Abstract
Controversy exists regarding the association between Iron Deficiency Anemia (IDA), iron status, and Febrile Convulsion (FC) during childhood. In this article, a systematic review and meta-analysis is conducted in order to determine possible association and the degree of association between these statuses and FC. To identify all studies related to IDA and FC, various references such as MEDLINE (PubMed), Embase (OVID), Web of sciences (Thomson Reuters) and Google scholar were searched (up until 15 January 2013). Heterogeneity was assessed using the Q statistic, Tau2, and I2. Additionally, subgroup analyses were performed. The outcome of primary interest was the overall Odds Ratio (OR) of FC for IDA and standard mean differences (SMD) of ferritin level. In total, 21 articles were considered to assess the association between IDA and FC. Anemia was more prevalent among the FC patients compared with the controls and the overall OR was 1.52 (95% CI=1.03 to 2.25). In addition, the pooled OR for 17 studies performed in the populations with low and moderate prevalence of anemia was 2.04 (95% CI=1.46 to 2.85). Furthermore, 12 studies assessed the association between the ferritin level and FC. The overall SMD was -0.02 with a 95% CI of -0.09 to 0.06. Besides, the pooled SMD of ferritin was -0.57 (95% CI=-0.7 to -0.46) in 6 studies reporting no difference between the FC and the control group with respect to temperature. IDA was associated with a moderate increased risk of FC in children, particularly in the areas with low and moderate prevalence of anemia.
Keywords: Febrile, Children, Iron deficiency anemia
Introduction
Febrile Convulsions (FC) refer to the convulsions that occur in children between the ages of 6 months and five years, with body temperature of 38ºC or higher not resulting from Central Nervous System (CNS) infection or any metabolic imbalance without any prior afebrile seizures. This condition occurs in 2-5% of the children who are neurologically healthy.1 The precise cause of FC is not known, but several genetic and environmental factors have been implicated.2 The maximum age of FC occurrence is 14-18 months, which overlaps with the maximum prevalence of Iron Deficiency Anemia (IDA) which is 1-2 years old.3
IDA is the most common nutritional deficiency in the world. Iron is an important micronutrient which is used by roughly all the cells in the human body. It is well understood that iron is a cofactor for several enzymes in the body and has a role in the neurotransmitters production and function, hormonal function and DNA duplication.4
Iron deficiency stimulates the function of neurons and, consequently, increases the risk of convulsions.3,5 Similar conditions are observed in Attention Deficit Hyperactivity Disorder (ADHD) and Restless Leg Syndrome (RLS).6 Animal studies have shown the pathophysiology of this malfunction. The relationship between IDA and FC is unknown. While some studies have shown IDA as a risk factor for the development of FC,7-10 this relationship has not been confirmed by other studies.5,11 On the other hand, few reports have claimed that IDA may have a protective effect on FC development.2,12,13
With respect to the high prevalence of FC and IDA in children and considering the fact that IDA is a probable risk factor for FC occurrence, as well as controversy in previous studies on this subject, this meta-analysis is carried out to determine the role of IDA in FC development by comparing IDA and ferritin level between FC patients and controls.
Methods
Data Source
MEDLINE (PubMed), Embase (OVID), Web of sciences (Thomson Reuters) and Google scholar were searched by NH and AA for abstracts using a combination of text words. The following index (MeSH) terms were sought for ‘‘Iron deficiency anemia” [MeSH Terms] OR ‘‘iron status’’ [All Fields] AND ‘‘febrile convulsion’’ [MeSH Terms] OR ‘‘febrile seizure’’ [All Fields]. No limitations or time period restrictions were applied during the search. A manual search of the bibliography of the retrieved papers was also carried out and the experts in this field were contacted for additional references. The latest date for the search was on January 15, 2013.
Study Selection
The analysis was restricted to human studies and no restriction was placed with respect to the language (figure 1). Studies, which did not follow a case-control or comparative design with clear comparative groups of cases with seizures and controls without seizures were excluded from the analysis. The studies in which the cases did not have a fever, the cases and controls having other hematologic conditions, or the controls which have had any kind of seizure were excluded. Additionally, the studies that proposed no specified criteria for defining iron deficiency were excluded. The abstracts of these articles were thoroughly checked in order to select the most appropriate investigation. Attempts were also made to identify additional articles by searching the reference lists of the studies. Authors were contacted for additional information if data were not reported in a suitable format for data synthesis. Eventually, EndNote software was used to merge the retrieved citations and eliminate the duplications.
Figure 1.
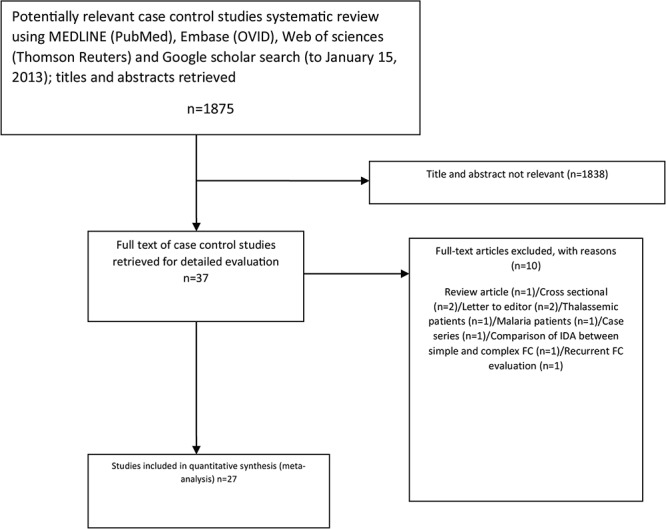
MOOSE flowchart showing selection of studies for meta-analysis of association between Iron Deficiency Anemia and Febrile Convulsion.
