Abstract
Gastrin-releasing peptide (GRP) is a mammalian neuropeptide that acts through the G protein-coupled receptor, GRP receptor (GRPR). Increasing evidence indicates that GRPR-mediated signaling in the central nervous system plays an important role in many physiological processes in mammals. Additionally, we have recently reported that the GRP system within the lumbosacral spinal cord not only controls erection but also triggers ejaculation in male rats. This system of GRP neurons is sexually dimorphic, being prominent in male rats but vestigial or absent in females. It is suggested that the sexually dimorphic GRP/GRPR system in the lumbosacral spinal cord plays a critical role in the regulation of male sexual function. In parallel, it has been reported that the somatosensory GRP/GRPR system in the spinal cord contributes to the regulation of itch specific transmission independently of the pain transmission. Interestingly, these two distinct functions in the same spinal region are both regulated by the neuropeptide, GRP. In this report, we review findings on recently identified GRP/GRPR systems in the spinal cord. These GRP/GRPR systems in the spinal cord provide new insights into pharmacological treatments for psychogenic erectile dysfunction as well as for chronic pruritus.
Keywords: Chronic pruritus, erectile dysfunction, gastrin-releasing peptide, gastrin-releasing peptide receptor, sexual function, somatosensory, spinal cord.
1. INTRODUCTION
Substantial evidence indicates that neuropeptide-mediated signal transduction in the central nervous system plays an important role in regulating neuroendocrine functions and behaviors. Released neuropeptides function by activating specific 7-transmembrane domain receptors that are members of the G protein-coupled receptor (GPCR) superfamily. This, in turn, leads to stimulation of downstream protein kinase signaling pathways and ultimately alters gene expression.
The fourteen-amino acid peptide bombesin was initially described as an antibacterial peptide from the skin of the European fire-bellied toad, Bombina bombina, and was shown to have potent bioactivity in the mammalian nervous system [1, 2]. Subsequently, the mammalian bombesin-like peptides, gastrin-releasing peptide (GRP) [2] and neuromedin B (NMB) [3], were isolated. GRP is a 27-amino acid peptide (29-amino acids in rodents), originally isolated from the porcine stomach, and believed to be a mammalian counterpart of the amphibian peptide, bombesin [2]. Many studies have demonstrated that GRP is widely expressed in the central nervous system as well as in the gastrointestinal tract in mammals [4]. Bombesin-like peptides appear to function via a family of three GPCRs [5], namely the GRP- preferring receptor (GRPR) [6], the NMB-preferring receptor (NMBR) [7], and the orphan receptor, bombesin receptor subtype-3 in mammals [8]. To date, it has been reported that GRP is integral in a variety of physiological functions, including food intake [9-11], circadian rhythms [12-14], and fear memory consolidation [15-17], through specific GPCR, GRPR-mediated mechanisms [6]. GRPR activation at the plasma membrane is coupled to Gq protein activity and increases in [Ca2+] lead to stimulation of the PLC/PKC and ERK/MAPK pathways [6, 18]. One of the most exciting recent developments in GRPR research is the identification of a sexually dimorphic GRP/GRPR system in the lumbosacral spinal cord [19]. Namely, we found evidence that neurons within the spinal ejaculation generator [20] (lumbar segments 3 and 4; L3–L4 level) project GRP-expressing axons to the lower lumbar and the upper sacral spinal cord (L5–L6, and S1 level) involved in the somatic and autonomic functions to generate both erection and ejaculation (Fig. 1) [19, 21, 22]. All these target regions in the lumbosacral spinal cord express GRPR [19]. Pharmacological stimulation of GRPRs in the lumbosacral spinal cord restores both penile reflexes and ejaculation rate in castrated male rats. Furthermore, intrathecal administration of GRPR antagonists to this spinal region decreases penile reflexes and the ejaculation frequency compared to controls [19]. In parallel, another important finding has demonstrated that spinal itch transmission is independent of pain transmission and relies on GRP/GRPR signaling in the dorsal root ganglion (DRG)-dorsal horn of the spinal cord [23, 24] as well as in the trigeminal sensory system (trigeminal ganglion-trigeminal sensory nuclei) (Fig. 1) [25]. The itch and erectile systems are localized to distinct anatomical regions within the spinal cord which likely what allows for the two GRP/GRPR systems to function independent of each other. However, it is interesting that two distinct functions in the same spinal region are regulated by the same neuropeptide, GRP (see Fig. 1). In this report, we review findings on recently identified spinal GRP/GRPR systems involved in both male sexual function and the itch sensation. These discrete GRP/GRPR systems in the spinal cord provide new insights into potential pharmacological treatments for psychogenic erectile dysfunction (ED) as well as for chronic pruritus.
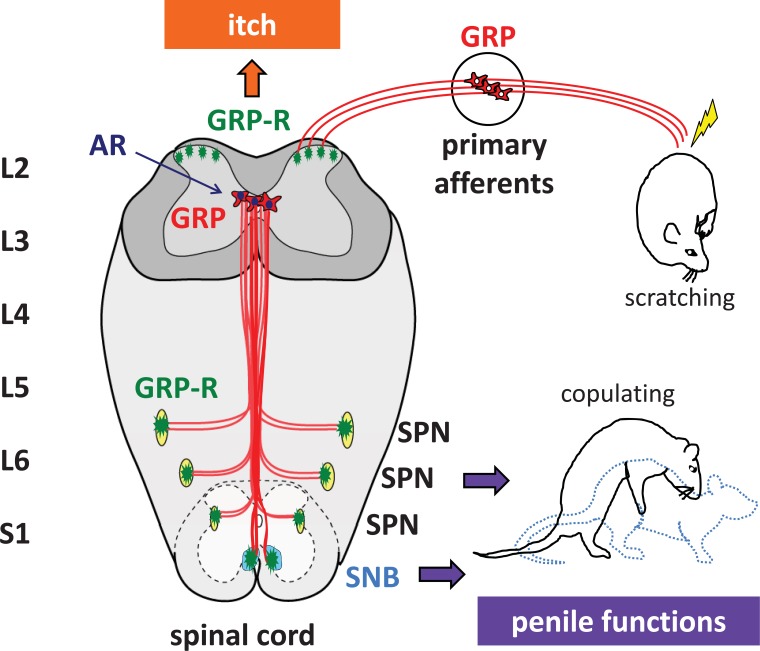
Fig. (1).
