirshafiey A, Movahedian M, Khorramizadeh MR. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicenna J Med Biotechnol. 2010;2:47. [PMC free article] [PubMed] [Google Scholar]
- 11.Ghaemi A, Soleimanjahi H, Bamdad T, Soudi S, Arefeian E, Hashemi SM, et al. Induction of humoral and cellular immunity against latent HSV-1 infections by DNA immunization in BALB/c mice. Comp Immunol Microbiol Infect Dis. 2007;30:197–210. doi: 10.1016/j.cimid.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- 13.Costa O, Divoux D, Ischenko A, Tron F, Fontaine M. Optimization of an animal model of experimental autoimmune encephalomyelitis achieved with a multiple MOG 35–55 peptide in C57BL6/J strain of mice. J Autoimmun. 2003;20:51–61. doi: 10.1016/s0896-8411(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 14.Oyebamiji AI, Finlay TM, Hough RM, Hoghooghi V, Lim E MF, Wong CH, et al. Characterization of migration parameters on peripheral and central nervous system T cells following treatment of experimental allergic encephalomyelitis with CRYAB. J Neuroimmunol. 2013;259:66–74. doi: 10.1016/j.jneuroim.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 16.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–2768. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 17.Olsson T, Zhi WW, Hojeberg B, Kostulas V, Jiang YP, Anderson G, et al. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma. J Clin Invest. 1990;86:981–985. doi: 10.1172/JCI114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–10102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 19.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 20.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 21.Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, et al. Cytokine mRNA levels in mononuclear blood cells from patients with multiple sclerosis. Neurology. 1994;44:1523–1526. doi: 10.1212/wnl.44.8.1523. [DOI] [PubMed] [Google Scholar]
- 22.Balashov KE, Olek MJ, Smith DR, Khoury SJ, Weiner HL. Seasonal variation of interferon-gamma production in progressive multiple sclerosis. Ann Neurol. 1998;44:824–828. doi: 10.1002/ana.410440519. [DOI] [PubMed] [Google Scholar]
- 23.Bright JJ, Musuro BF, Du C, Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J Neuroimmunol. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- 24.van Boxel-Dezaire AH, Hoff SC, van Oosten BW, Verweij CL, Drager AM, Ader HJ, et al. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol. 1999;45:695–703. doi: 10.1002/1531-8249(199906)45:6<695::aid-ana3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]