Data Extraction
Among the selected articles, the data in the study design, the raw number of FC patients and controls who had or had not experienced IDA, the patients’ age and sex, the diagnostic criteria used for definition of IDA, and source of cases and controls were extracted independently. Any discrepancy on the suitability for inclusion of a study was resolved by discussion among the authors.
Statistical Analysis
In this analysis, STATA (v. 10) and the inverse variance model was used to calculate the overall Odds Ratio (OR), 95% confidence interval (CI) and test statistic for the relationship. Because serum ferritin was continuous measurements, we used standard mean differences (SMD) as the effect size.
Statistical heterogeneity of studies was assessed through the calculation of tau2 and I2. A random effects model was applied unless I2 was <25% in which a fixed effects model would be used.14 To dissipate any heterogeneity, subgroup analysis was performed on IDA definition. Generation of a funnel plot and the Egger and Begg P value allowed determination of the potential publication bias. In addition, the “trim-and-fill” method was utilized to obtain the estimates of ORs corrected for a possible publication bias (metatrim command). Furthermore, the quality of the studies was evaluated using the Newcastle-Ottawa Scale (NOS).15 To assess the effects of the study characteristics on risk, random-effects meta-regression analyses were performed on the ORs adjusted for the year of study and each study characteristics was assessed by the Newcastle-Ottawa Scale for case-control studies.
Results
Included Studies
The initial search for studies involving the association between FC and anemia (or iron status) yielded 1,875 articles. Yet, after reviewing the abstracts and exclusion of irrelevant and duplicated articles, 37 articles remained in the analysis (figure 1). Out of the 37 examined articles, one review article,16 two cross-sectional studies,17,18 two letters to the editor,4,19 two case-control studies which included thalassemic20 and malaria patients,21 one case series,22 one study that compared simple and complex FC regarding IDA,23 one study which included FC patients and evaluated the association between the paraclinical findings (e.g. anemia) and seizure recurrence,24 were excluded. Additionally, one study assessing the iron status via serum iron25 was also excluded. Furthermore, one article7 was counted as two studies since it contained two different control groups (hospital and population) and the association was assessed twice. The information about the selected studies can be found in table 1 and the assessed quality of each study using NOS is presented in table 2.
Table 1.
Information about the included articles in the final meta-analysis
| First author and year of publication | Iron deficiency anemia (IDA) criteria | Kind of febrile convulsion (FC) | Cases | Controls |
OR
(95% CI) |
Case source | Control source | Age range and (mean age of cases) |
|---|---|---|---|---|---|---|---|---|
| Kobrinsky et al.,12 1995 | Hb<11 g/dl | Simple FC | 26 | 25 |
0.39 (0.1-1.5) |
Hospital | Hospital |
6-36 m/o (18.9±7.6) |
| Pisacane et al.,7 1996 | Hb<10.5 g/dl, MCV<70 fL, Serum Iron<5.4 µmol/L | unknown | 146 | 146 |
2.57 (1.43-4.59) |
Hospital | Hospital |
6-24 m/o (15±5.6) |
| Pisacane et al.,7 1996 | Hb<10.5 g/dl, MCV<70 fL, Serum Iron<5.4 µmol/L | unknown | 146 | 147 |
3.3 (1.78-6.11) |
Hospital | Population |
6-24 m/o (15±5.6) |
| Daoud et al.,26 2002 | Hb<11 g/dl |
First FC Simple (n=66) Complex (n=9) |
75 | 75 |
1.46 (0.73-2.93) |
Hospital | Hospital | 18.8 m/o |
| Guzman et al.,9 2005 | Hb<2SD of normal value for age | First FC | 40 | 40 |
3.89 (1.53-9.87) |
Hospital | Hospital | 3 m/o–5 y/o |
| Rehman et al.,8 2005 | Hb<10 g/dl | First FC | 30 | 30 |
7.67 (2.42-24.24) |
Hospital | Hospital |
8-36 m/o (22.97±9.52) |