Schematic diagram of gastrin-releasing peptide (GRP)/GRP receptor (GRPR) systems, which control male sexual functions and itch transmission at the spinal cord level. This spinal center mediates penile reflexes and triggers ejaculation through androgen receptor (AR)-mediated mechanisms expressed in spinal GRP neurons. The somatosensory GRP system is located in the primary afferents, and the central axons of the primary afferents project to the spinal dorsal horn where GRPR is expressed at all spinal levels. (SNB, spinal nucleus of the bulbocavernosus; SPN, sacral parasympathetic nucleus).
2. GRPR TARGETING FOR ED AND EJACULATION DISORDERS
2.1. Discovery of a Local Neural Circuit in the Lumbosacral Spinal Cord that Controls Male Sexual Functions
Neural networks controlling male sexual functions are extremely complex, and are composed of various regions of both the brain and spinal cord. Lower spinal cord injuries frequently cause sexual dysfunction in men, including ED and an ejaculation disorder [26, 27]. Therefore, this indicates that the critical neural centers for male sexual function are located within the lower spinal cord [28-33]. However, little is known about the neural basis of male sexual function at this spinal cord level during copulatory behavior. Using GRP-immunohistochemistry, we recently identified a spinal GRP/GRPR system that controls male sexual function. A group of neurons in the lumbar spinal region (L3–L4 level) appear to play a role in both erection and ejaculation (Fig. 2) [19]. These GRP-expressing neurons project axons to the more caudal lumbosacral spinal cord (Fig. 2). The number of GRP-expressing neurons is greater in males than in females, with males showing much greater expression in the lumbosacral spinal cord [19, 34]. We also demonstrated the presence of GRPR in GRP neuron-projecting neurons located in the lumbosacral spinal cord using several methods, including the specific binding of GRP, Western immunoblotting, double immunohistochemistry, and reverse transcription-PCR [19, 35]. These results further indicate that the functional expression of GRPR is detected on both autonomic and somatic neurons, including neurons in the sacral parasympathetic nucleus (SPN) and motoneurons in the spinal nucleus of the bulbocavernosus (SNB), that innervate the striated perineal muscles bulbocavernosus (BC), the levator ani (LA), and the external anal sphincter (Fig. 1) [19, 35]. These target nuclei are reported to play critical roles in the regulation of penile reflexes (Fig. 1) [29-33].
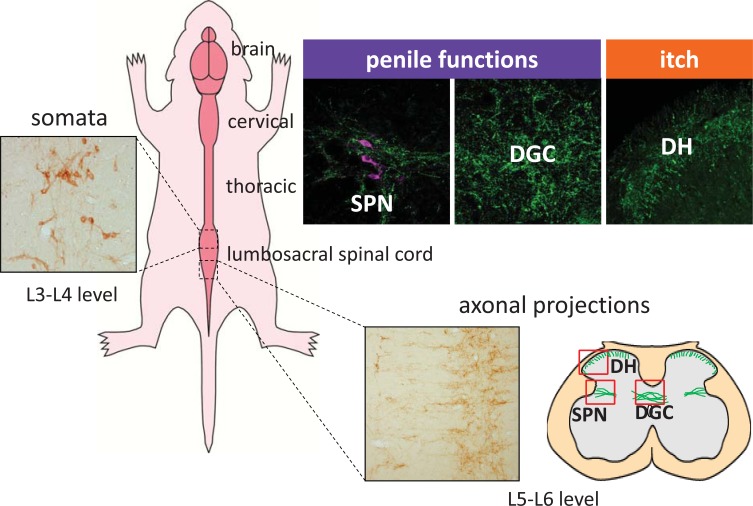
Fig. (2).
Distribution of GRP, which controls penile functions, in the lumbosacral spinal cord. A subset of neuronal somata expressing GRP is located in the lumbar spinal region (L3–L4 level) and shows an obvious male dominant sexual dimorphism. The spinal GRP neurons project to the more caudal region (L5–L6 and S1 level) through the dorsal gray commissure (DGC), expanding to the sacral parasympathetic nucleus (SPN) in the lower spinal cord. Another population of GRP-positive fibers is observed in the dorsal horn (DH) area and is involved in itch sensation.
2.2. Androgenic Regulation of GRP-Containing Neurons in the Lumbosacral Spinal Cord
Four weeks after orchidectomy of adult male rats, there is a significant reduction in the intensity of GRP-immuno-reactive fibers in the lumbosacral spinal cord and this decrease is prevented by long term (4 weeks) androgen replacement [19]. The intensity of both GRP-immuno-positive somata and dendrites in castrates was reduced compared to controls 4 weeks after surgery [35]. Chronic testosterone replacement (for 4 weeks) eliminated the effects of orchiectomy on GRP immunolabeling [35]. In contrast, long term testosterone treatment (4 weeks) of ovariectomized adult females did not fully masculinize the spinal GRP system, indicating a substantial sex difference in the number of GRP-expressing neurons in adult rats [19]. GRP-expressing neurons in males also express the androgen receptor (AR), but do not express the estrogen receptor alpha subtype [19, 34], suggesting that the expression of GRP in the lumbosacral spinal cord is androgen-dependent [35]. Therefore, these results indicate that the male-dominant sex difference in the number of GRP neurons might develop during a critical period (at the time of the “androgen shower”), and that this sexual difference in the neuron numbers persists into adulthood. Additional studies involving androgen manipulation (or androgen antagonists) during embryonic and/or neonatal development are needed to draw a firm conclusion as to the role of androgens in GRP neuronal development and function.