| Al-Zwaini et al.,27 2006 | Hct<33% | First and recurrent FC | 100 | 100 |
2.66 (1.46-4.84) |
Hospital | Hospital |
6-60 m/o (25.8±15.19) |
| Talebian et al.,28 2008 | Hb<2SD of normal value for age |
Simple FC (n=56) Complex FC (n=4) |
60 | 60 |
0.62 (0.23-1.63) |
Hospital | Hospital | under 5 y/o |
| Abaskhanian et al.,13 2009 | Hb and Hct<2 SD of normal values for agea | First simple FC | 100 | 100 |
0.48 (0.27-0.85) |
Hospital | Hospital |
6 m/o-5 y/o (21.9±14.1) |
| Hartfield et al.,3 2009 | Hb<11 g/dl, MCV<70 fL and RDW>15.6% | Simple or complex FC | 361 | 390 |
1.42 (0.74-2.73) |
Hospital | Hospital | 6-36 m/o |
| Bidabadi et al.,11 2009 | Hb and Hct <2 SD of normal values for agea |
First FC Simple (n=132) Complex (n=68) |
200 | 200 |
0.85 (0.57-1.26) |
Hospital | Hospital |
6 m/o-5 y/o (22.86-12.86) |
| Abdurrahman et al.,29 2009 | Hb<10.5 g/dl, MCV<70 fL, serum iron<22 μg/dl, and TIBC>400 μg/dl | First FC | 112 | 120 |
3.44 (1.73-6.83) |
Hospital or who visited private office of the authors | unknown | 5 m/o-4 y/o |
| Amirsalari et al.,30 2010 | Hb<10.5 g/dl | First FC | 132 | 88 |
0.43 (0.12-1.56) |
Hospital | Hospital | 9 m/o-5 y/o |
| Sherjil et al.,31 2010 | Hb<9 g/dl, serum ferritin<7, MCV<65, MCHC<28 | Unknown | 157 | 153 |
1.92 (1.14-3.23) |
Hospital | Hospital | 6 m/o-6 y/o |
| Jun et al.,322010 | Hb<10.5 g/dl |
First and recurrent FC Simple (n=59) Complex (n=41) |
100 | 100 |
1.64 (0.91-2.98) |
Hospital | Hospital | 15.8±6.1 m/o |
| Kumari et al.,10 2012 | Hb<11 g/dL, serum ferritin<12 and RDW>15% | Simple FC | 154 | 154 |
5.34 (3.27-8.74) |
Hospital | Hospital |
6 m/o-3 y/o (17.5±8.81) |
| Derakhshanfar et al.,2 2012 | Hb and Hct<2 SD of normal values for agea | Unknown | 500 | 500 |
0.57 (0.45-0.74) |
Hospital | Hospital |
6 m/o-5 y/o (26.49+12.65) |
| Heydarian et al.,5 2012 | Hb<10.5 g/dl | First simple FC | 120 | 120 |
1.04 (0.61-1.75) |
Hospital | Hospital |
6 m/o-5 y/o (20.7±14.8) |
| Zareifar et al.,35 2012 | Hb<2 SD of normal value for age | Simple FC | 300 | 200 |
0.42 (0.29-0.6) |
Hospital | Hospital |
6 m/o-5 y/o (26.4±13.8) |
| Majumdar et al.,33 2012 | Hb<11 g/dl, MCV<70 fL, MCH<27 pg, ferritin<12 μg/dl, serum ferrous<60 μg/dl, TIBC>450 μg/dl, transferrin<250 mg/dl | First FC | 50 | 50 |
6.29 (2.52-15.7) |
Hospital | Hospital | 6 m/o-6 y/o |
| Sadeghzadeh et al.,34 2013 | Hb<10.5 g/dl |
First and recurrent FC Simple (n=70) Complex (n=30) |
100 | 100 |
1.3 (0.64-2.64) |
Hospital | Health care center | 6 m/o-3 y/o |
| Mahyar et al.,36 2006 | - | First simple FC | 20 | 20 | - | Hospital | Hospital | 9-24 m/o |
| Salehi et al.,37 2009 | - | First FC | 90 | 90 | - | Hospital | Hospital |
9m/o-5 y/o (1.6±1.2) |
| Momen et al.,39 2010 | Hb<11 g/L, MCV<72 fL, ferritin<20 μg/dL and TIBC<440 μg/dL | First simple FC | 50 | 50 | - | Hospital | Hospital | 9 m/o-5 y/o |
| Vaswani et al.,40 2010 | hemoglobin<11 g /dL, MCV<70 fL, MCH<27 pg and serum ferritin<12 μg/dL | First FC | 50 | 50 | - | Hospital | Hospital |
6 m/o-6 y/o (1.73±0.94) |
| Talebian et al.,38 2011 | - | Unknown | 40 | 40 | - | Hospital | Hospital |
6 m/o-5 y/o (24.8±13.95) |
OR (95% CI): Odds ratio with 95% confidence intervals; SD: Standard deviation; Hb: Hemoglobin; Hct: Hematocrit; MCV: Mean corpuscular volume; MCHC: Mean corpuscular hemoglobin concentration; TIBC: Total iron binding capacity; m/o: Months old; y/o: years old; aHb<10.5 g/dl for 6–24 months and <11.5 g/dl for 24–60 months old, Hct<33% for 6–24 months and <34% for 24–60 months old, MCV<70 fL for 6–24 months and <75 fL for 24–60 months old, MCH<23 pg for 6–24 months and <24 pg for 24–60 months old, MCHC<30 g/dl for 6–24 months and <31 g/dl for 24–60 months old, RBC<3.7×106 for 6–24 months and <3.9×106 for 24–60 months old, serum iron concentration <40 mg/dl before 1 year old and <50 mg/dl after 1 year old, PF<7 mg/l, and TIBC>430 mcg/dl
Table 2.
Quality assessment of the included studies
| Quality Indicators From Newcastle-Ottawa Scalea | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | |
| Kobrinsky et al.,12 1995 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Pisacane1 et al.,7 1996 | Yes | No | No | Yes | Yes | No | Yes | Yes | No |
| Pisacane2 et al.,7 1996 | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No |
| Daoud et al26, 2002 | Yes | No | No | Yes | Yes | No | Yes | Yes | No |
| Guzman et al.,9 2005 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Rehman et al.,8 2005 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Al-Zwaini et al.,27 2006 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Talebian et al.,28 2008 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Abaskhanian et al.,13 2009 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Hartfield et al.,3 2009 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Bidabadi et al.,11 2009 | Yes | No | No | Yes | Yes | yes | No | Yes | No |
| Abdurrahman et al.,29 2009 | Yes | No | No | Yes | Yes | Yes | No | Yes | No |
| Amirsalari et al.,30 2010 | Yes | yes | No | Yes | Yes | No | No | Yes | No |
| Sherjil et al.,31 2010 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Jun et al.,322010 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Kumari et al.,10 2012 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Derakhshanfar et al.,2 2012 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Heydarian et al.,5 2012 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Zareifar et al.,35 2012 | Yes | No | No | Yes | Yes | Yes | No | Yes | No |
| Majumdar et al.,33 2012 | Yes | No | No | Yes | Yes | No | No | Yes | No |
| Sadeghzadeh et al.,34 2013 | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
a1: Indicates cases independently validated; 2: Cases are representative of population; 3: Community controls; 4: Controls have no history of Febrile Convulsion; 5A: Study controls for age; 5B: Study controls for additional factor(s) e.g. Iron supplement, Temperature, etc.; 6: Ascertainment of exposure by blinded interview or record; 7: Same method of ascertainment used for cases and controls; 8: Non-response rate the same for cases and controls
Anemia in FC Patients vs. Controls
According to the results, a statistically significant association was found between anemia and FC. The overall OR upon the inclusion of all the 21 studies2,3,5,7-13,26-35 was 1.52 (95% CI=1.01 to 2.28) [I2=89.7; P<0.001; tau2=0.72] (figure 2).
Figure 2.

The figure demonstrates the pooled odds ratios and 95% confidence intervals for Iron deficiency anemia when comparing febrile convulsions (FC) patients with control groups and overall and subgroup analysis according to prevalence of anemia in population of studies (higher vs. lower than 40%). The studies are listed based on date
The evidence of publication bias was provided by a funnel plot. The Egger test was significant for publication bias (P=0.02) but not the Begg test (P=0.43).
The stratified analyses for anemia criteria according to the IDA definition yielded pooled OR estimates of 1.84 (95% CI=1.02 to 3.33; z=2.04; P=0.04) [I2=92.7; P<0.001; tau2=0.81] for 10 studies with anemia criteria according to iron status and 1.26 (95% CI=0.73 to 2.1; z=0.84; P=0.4) [I2=84.6; P<0.001; tau2=0.63] for 11 studies with anemia criteria according to Hb and hematocrit (Hct). Furthermore, the pooled OR was 0.56 (95% CI=0.42 to 0.75; z=3.87; P<0.0001) [I2=57.9; P<0.001; tau2=0.05] for 4 studies performed in the populations with a high prevalence of anemia (>40% in the control groups), but 2.04 (95% CI=1.46 to 2.85; z=4.16; P<0.0001) [I2=73.6; P<0.001; tau2=0.34] for 17 studies conducted among those with low and moderate prevalence of anemia (<40% in the control groups) (figure 2).
The influence analyses were completed by recalculating the pooled ORs for the sample on multiple occasions, while removing one study at each iteration. These analyses were particularly important because several studies included samples that were substantially larger than most of the other studies and thus could have exerted large effects on the overall effect estimates. For all studies, these analyses yielded ORs ranging from 1.43 (95% CI=0.97 to 2.1) to 1.64 (95% CI=1.1 to 2.4).
The present analysis also assessed the relationship between the quality of the study, year of the study, IDA definition, FC definition, the prevalence of anemia in the study population and the effect size (log OR) via random effects meta-regression. This analysis showed that even after correction for these elements, the relation between IDA definition (coefficient=0.76; SE=0.36; P=0.04) and the prevalence of anemia in the study population (coefficient=-1.74; SE=0.46; P=0.003) and FC was significant (I2=72.7; tau2=0.33). Nevertheless, the regression model revealed that none of the NOS scores and the year of the study was significantly related to OR.
Ferritin Level in FC Patients vs. Controls
Data on ferritin level were available for 12 studies.2,11,13,26,33,35-41 Four studies (Daoud et al.,26 Momen et al.,39 Vaswani et al.,40 and Modaresi et al.41) reported a significant decrease in the ferritin level in FC patients. On the other hand, three studies (Abaskhanian et al.,13 Bidabadi et al.,11 Derakhshanfar et al.2) reported a significant increase in the ferritin level in the FC patients compared with controls. The results of a random effects model for the 12 case-control studies included in the present meta-analysis are presented in figure 3. The overall Standard Mean Difference (SMD) with a 95% CI was -0.02 (-0.09 to 0.06; z=0.47; P=0.64) [I2=94.4; P<0.001; tau2=0.31].
Figure 3.

The figure demonstrates standard mean difference (SMD) of ferritin level and 95% confidence intervals when comparing febrile convulsions (FC) patients with control groups and overall and subgroup analysis according to significance difference between temperature of case and control. The studies are listed based on date.
The publication bias was evaluated by a funnel plot. Neither Egger test (P=0.54) nor Begg test (P=0.49) was statistically significant for the publication bias.