2.3. Analyses of the GRP/GRPR System in the Lumbosacral Spinal Cord Using AR Mutant Models
To study whether ARs regulate the sexual dimorphism of these neurons, we used two different AR-related genetically-modified animal models, including genetically XY male rats carrying the testicular feminization mutation (Tfm) of the Ar gene [36, 37] and a conditional knockout (KO) mouse line specifically lacking the Ar gene in the nervous system (ARNesCre) [38]. Tfm males develop testes during embryonic development according to their chromosomal sex (XY) and prenatally secrete testosterone. However, the AR protein in Tfm males is largely dysfunctional, and they develop a clitoris rather than a penis, and therefore present a completely feminine appearance [36, 37]. The spinal cord of Tfm males is hyperfeminine, having even fewer GRP-immunopositive neurons in the lumbar spinal cord than not only control males, but also control females [19, 34]. These results in the Tfm model indicate that androgen effects on the lumbosacral spinal cord are specifically mediated by the AR. Furthermore, the hyperfeminine appearance of the Tfm male rats indicates that the spinal GRP system is dependent on the AR but not on the estrogen receptor.
Subsequently, we used an ARNesCre mouse line to test whether the AR directly regulates sexual differentiation of the spinal GRP system. Although adult ARNesCre males exhibit higher levels of circulating testosterone than controls [38], the number of SNB motoneurons is unaltered in the ARNesCre mice [39]. In addition, the amount of AR protein in both BC and LA muscles is also similar, and there is no difference in BC-LA muscle weights between adult control and mutant males [39]. In the SNB, the AR participates in the developmental regulation of both soma size and dendritic length but not in the survival of SNB motoneurons [39]. Immunohistochemical studies in the lumbosacral spinal cord also demonstrated the expression of the AR in the cellular nuclei of SNB motoneurons in controls but not in ARNesCre males [39]. However, loss of Ar expression in the nervous system resulted in a significant decrease in the number of GRP-immunoreactive neurons in the lumbosacral spinal cord compared with that in control males [40]. Consequently, the intensity of GRP axonal projections to both the lower lumbar and upper sacral spinal cord was also greater in control males compared to ARNesCre males [40]. Thus, these results suggest that nervous system AR activation during neonatal development regulates both morphological differentiation and adult activation of SNB motoneurons, but does not directly affect the survival of SNB motoneurons [39]. On the other hand, central but not peripheral ARs are critical for developmental regulation and maintenance of the male dominant cell number and dendritic arborization in the spinal GRP system [40]. Nevertheless, we cannot rule out the possibility that both the number of GRP neurons and possibly the immunoreactivity for GRP in the GRP neurons are also attenuated in ARNesCre males in adulthood [40]. The role (directly or indirectly) of GRP neuronal survival in the lumbosacral spinal cord remains unknown.
2.4. In Vivo Physiological Significance of the GRP/GRPR System in the Lumbosacral Spinal Cord
Pharmacological stimulation of GRPRs by the rodent specific agonist, rat neuromedin C (a 10-amino-acid C-terminal fragment of rat GRP, termed rat GRP20–29 [41]), ameliorates both penile reflexes and the ejaculation frequency in castrates [19]. Intrathecal administration of RC-3095, a specific antagonist for GRPR to this spinal region drastically inhibits both penile reflexes and the ejaculation frequency in normal male rats [19]. Considerable changes in the lumbosacral spinal cord were found in a post-traumatic stress disorder (PTSD) rat model (single-prolonged stress-exposed) where there was a decrease not only in the GRP mRNA and protein expression but also in the AR protein levels [42]. We further observed that the trauma-exposed rats showed significant reductions in penile reflexes [42]. These molecular as well as anatomical changes may be directly linked to a decrease in the number of penile reflexes in rats [42]. Thus, it is possible that a severe mental stress attenuates two aspects of the GRP system that regulates penile reflexes. Namely, the expressions of GRP and AR in the lumbar spinal cord are significantly weakened by a psychological trauma [42]. It is significant that in vivo pharmacological stimulation of GRPRs also improves penile function in a dose-dependent manner, and this effect is attenuated significantly by traumatic stress in a rat PTSD model [42]. Although the spontaneous ejaculation per se is often observed in unstressed control males, GRPR agonistic agents significantly increase in the frequency of the spontaneous ejaculation in trauma-exposed males [43].
In order to examine the actions of GRPR in vivo, we have recently generated a transgenic rat expressing the red fluorescent protein under control of the GRPR promoter. Preliminarily, in the lumbosacral spinal cord of the transgenic rat, we observed higher fluorescent signals in SNB motoneurons. Furthermore, the fluorescent signal was greater in males than females (our unpublished observation). These observations strongly suggest that a spinal GRP/GRPR system could generate an ejaculatory response by activating autonomic and somatic centers; e.g. the SPN and SNB in the lumbosacral spinal cord. Findings from this set of studies support the hypothesis that the GRP/GRPR system may regulate male sexual behavior via afferents to both SPN and SNB neurons, and also coordinate autonomic and somatic functions in response to penile responses during male copulatory behavior.
2.5. Therapeutic Treatments Targeting GRPR in the Lumbosacral Spinal Cord
Androgens, including testosterone and 5alpha-dihydrotestosterone, play an important role in ejaculation behavior in rodents [44] as well as in humans [45]. AR expression in GRP-expressing neurons of the ejaculation center may offer a new avenue for androgenic modulation of penile reflexes regulated at the spinal cord level [19]. Further studies investigating GRP might also aid in the development of new pharmacological interventions to relieve psychogenic ED at the spinal cord level, since this system in the lumbosacral spinal cord specifically relies on the neuropeptide GRP [43]. The effects of GRPR agents in controlling male reproductive functions are robust, at least within the lumbosacral spinal cord, and are mediated by effects on the spinal GRP/GRPR system. Single-prolonged stress-model may also serve as a viable paradigm to study the pathophysiology and neuropathy of psychogenic ED, because a significant reduction in the frequency of penile reflexes was observed in rodent PTSD models [42, 43]. In addition, the administration of a specific GRPR agonist might improve ejaculation frequency in castrated as well as trauma-exposed rats [42, 43]. This is the first observation of a neuronal pathway to restore penile reflexes without androgens in mammals [43].
In rodents, a hypothesis is proposed that GRPR signaling in the lumbosacral spinal cord plays an important role in the regulation of male sexual functions. Clinically, however, the next question is: Does the spinal GRP/GRPR system exist and function in the lower spinal cord in humans as well? Future studies should include an emphasis on comparative studies for the spinal GRP/GRPR system, involving other mammals, particularly humans and/or primates.