The pooled SMD of ferritin was -0.57 (with 95% CI of -0.7 to -0.46; z=9.23; P<0.001) [I2=90.6; P<0.001; tau2=0.26] for the six studies reporting no difference between FC and control groups regarding the temperature. On the other hand, it was measured as 0.29 (95% CI=0.2 to 0.38; z=6.26; P<0.001) [I2=86.6; P<0.001; tau2=0.04] for the other 6 studies showing a significant difference between FC and control groups regarding temperature (figure 3).
Discussion
The results revealed a statistically significant relationship between FC and IDA; IDA was 1.52 times more prevalent among the FC patients compared with controls. We observed a significant heterogeneity in this meta-analysis. Some parts of this heterogeneity can be explained by variations in the demographic characteristics, the primary illness resulting in fever, fever severity (temperature), family history of seizures, selection bias (especially in the control groups), etc. (table 1).
Yet, meta-regression and subgroup analyses showed that the state of IDA prevalence in the control group, which should reflect the prevalence of IDA in the general population, could be one of the main reasons for the observed heterogeneity. Of course, this relationship was more significant and heterogeneity showed a decreasing trend in the subgroup analysis which did not have a high prevalence of IDA (<40%) (OR: 2.07). In other words, the association between IDA and FC was significant in the regions with a low/medium prevalence of IDA, but not in those with a high prevalence of IDA (>40%).
One possible explanation is that when the rate of IDA is high in a particular population, the difference between the ratio of IDA in FC patients and controls is not high enough to show a significant difference. Moreover, most studies with high rates of IDA were done in Iran. This indicates the role of genetics, besides IDA, as a cofactor in FC.2 In other words, since FC is a multifactorial disease, it can be concluded that genetics trigger the effects of IDA on FC.42,43 Furthermore, reports have suggested that FC development is associated with the socio-economic level of patient’s family.4,44 It is obvious that the socio-economic status is associated with IDA; therefore, it is anticipated that IDA has an intermediate effect on FC and is a negative confounder with other factors related to low levels of socio-economic status.45 Overall, the associated factors with low socio-economic status includes zinc,46,47 magnesium.48,49 selenium,48,50 copper deficiencies47 and high levels of lead.8
The ferritin level is affected by the severity of fever and increases with fever. In some studies, the severity of fever was not similar in the FC patients and controls. Consequently, ferritin level could not be compared between the two groups. Nonetheless, when groups with similar fever severity (temperature) were compared, ferritin level was found to be lower in the FC children compared with the healthy ones. This is consistent with other findings from our meta-analysis.
According to the findings, iron deficiency leads to dysfunction of myelination as well as tyrosine and tryptophan hydroxylase synthesis, which are necessary for neurotransmitter production as well as the release of neurotransmitters from vesicles.21,51 The role of iron has also been documented in the production of serotonin, dopamine, and Gamma-Butyric Acid (GABA).5
Bradford Hill’s criterion is among the best for showing a causative relationship.52,53 Generally, it is difficult to draw conclusions about the causal relationships in observational studies. However, this meta-analysis could address some of the Hill’s criteria.53 The strength of the association was 2.07 (in populations with low and moderate prevalence of anemia) and the criteria could be accepted. Some studies have revealed the temporality and earlier occurrence of IDA compared to FC. In most studies, the primary event was the first FC and, as a result, we can accept that IDA/iron deficiency occurred before FC. Yet, it is difficult to draw such conclusion in the studies where the evaluated event was both the first and the recurrent FC. The association between IDA/iron deficiency and FC is consistent with the current knowledge and theories (coherence criteria). The biological plausibility evidence demonstrates the role of iron as an important cofactor for normal functioning of the brain neurotransmitters. However, limited data are available on the biological gradient between the cause (IDA) and the effect (FC). Therefore, further study on this topic is recommended. Since FC is a multi-factorial disease, specificity criteria cannot be adhered. According to these criteria, IDA should only cause an effect (FC) to be concluded as a cause for FC. Evidence regarding the administration of iron for FC children has also been controversial and requires more research.20,54,55 Overall, it can be stated that IDA causes FC through a similar mechanism that causes ADHD, Breath Holding Spells (BHS) and RLS (analogy).6
In addition to the observational nature of the articles used here, this meta-analysis had other limitations. First, the quality of some of the articles was not high enough. For example, the characteristics of the drop out patients were not clear. Besides, in most of the studies, data gathering was not done in a blinded fashion and selection of the patients was hospital-based rather than population-based.56 Additionally, the patients were not selected randomly in few studies. Furthermore, in a few studies, the two study groups were not similar regarding the important factors and the role of the confounding variables was not well controlled. Thus, we attempted to control the role of the quality of these items using meta-regression. Another limitation of this meta-analysis was different markers and definitions for IDA and FC. The odds ratio of heterogeneity was large between the studies and some part of this heterogeneity might be related to different definitions of IDA and/or FC. We tried to control this issue by performing a meta-regression analysis and then subgroup analysis. The funnel plot indicated the lack of symmetry and the possibility of bias. However, using “trim and fill” sensitivity analysis, no considerable change was observed in the results indicating the low level of bias in the performed meta-analysis.
Further high-quality studies (cohort or case-control) are recommended on this topic. In future studies, IDA and FC should be defined more precisely and the role of the important factors, such as socio-economic status and the serum levels of zinc, manganese, lead, etc., should be controlled to determine whether the association between IDA and FC is causal or not. After documenting a causative relationship, interventional studies can also be performed to assess the effect of iron administration on the FC patients.
Conclusion
Thus far, the combined results of the case control studies suggest that iron deficiency anemia is associated with a moderate increased risk of FC in children, particularly in areas with low and moderate prevalence of anemia. Future work should be carried out on interventional studies and the implication for public health.
Acknowledgment
This study was financially supported by Vice Chancellor for Research Affairs, Mazandaran University of Medical Sciences. We also would like to thank the authors who helped to make this review more comprehensive by providing data beyond those published in their original reports.
Conflict of Interest: None declared.
References
- 1.Kliegman RM, Stanton BF, Geme III JWS, Schor NF, Behrman RE. Nelson textbook of PEDIATRICS. 19th ed. Filladelphia: Elsevier Saunders; 2011. [Google Scholar]
- 2.Derakhshanfar H, Abaskhanian A, Alimohammadi H, ModanlooKordi M. Association between iron deficiency anemia and febrile seizure in children. Med Glas (Zenica) 2012;9:239–42. PubMed PMID: 22926357. [PubMed] [Google Scholar]
- 3.Hartfield DS, Tan J, Yager JY, Rosychuk RJ, Spady D, Haines C, et al. The association between iron deficiency and febrile seizures in childhood. Clin pediatr. 2009;48:420–6. doi: 10.1177/0009922809331800. doi: 10.1177/0009922809331800. PubMed PMID: 19229063. [DOI] [PubMed] [Google Scholar]
- 4.Hartfield D. Iron deficiency is a public health problem in Canadian infants and children. Paediatr Child Health. 2010;15:347–50. doi: 10.1093/pch/15.6.347. PubMed PMID: 21731416; PubMed Central PMCID: PMC2921732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heydarian F, Vatankhah H. The role of anemia in first simple febrile seizure in children aged 6 months to 5 years old. Neurosciences (Riyadh) 2012;17:226–9. PubMed PMID: 22772927. [PubMed] [Google Scholar]
- 6.Johnston MV. Iron deficiency, febrile seizures and brain development. Indian Pediatr. 2012;49:13–4. doi: 10.1007/s13312-012-0003-y. PubMed PMID: 22318097. [DOI] [PubMed] [Google Scholar]
- 7.Pisacane A, Sansone R, Impagliazzo N, Coppola A, Rolando P, D’Apuzzo A, et al. Iron deficiency anaemia and febrile convulsions: case-control study in children under 2 years. BMJ. 1996;313:343. doi: 10.1136/bmj.313.7053.343. doi: 10.1136/bmj.313.7053.343. PubMed PMID: 8760744; PubMed Central PMCID: PMC2351736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naveed-ur-Rehman, Billoo AG. Association between iron deficiency anemia and febrile seizures. J Coll Physicians Surg Pak. 2005;15:338–40. PubMed PMID: 15924837. [PubMed] [Google Scholar]
- 9.Guzman AR, Castillejos EL, Vicuña GL, Lagui VL, Balarezo ML, Gurreonero RL, et al. La anemia: un posible factor de riesgo para la primera convulsión febril. Paediatrica. 2005;7:62–5. [Google Scholar]
- 10.Kumari PL, Nair MK, Nair SM, Kailas L, Geetha S. Iron deficiency as a risk factor for simple febrile seizures--a case control study. Indian Pediatr. 2012;49:17–9. doi: 10.1007/s13312-012-0008-6. PubMed PMID: 21719928. [DOI] [PubMed] [Google Scholar]
- 11.Bidabadi E, Mashouf M. Association between iron deficiency anemia and first febrile convulsion: A case-control study. Seizure. 2009;18:347–51. doi: 10.1016/j.seizure.2009.01.008. doi: 10.1016/j.seizure.2009.01.008. PubMed PMID: 19223207. [DOI] [PubMed] [Google Scholar]
- 12.Kobrinsky NL, Yager JY, Cheang MS, Yatscoff RW, Tenenbein M. Does iron deficiency raise the seizure threshold? J Child Neurol. 1995;10:105–9. doi: 10.1177/088307389501000207. doi: 10.1177/088307389501000207. PubMed PMID: 7782598. [DOI] [PubMed] [Google Scholar]
- 13.Abaskhanian A, Vahid Shahi K, Parvinnejad N. The association between iron deficiency and the first episode of febrile seizure. J Babol Univ Med Sci. 2009;11:32–6. [Google Scholar]
- 14.Ried K. Interpreting and understanding meta-analysis graphs--a practical guide. Aust Fam Physician. 2006;35:635–8. PubMed PMID: 16894442. [PubMed] [Google Scholar]