3. GRPR TARGETING FOR CHRONIC PRURITUS
3.1. Discovery of the GRPR as an Itch-Specific Receptor in the Somatosensory System
Itch or pruritus has been defined as an unpleasant sensation of the skin that provokes the urge to scratch in order to relieve the stress of itch. Itch followed by scratching worsens skin inflammation, inducing an itch-scratch cycle. Pruritus can be distinguished as acute or chronic, with the latter defined as pruritus lasting 6 or more weeks based on a recommendation by the International Forum for the Study of Itch [46]. Chronic itch associated with inflammatory skin diseases, neurological diseases, psychiatric diseases, and systemic diseases markedly decreases work productivity and quality of life because of the discomfort, inability to concentrate, stress, and insomnia. The neural circuit of itch and the molecular basis of itch are associated with those of pain, however, itch-specific mediators have not been identified. Therefore, no fundamental therapeutic agent for chronic pruritus has been developed. It was reported in the 1980s that intracerebroventricular injection of various peptides induced scratching and grooming behavior in various experimental animals. Bombesin, an exogenous ligand for GRPR in mammals, significantly elicited scratching behavior in mice, rats, guinea pigs, rabbits, and monkeys [47-49]. However, the molecular mechanisms underlying the initiation of the scratching behavior remain unknown.
The exciting discovery of Sun and Chen [23] presented a major breakthrough in research on the molecular basis of itch. They reported that intradermal injection of some pruritogens, such as protease-activated receptor 2 (PAR2) agonist SLIGRL-NH2, chloroquine, and compound 48/80, markedly decreased the scratching behaviors in GRPR KO animals compared with those of controls. In contrast, the scratching behaviors elicited by histamine, endothelin-1, and serotonin in GRPR KO animals were comparable with that of the controls [24]. Furthermore, GRPR KO animals showed normal nociceptive responses to mechanical, thermal, inflammatory, and neuropathic stimulation as well as normal motor function [23]. Intrathecal administration of a GRPR antagonist also reduced scratching behavior in control mice upon administration of some peripheral pruritogens [23]. Another group demonstrated that intracerebroventricular administration of GRP dose-dependently elicited marked scratching in both mice [50] and rats [51]. Furthermore, intracerebroventricular injection of GRPR antagonist inhibited GRP-induced scratching behavior [50, 51]. In addition, GRP is expressed in several unmyelinated primary afferents, and the central axon terminals of primary afferents showed intense GRP-immunoreactivity in the superficial layers of the spinal dorsal horn [23]. Specific ablation of the GRPR-expressing cells in the lamina I of the spinal dorsal horn dramatically suppressed the scratching behavior induced by histamine-dependent pruritonic agents, such as histamine, compound 48/80, serotonin, and endothelin-1, by histamine-independent pruritonic agents, such as a PAR2 agonist and chloroquine, and by diphenylcyclopropenone, which induce chronic itch [24]. Subsequently, Liu et al. [52] reported the co-expression of GRPR and the mu-opioid receptor isoform MOR1D which is necessary for the scratching behavior evoked by morphine. They also demonstrated functional heterodimerization of GRPR and MOR1D in the lamina I of the spinal cord. Furthermore, they showed that both GRPR and MOR1D internalization were induced by morphine [52]. Pharmacological behavioral tests further demonstrated that GRPR plays an important role in pruriception mediated by opioid, but not in antinociception mediated by opioid [52]. Together these findings indicate that GRPR in the spinal cord takes a central role as the relay point of itch transmission from reception of various stimuli in peripheral tissues to the higher brain regions.
3.2. Expression and Distribution of GRP/GRPR in Somatosensory Systems
Using immunohistochemical analysis, several groups reported that either bombesin- or GRP-immunoreactivity was observed in 2-9% of the DRG neurons in mice [23, 53-58], rats [25], and monkeys (our unpublished observations), and was primarily localized in small- or medium-sized cells (Fig. 3). GRP often co-localized with other peptides, such as substance P and calcitonin gene-related peptide, and also co-localized with some itch mediators, including Mas-related GPCR A3 (MrgprA3), Toll-like receptor 7 (TLR7), and transient receptor potential vanilloid 1 (TRPV1), but rarely co-localized with the non peptidergic marker, isolectin B4 [23, 25]. One of the Mrgpr family, MrgprA3, is a receptor for chloroquine, an anti-malaria drug that induces scratching [59]. MrgprA3-expressing neurons are responsive to both histamine and chloroquine [59]. Histological studies indicate that 93% of the MrgprA3-positive neurons also expressed GRP [59]. Moreover, abundant synapses functionally connect GRPR-positive cells and MrgprA3-positive primary sensory neurons in the spinal dorsal horn [60]. TLR7, a member of the TLR family, is mainly expressed in the small-sized DRG neurons and is important for inducing pruritus elicited by nonhistaminergic pruritogens, but is not necessary for pain in mice [54]. Single-cell real-time PCR analysis indicated that TLR7-positive cell populations also expressed GRP, MrgprA3, and TRPV1 in the DRG of the mouse [54]. TRPV1 is considered to be a nociceptive primary afferent marker and is activated by several factors, including capsaicin, noxious heat, and many inflammatory mediators. A recent report indicated that a particular TRPV1-positive primary afferent responded to some pruritogens, such as chloroquine, histamine, and imiquimod, through diverse intracellular signal transduction pathways [61-64]. Neurons co-expressing GRP and TRPV1 in the DRG have been identified [23, 25, 53].