- 15.The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis [Internet] Ottawa: The Ottawa Hospital Foundation; [cited 2013 Jan 15]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 16.Daoud AS. Febrile convulsion: Review and update. Journal of Pediatric Neurology. 2004;2:9–14. [Google Scholar]
- 17.Al-Zwaini EJ. Epidemiological and clinical features of hospitalized patients with febrile seizures in Ramadi, West of Iraq. J Pediatr Neurol. 2007;5:311–15. [Google Scholar]
- 18.Fallah R, Golestan M. Role of laboratory diagnostic tests in first febrile seizure. J Pediatr Neurol. 2008;6:129–32. [Google Scholar]
- 19.Macdonald CE, Playford RJ. Iron deficiency anaemia and febrile convulsions. ...and coeliac disease. BMJ. 1996;313:1205. doi: 10.1136/bmj.313.7066.1205a. PubMed PMID: 8916767; PubMed Central PMCID: PMC2352513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaloo S, Haghbin S, Karimi S. Febrile seizure in thalassemic patients. Iran J Child Neurology. 2010;4:23–6. [Google Scholar]
- 21.Idro R, Gwer S, Williams TN, Otieno T, Uyoga S, Fegan G, et al. Iron deficiency and acute seizures: results from children living in rural Kenya and a meta-analysis. PLoS One. 2010;5:e14001. doi: 10.1371/journal.pone.0014001. doi: 10.1371/journal.pone.0014001. PubMed PMID: 21103365; PubMed Central PMCID: PMC2982825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eseigbe EE, Adama SJ, Eseigbe P. Febrile seizures in Kaduna, north western Nigeria. Niger Med J. 2012;53:140–4. doi: 10.4103/0300-1652.104383. doi: 10.4103/0300-1652.104383. PubMed PMID: 23293414; pubMed Central PMCID: PMC3531033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaydin E, Arhan E, Cetinkaya B, Ozdel S, Değerliyurt A, Güven A, et al. Differences in iron deficiency anemia and mean platelet volume between children with simple and complex febrile seizures. Seizure. 2012;21:211–4. doi: 10.1016/j.seizure.2011.12.014. PubMed PMID: 22251926. [DOI] [PubMed] [Google Scholar]
- 24.Fallah R, Akhavan Karbasi S. The relationship between paraclinical findings of first febrile seizure and recurrence of seizure. Kowsar Medical Journal. 2008;13:147–52. [Google Scholar]
- 25.Tang W, Zhao K. The relationship between serum trace elements and children febrile convulsion. Chinese Journal of Hemorheology. 2007;4:640–1. [Google Scholar]
- 26.Daoud AS, Batieha A, Abu-Ekteish F, Gharaibeh N, Ajlouni S, Hijazi S. Iron status: a possible risk factor for the first febrile seizure. Epilepsia. 2002;43:740–3. doi: 10.1046/j.1528-1157.2002.32501.x. doi: 10.1046/j.1528-1157.2002.32501.x. PubMed PMID: 12102677. [DOI] [PubMed] [Google Scholar]
- 27.AL-Zwaini EJ, AL-Ani SS, AL-Khalidi MJ, AL-Ta’ie MF. Risk Factors for Febrile Seizures: A Matched Case Control Study. The Iraqi Postgraduate Medical Journal. 2006;5:353–58. [Google Scholar]
- 28.Talebian A, Momtazmanesh N. Febrile Seizure And Anemia. Iran J Child Neurology. 2007;2:31–33. [Google Scholar]
- 29.Abdurrahman KN, Ficms MD, Al-Atrushi A, Ficms M. The association between iron deficiency anemia and first febrile seizure: a case control study. Duhok Med J. 2010;4:60–6. [Google Scholar]
- 30.Amirsalari S, Keihani Doust ZT, Ahmadi M, Sabouri A, Kavemanesh Z, Afsharpeyman S, et al. Relationship between iron deficiency anemia and febrile seizures. Iran J Child Neurology. 2010;4:27–30. [Google Scholar]
- 31.Sherjil A, us Saeed Z, Shehzad S, Amjad R. Iron deficiency anaemia--a risk factor for febrile seizures in children. J Ayub Med Coll Abbottabad. 2010;22:71–3. PubMed PMID: 22338422. [PubMed] [Google Scholar]
- 32.Jun YS, Bang HI, Yu ST, Shin SR, Choi DY. Relationship between iron deficiency anemia and febrile convulsion in infants. Korean J Pediatr. 2010;53:392–6. doi: 10.3345/kjp.2010.53.3.392. [Google Scholar]
- 33.Majumdar R. Iron deficiency anemia as a risk factor for first febrile seizure [dissertation] Bangalore: Rajiv Gandhi University of Health Sciences; 2012. 94 pp. [Google Scholar]
- 34.Sadeghzadeh M, Khoshnevis Asl P, Mahboubi E. Iron status and febrile seizure- a case control study in children less than 3 years. Iran J Child Neurol. 2012;6:27–31. PubMed PMID: 24665277; PubMed Central PMCID: PMC3943016. [PMC free article] [PubMed] [Google Scholar]
- 35.Zareifar S, Hosseinzadeh HR, Cohan N. Association between iron status and febrile seizures in children. Seizure. 2012;21:603–5. doi: 10.1016/j.seizure.2012.06.010. doi: 10.1016/j.seizure.2012.06.010. PubMed PMID: 22796045. [DOI] [PubMed] [Google Scholar]
- 36.Mahyar A, Rezae MA. Ferritin Level in Children with and without Febrile Convulsion. Journal of Kermanshah University of Medical Sciences. 2006;10:204–9. [Google Scholar]