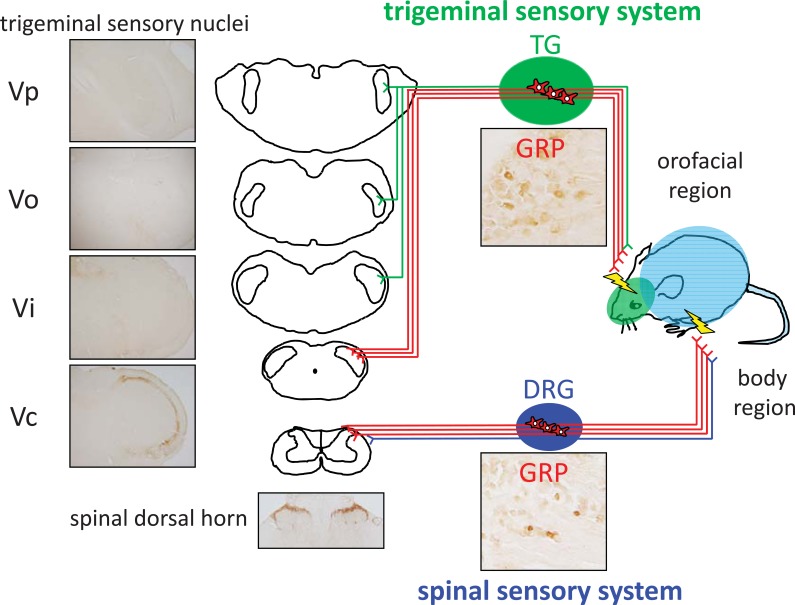
Fig. (3).
Distribution of gastrin-releasing peptide (GRP) in both the trigeminal and spinal somatosensory systems. GRP is expressed in both TG and DRG neurons, and expression is specific to the small- and medium-sized cells. GRP-positive fibers are rarely observed in the trigeminal sensory nucleus principalis (Vp), the trigeminal spinal nucleus oralis (Vo), and interpolaris (Vi). On the other hand, GRP-expressing fibers are predominant in the superficial layers of the trigeminal spinal nucleus caudalis (Vc), similar to that in the spinal dorsal horn. (DRG, dorsal root ganglion; TG, trigeminal ganglion).
GRP protein expression in the DRG has been demonstrated, but there is some debate involving expression of mRNA for GRP in the DRG. Several groups detected the expression of GRP mRNA in the DRG by reverse transcription-PCR analysis [54, 55, 57, 65] and in situ hybridization analysis [57]. In contrast, some groups reported that it is difficult to detect the expression of GRP mRNA in the DRG, but they could detect robust expression of GRP in the spinal dorsal horn [55, 66]. Mishra et al. [66] argued that GRP-positive neurons in the spinal cord are downstream of natriuretic peptide B-expressing primary afferents which are directly activated by peripheral stimulation. Other groups determined the origin of the GRP-fibers in the spinal cord by spinal dorsal root rhizotomy. They demonstrated that GRP-containing fibers in the spinal dorsal horn were prominently decreased in the surgically treated side of both mice [23, 58] and rats [25]. Thus, it has been suggested that the majority of the GRP-containing fibers in the spinal dorsal horn may be derived from primary afferents [23, 25, 58].
Peripheral axon terminals of primary afferents expressing GRP enter both the epidermis and dermis in mice [56, 67] and the dermoepidermal junction in both monkeys [68] and humans [69, 70]. Not all, but some GRP-containing fibers express TRPV1, PAR2, mu-opioid receptor, and tropomyosin-related-kinase A in the skin of an NC/Nga atopic mouse model [67]. In an investigation into the function of GRP in the skin, Andoh et al. [71] reported that an intradermal injection of GRP not only evoked scratching behavior but also led to degranulation of mast cells via GRPR in the mast cells. Cutaneous GRP-induced scratching was markedly reduced by a variety of factors, including the GRPR antagonist RC-3095, the mu-opioid receptor antagonist naltrexone hydrochloride, the H1 histamine receptor antagonists chlorpheniramine maleate and fexofenadine hydrochloride, and the PAR2 antagonist FSLLRY-NH2 [71]. Taken together, these findings revealed that GRP is distributed in both the peripheral and central terminals of primary afferents and is involved in the response to the itch sensation.
In regard to the role of the trigeminal sensory system which transmits the sensations from the orofacial regions, we found that 12% of trigeminal neurons as well as DRG neurons were GRP-positive in rats [25]. These GRP-positive neurons were primarily small- or medium-sized cells and are identified in rats (Fig. 3) [25] as well as in monkeys (our unpublished observations). The superficial laminae of the trigeminal spinal nucleus caudalis (Vc) are regarded as the projection site of the trigeminal nociceptive afferents. Electrophysiological responses were observed in the Vc superficial layers by both nociceptive and pruriceptive stimulation in the orofacial receptive fields [72, 73]. Recently, we demonstrated that GRP-containing fibers are abundant in the superficial layers of the Vc, but are found in very low-density in the rostral part of the trigeminal sensory nucleus principalis, the trigeminal spinal nucleus oralis, and interpolaris (Fig. 3) [25]. The expression of GRPR mRNA was observed in the spinal dorsal horn in both mice and rats [19, 23, 55] and also observed in the Vc dorsal horn in rats (our unpublished observations). Therefore, these results indicate that there are GRP/GRPR neuronal synaptic contacts in the Vc, which are involved in itch transduction at the brainstem level.
In terms of expression of GRP and GRPR in chronic pruritus, an atopic dermatitis NC/Nga mouse model showed high densities of GRP-containing fibers in the epidermis [67]. GRP expression in the DRG and GRPR expression in the spinal dorsal horn were both increased in allergic contact dermatitis and in the dry skin mouse model [58]. Similar results were also reported in monkeys with chronic pruritus [68]. Atopic dermatitis patients have elevated serum GRP levels and show increases in the visual analogue scale itch score compared with healthy individuals [69]. Thus, the expression of GRP/GRPR in the sensory nervous system might be a conserved property in mammals, and abnormal regulation of the GRP/GRPR system has been indicated in chronic pruritus.
3.3. In Vivo Physiological Significance of the GRP/GRPR System in Somatosensory Systems
Several groups have determined the function of both GRP and GRPR in somatosensory systems using a combination of calcium imaging, electrophysiology, and a behavioral pharmacological test. Calcium imaging and histological analyses have demonstrated that a substantial population (20-40% each) of pruritogen (e.g., chloroquine, bovine adrenal medulla peptide 8-22, histamine, and SLIGRL-NH2)-responsive DRG neurons expressed GRP [74]. Whereas scratching behavior induced by chloroquine and SLIGRL-NH2 was decreased by systemic or intrathecal administration of a GRPR antagonist, the activity generated by either histamine or the bovine adrenal medulla peptide 8-22 was not decreased by administration of a GRPR antagonist. This implyies that GRP is the spinal neuropeptide transmitter for the non-histaminergic itch [74]. Furthermore, using single-unit recording electrophysiology, Akiyama et al. [75] found that GRP, substance P, and glutamate play a role in the spinal neurotransmission of histamine-dependent and -independent itch. Intradermal injection of chloroquine did not reduce the responses of single superficial dorsal horn neurons to either a GRPR antagonist or the neurokinin-1 receptor (NK-1R), a substance P receptor, antagonist. However, the responses were completely inhibited by the co-application of a GRPR antagonist, an NK-1R antagonist, and an AMPA/kainate receptor antagonist CNQX [75]. Intrathecal administration of each GRPR, NK-1R, or AMPA/kainate receptor antagonists alone markedly decreased the scratching behavior evoked by chloroquine and administration of all 3 antagonists abolished scratching [75]. To provide direct evidence that GRP actually mediated the responses between primary afferent fibers and dorsal horn neurons, Koga et al. [76] performed electrophysiological and calcium imaging analyses. They found that 9% of the dissociated rat dorsal horn neurons showed an increase in calcium signaling induced by GRP application [76]. GRP application increased action potential firing in approximately 25% of superficial dorsal horn neurons in both adult rat and mouse spinal cord slice preparations by whole-cell patch clamp recordings, and these neurons exclusively received the input from primary afferent C-fibers [76]. On the other hand, few GRP-responsive neurons received the input from primary afferent Aδ-fibers [76]. Bath application of RC-3095 blocked GRP-induced action potentials firing. Surprisingly, they revealed that CNQX completely inhibited the excitatory postsynaptic currents evoked by unmyelinated primary afferents in GRP responsive dorsal horn neurons, suggesting that the excitatory neurotransmitter, glutamate, mediates synaptic transmission between primary afferent C-fibers and GRP-responsive dorsal horn neurons [76]. Glutamate is the most abundant excitatory neurotransmitter in the primary afferents. The vesicular glutamate transporter subtype 2 (VGLUT2) is highly expressed in the small- and medium-sized DRG neurons and dominates the nociceptive primary afferents [77, 78]. Since the distribution of GRP and VGLUT2 overlap significantly, it is suggested that VGLUT2-mediated neurotransmission in the GRP population plays a role in regulating itch sensation [53]. Using immunoelectron microscopy, Takanami et al. [25] recently reported that many clear and spherical-shaped microvesicles and large dense-core vesicles were observed in GRP-positive presynaptic terminals in the superficial layers of the Vc and spinal cord. Although the contents of the clear microvesicles were not determined, these results suggested that glutamate and/or other excitatory neurotransmitters are contained in the GRP-positive primary afferents and participate in the regulation of both itch and pain in the Vc as well as in the spinal dorsal horn. Other studies suggested that the synaptic glutamate released from a group of peripheral nociceptors is required for pain sensation and suppresses itch, and that glutamate controls the receptivity balance of itch/pain [53, 79]. Further studies are needed to understand how pain and itch sensations are transmitted via GRP and glutamate in both the spinal and trigeminal systems in vivo.
3.4. Therapeutic Treatments for Chronic Pruritus Targeting GRPR in Somatosensory Systems
Directly targeting spinal GRPR can control itch more efficiently than regulating each peripheral pruritogen. Combined administration of an AMPA/kainate receptor antagonist, an NK-1R antagonist, and/or a GRPR antagonist might abolish the scratching behavior compared with the single administration of each drug. Indeed, the combination of a GRPR antagonist with other drugs is becoming more widely used to reduce itch [74, 75]. However, questions remain about the transmission mechanism of itch involved in GRPR-mediated pathways. Although it has been reported that GRP is responsible for both acute and chronic itch transmission, it was shown that using certain genetically modified mice [58], the administration of a GRPR antagonist only decreased the scratching behavior induced by histamine-independent pruritonic agents [74] and GRPR null animals showed similar results [23, 24]. On the other hand, the specific deletion of GRPR-expressing neurons in the lamina I of the spinal dorsal horn using the toxin bombesin-saporin abolished histamine-dependent and histamine-independent itch and chronic itch sensation [24]. Taken together, these results indicated that GRPR is an itch-specific receptor and the targeting GRPR in the spinal cord is critical for the development of clinical treatments. Therefore, it is necessary to further investigate the mechanisms of synapse formation in GRPR-expressing cells. Elucidation of the relationship among GRP, MrgprA3, and TRPV1, and also control of the balance of itch/pain by VGLUT2 and/or Bhlhb5 [80] might lead to a greater understanding of the treatment of acute and chronic pruritus.
Recently, it was suggested that modulation of MAPK signaling pathways in the DRG is involved in the pathology of chronic pruritus [58]. The expression of GRP, MrgprA3, and phosphorylated ERK in DRG and the expression of GRPR in the spinal dorsal horn were up-regulated in an allergic contact dermatitis model and in a dry skin murine model suffering from chronic pruritus [58]. Excessive spontaneous scratching was reduced by the intrathecal administration of an MEK inhibitor in these chronic pruritus models [58]. The B-RAF/MEK/ERK pathway in the sensory system was identified as an upstream regulator of expression of various itch mediators in mice [58]. Although it remains unknown whether the modulation of MAPK signaling pathways is induced by epigenetic changes due to chronic inflammation in the chronic itch model, GRPR is a potential drug target for these signaling pathways that modulate itch sensation.
4. CONCLUSION
We have reviewed some of the data on the functional activity of the GRPR in the spinal cord, including pharmacological treatments for psychogenic ED as well as for chronic pruritus. Two different research groups, including us, have simultaneously reported that the GRP/GRPR systems within the spinal cord not only control male sexual functions [19, 35] but also convey the itch sensation [23, 24]. Coincidently, therefore, these two distinct functions: sexual and sensory roles in the same spinal region are regulated by the same neuropeptide, GRP. The pioneering discoveries of two GRP/GRPR systems in the spinal cord may open the door to a new field of research for many pharmacologists as well as clinicians. In the future, a further understanding of the interaction between the brain and these 2 previously unrecognized spinal local networks will provide a strong impact on various research fields, including neuroscience, pharmacology, andrology, dermatology, etc.