- 37.Salehi Omran MR, Tamaddoni A, Nasehi MM, Babazadeh H, Alizadeh Navaei R. Iron status in febrile seizure: a case-control study. Iran J Child Neurology. 2009;3:39–42. [Google Scholar]
- 38.Talebian A, Andalib S, Moravveji SA, Vakili Z. Serum ferritin level in febrile children with and without seizures. KAUMS Journal (FEYZ) 2011;15(4):389–93. [Google Scholar]
- 39.Momen AA, Nikfar R, Karimi B. Evaluation of iron status in 9-month to 5-year-old children with febrile seizures: a case control study in the south west of Iran. Iran J Child Neurology. 2010;4:45–50. [Google Scholar]
- 40.Vaswani RK, Dharaskar PG, Kulkarni S, Ghosh K. Iron deficiency as a risk factor for first febrile seizure. Indian Pediatr. 2010;47:437–9. doi: 10.1007/s13312-010-0080-8. doi: 10.1007/s13312-010-0080-8. PubMed PMID: 19736364. [DOI] [PubMed] [Google Scholar]
- 41.Modaresi M, Mahmoudian T, Yaghini O, Kelishadi R, Golestani H, Tavasoli A, et al. Is Iron Insufficiency Associated With Febrile Seizure? Experience in an Iranian Hospital. J Compr Ped. 2012;3:21–4. doi: 10.5812/jcp.6946. [Google Scholar]
- 42.Ottman R. Analysis of genetically complex epilepsies. Epilepsia. 2005;46:7–14. doi: 10.1111/j.1528-1167.2005.00350.x. doi: 10.1111/j.1528-1167.2005.00350.x. PubMed PMID: 16359464; PubMed Central PMCID: PMC1352332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graves RC, Oehler K, Tingle LE. Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician. 2012;85:149–53. PubMed PMID: 22335215. [PubMed] [Google Scholar]
- 44.Aydin A, Ergor A, Ozkan H. Effects of sociodemographic factors on febrile convulsion prevalence. Pediatr Int. 2008;50:216–20. doi: 10.1111/j.1442-200X.2008.02562.x. doi: 10.1111/j.1442-200X.2008.02562.x. PubMed PMID: 18353063. [DOI] [PubMed] [Google Scholar]
- 45.Szklo M, Nieto FJ. Epidemiology: beyond the basics. 2nd ed. Massachusetts: Jones and Bartlett Publishers; 2007. [Google Scholar]
- 46.Lee JH, Kim JH. Comparison of serum zinc levels measured by inductively coupled plasma mass spectrometry in preschool children with febrile and afebrile seizures. Ann Lab Med. 2012;32:190–3. doi: 10.3343/alm.2012.32.3.190. PubMed PMID: 22563553; PubMed Central PMCID:PMC3339298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amiri M, Farzin L, Moassesi ME, Sajadi F. Serum trace element levels in febrile convulsion. Biol Trace Elem Res. 2010;135:38–44. doi: 10.1007/s12011-009-8487-6. doi: 10.1007/s12011-009-8487-6. PubMed PMID: 19669113. [DOI] [PubMed] [Google Scholar]
- 48.Akbayram S, Cemek M, Büyükben A, Aymelek F, Karaman S, Yilmaz F, et al. Major and minor bio-element status in children with febrile seizure. Bratisl Lek Listy. 2012;113:421–3. doi: 10.4149/bll_2012_095. PubMed PMID: 22794517. [DOI] [PubMed] [Google Scholar]
- 49.Sadinejad M, Mohsenzadeh A. Determinatine serum level of magnesium In children with febrile seizures. J Nutr Sci Vitaminol (Tokyo) 2005;7:105–8. [Google Scholar]
- 50.Mahyar A, Ayazi P, Fallahi M, Javadi A. Correlation between serum selenium level and febrile seizures. Pediatr Neurol. 2010;43:331–4. doi: 10.1016/j.pediatrneurol.2010.05.024. doi: 10.1016/j.pediatrneurol.2010.05.024. PubMed PMID: 20933176. [DOI] [PubMed] [Google Scholar]
- 51.Schulz K, Kroner A, David S. Iron efflux from astrocytes plays a role in remyelination. J Neurosci. 2012;32:4841–7. doi: 10.1523/JNEUROSCI.5328-11.2012. doi: 10.1523/JNEUROSCI.5328-11.2012. PubMed PMID: 22492039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. PubMed PMID: 14283879; PubMed Central PMCID: PMC1898525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Susser M. What is a cause and how do we know one? A grammar for pragmatic epidemiology. Am J Epidemiol. 1991;133:635–48. doi: 10.1093/oxfordjournals.aje.a115939. PubMed PMID: 2018019. [DOI] [PubMed] [Google Scholar]
- 54.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43. doi: 10.1016/S0140-6736(06)67962-2. doi: 10.1016/S0140-6736(06)67962-2. PubMed PMID: 16413877. [DOI] [PubMed] [Google Scholar]
- 55.Smith HJ, Meremikwu M. Iron chelating agents for treating malaria. Cochrane Database Syst Rev. 2003:CD001474. doi: 10.1002/14651858.CD001474. doi: 10.1002/14651858.CD001474. PubMed PMID: 12804409. [DOI] [PubMed] [Google Scholar]
- 56.Ellenberg JH, Nelson KB. Sample selection and the natural history of disease. Studies of febrile seizures. JAMA. 1980;243:1337–40. doi: 10.1001/jama.1980.03300390021015. PubMed PMID: 7359696. [PubMed] [Google Scholar]