ACKNOWLEDGEMENTS
We are grateful to Dr. D. G. Zuloaga (Oregon Health and Science University, Oregon) for his valuable discussion and for reading this manuscript. This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI): Grant-in-Aid for Young Scientists (A): no. 24680039; no. 21680031; Grant-in-Aid for Challenging Exploratory Research: no. 23659758 (to H.S); Grant-in-Aid for Young Scientists (B): no. 26870496 (to K.T.) from the Ministry of Education, Science, Sports, Culture and Technology, Japan; Grants-in-Aid for the Adaptable and Seamless Technology Transfer Program through target-driven R&D: no. AS242Z02632Q from the Japan Science and Technology Agency, Japan (to K.T.); and by a Research Grant from the Naito Memorial Grant for Natural Science Researches, Japan (to H.S. and K.T); and a Research Grant from the Kato Memorial Bioscience Foundation, Japan (to H.S.).
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest.
REFERENCES
- 1.Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina Alytes. Experientia. 1971;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 2.McDonald TJ, Jornvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979;90(1):227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- 3.Minamino N, Kangawa K, Matsuo H. Neuromedin B a novel bombesin-like peptide identified in porcine spinal cord. Biochem Biophys. Res. Commun. 1983;114(2):541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- 4.Panula P, Nieminen O, Falkenberg M, Auvinen S. Localization and development of bombesin/GRP-like immunoreactivity in the rat central nervous system. Ann. N. Y. Acad. Sci. 1988;547:54–69. doi: 10.1111/j.1749-6632.1988.tb23875.x. [DOI] [PubMed] [Google Scholar]
- 5.Kroog GS, Jensen RT, Battey JF. Mammalian bombesin receptors. Med. Res. Rev. 1995;15(5):389–417. doi: 10.1002/med.2610150502. [DOI] [PubMed] [Google Scholar]
- 6.Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991;14(12):524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- 7.Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, Battey J. cDNA cloning, characterization and brain region-specific expression of neuromedin B-preferring bombesin receptor. Neuron. 1991;6:421–430. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]
- 8.Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville EA, Battey JF. BRS-3 a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J. Biol. Chem. 1993;268(8):5979–5984. [PubMed] [Google Scholar]
- 9.Ladenheim EE, Taylor JE, Coy DH, Moore KA, Moran TH. Hindbrain GRP receptor blockade antagonizes feeding suppression by peripherally administered GRP. Am. J. Physiol. 1996;271(1 Pt 2):R180–184. doi: 10.1152/ajpregu.1996.271.1.R180. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Wada E, Wada K. Bombesin-like peptides: studies on food intake and social behaviour with receptor knock-out mice. Ann. Med. 2000;32(8):519–529. doi: 10.3109/07853890008998831. [DOI] [PubMed] [Google Scholar]
- 11.Ladenheim EE, Behles RR, Bi S, Moran TH. Gastrin-releasing peptide messenger ribonucleic acid expression in the hypothalamic paraventricular nucleus is altered by melanocortin receptor stimulation and food deprivation. Endocrinology. 2009;150(2):672–678. doi: 10.1210/en.2008-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J. Neurosci. 1993;13(2):793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamura H, Ibata Y. GRP immunoreactivity shows a day-night difference in the suprachiasmatic nuclear soma and efferent fibers comparison to VIP immunoreactivity. Neurosci. Lett. 1994;181(1-2):165–168. doi: 10.1016/0304-3940(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 14.Karatsoreos IN, Romeo RD, McEwen BS, Silver R. Diurnal regulation of the gastrin-releasing peptide receptor in the mouse circadian clock. Eur. J. Neurosci. 2006;23(4):1047–1053. doi: 10.1111/j.1460-9568.2006.04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111(6):905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 16.Roesler R, Lessa D, Venturella R, Vianna MR, Luft T, Henriques JA, Izquierdo I, Schwartsmann G. Bombesin/gastrin-releasing peptide receptors in the basolateral amygdala regulate memory consolidation. Eur. J. Neurosci. 2004;19(4):1041–1045. doi: 10.1111/j.0953-816x.2004.03175.x. [DOI] [PubMed] [Google Scholar]
- 17.Merali Z, Bedard T, Andrews N, Davis B, McKnight AT, Gonzalez MI, Pritchard M, Kent P, Anisman H. Bombesin receptors as a novel anti-anxiety therapeutic target BB1 receptor actions on anxiety through alterations of serotonin activity J. Neurosci. 2006;26(41):10387–10396. doi: 10.1523/JNEUROSCI.1219-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roesler R, Kent P, Schroder N, Schwartsmann G, Merali Z. Bombesin receptor regulation of emotional memory. Rev. Neurosci. 2012;23(5-6):571–586. doi: 10.1515/revneuro-2012-0046. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto H, Matsuda KI, Zuloaga DG, Hongu H, Wada E, Wada K, Jordan CL, Breedlove SM, Kawata M. Sexually dimorphic gastrin releasing peptide system in the spinal cord controls male reproductive functions. Nat. Neurosci. 2008;11(6):634–636. doi: 10.1038/nn.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297(5586):1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto H, Kawata M. Gastrin-releasing peptide system in the spinal cord controls male sexual behaviour. J. Neuroendocrinol. 2009;21(4):432–435. doi: 10.1111/j.1365-2826.2009.01847.x. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto H, Arii T, Kawata M. High-voltage electron microscopy reveals direct synaptic inputs from a spinal gastrin-releasing peptide system to neurons of the spinal nucleus of bulbocavernosus. Endocrinology. 2010;151(1):417–421. doi: 10.1210/en.2009-0485. [DOI] [PubMed] [Google Scholar]
- 23.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448(7154):700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 24.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325(5947):1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takanami K, Sakamoto H, Matsuda KI, Satoh K, Tanida T, Yamada S, Inoue K, Oti T, Sakamoto T, Kawata M. Distribution of gastrin-releasing peptide in the rat trigeminal and spinal somatosensory systems. J. Comp. Neurol. 2014;522(8):1858–1873. doi: 10.1002/cne.23506. [DOI] [PubMed] [Google Scholar]
- 26.Brown DJ, Hill ST, Baker HW. Male fertility and sexual function after spinal cord injury. Prog Brain Res. 2006;152:427–439. doi: 10.1016/S0079-6123(05)52029-6. [DOI] [PubMed] [Google Scholar]
- 27.Sipski ML. Sexual functioning in the spinal cord injured. Int. J. Impot Res. 1998;10(Suppl 2, S128-130 ):discussion S138–140. [PubMed] [Google Scholar]
- 28.Breedlove SM. Hormonal control of the anatomical specificity of motoneuron-to-muscle innervation in rats. Science. 1985;227(4692):1357–1359. doi: 10.1126/science.3975621. [DOI] [PubMed] [Google Scholar]
- 29.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7(10):1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda KI, Sakamoto H, Kawata M. Androgen action in the brain and spinal cord for the regulation of male sexual behaviors. Curr. Opin. Pharmacol. 2008;8(6):747–751. doi: 10.1016/j.coph.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system.I. Complete Demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J. Neurosci. 1983;3(2):417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system.II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J. Neurosci. 1983;3(2):424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakamoto H. Brain-spinal cord neural circuits controlling male sexual function and behavior. Neurosci. Res. 2012;72(2):103–116. doi: 10.1016/j.neures.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto H. The gastrin-releasing peptide system in the spinal cord mediates masculine sexual function. Anat. Sci. Int. 2011;86(1):19–29. doi: 10.1007/s12565-010-0097-z. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto H, Takanami K, Zuloaga DG, Matsuda K, Jordan CL, Breedlove SM, Kawata M. Androgen regulates the sexually dimorphic gastrin-releasing peptide system in the lumbar spinal cord that mediates male sexual function. Endocrinology. 2009;150(8):3672–3679. doi: 10.1210/en.2008-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bardin CW, Bullock L, Blackburn WR, Sherins RJ, Vanha-Perttula T. Testosterone metabolism in the androgen-insensitive rat a model for testicular feminization. Birth Defects Orig. Artic. Ser. 1971;7(6):185–192. [PubMed] [Google Scholar]
- 37.Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J. Biol. Chem. 1990;265(15):8893–8900. [PubMed] [Google Scholar]
- 38.Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J. Neurosci. 2009;29(14):4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raskin K, Marie-Luce C, Picot M, Bernard V, Mailly P, Hardin-Pouzet H, Tronche F, Mhaouty-Kodja S. Characterization of the spinal nucleus of the bulbocavernosus neuromuscular system in male mice lacking androgen receptor in the nervous system. Endocrinology. 2012;153(7):3376–3385. doi: 10.1210/en.2012-1001. [DOI] [PubMed] [Google Scholar]
- 40.Sakamoto H, Saito K, Marie-Luce C, Raskin K, Oti T, Satoh K, Tamura K, Sakamoto T, Mhaouty-Kodja S. Androgen regulates development of the sexually dimorphic gastrin-releasing peptide neuron system in the lumbar spinal cord: Evidence from a mouse line lacking androgen receptor in the nervous system. Neurosci. Lett. 2014;558:109–114. doi: 10.1016/j.neulet.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 41.Minamino N, Kangawa K, Matsuo H. Neuromedin C a bombesin-like peptide identified in porcine spinal cord. Biochem Biophys. Res. Commun. 1984;119(1):14–20. doi: 10.1016/0006-291x(84)91611-5. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto H, Matsuda K, Zuloaga DG, Nishiura N, Takanami K, Jordan CL, Breedlove SM, Kawata M. Stress affects a gastrin-releasing peptide system in the spinal cord that mediates sexual function: implications for psychogenic erectile dysfunction. PLoS One. 2009;4(1):e4276. doi: 10.1371/journal.pone.0004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakamoto H. The neurobiology of psychogenic erectile dysfunction in the spinal cord. J. Androl. 2010;31(6):519–526. doi: 10.2164/jandrol.110.010041. [DOI] [PubMed] [Google Scholar]
- 44.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm. Behav. 2007;52(1):45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meston CM, Frohlich PF. The neurobiology of sexual function. Arch. Gen. Psychiatry. 2000;57(11):1012–1030. doi: 10.1001/archpsyc.57.11.1012. [DOI] [PubMed] [Google Scholar]
- 46.Stander S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, Bergasa NV, Gieler U, Misery L, Wallengren J, Darsow U, Streit M, Metze D, Luger TA, Greaves MW, Schmelz M, Yosipovitch G, Bernhard JD. Clinical classification of itch a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87(4):291–294. doi: 10.2340/00015555-0305. [DOI] [PubMed] [Google Scholar]
- 47.Katz R. Grooming elicited by intracerebroventricular bombesin and eledoisin in the mouse. Neuropharmacology. 1980;19(1):143–146. doi: 10.1016/0028-3908(80)90181-1. [DOI] [PubMed] [Google Scholar]
- 48.Gmerek DE, Cowan A. Studies on bombesin-induced grooming in rats. Peptides. 1983;4(6):907–913. doi: 10.1016/0196-9781(83)90089-x. [DOI] [PubMed] [Google Scholar]
- 49.Cowan A, Khunawat P, Zhu XZ, Gmerek DE. Effects of bombesin on behavior. Life Sci. 1985;37(2):135–145. doi: 10.1016/0024-3205(85)90416-3. [DOI] [PubMed] [Google Scholar]
- 50.Sukhtankar DD, Ko MC. Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS One. 2013;8(6):e67422. doi: 10.1371/journal.pone.0067422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su PY, Ko MC. The role of central gastrin-releasing peptide and neuromedin B receptors in the modulation of scratching behavior in rats. J. Pharmacol. Exp. Ther. 2011;337(3):822–829. doi: 10.1124/jpet.111.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, Chen ZF. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147(2):447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallen-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68(3):529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010;13(12):1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol. Pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inan S, Dun NJ, Cowan A. Investigation of gastrin-releasing peptide as a mediator for 5'-guanidinonaltrindole-induced compulsive scratching in mice. Peptides. 2011;32(2):286–292. doi: 10.1016/j.peptides.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu XY, Wan L, Huo FQ, Barry DM, Li H, Zhao ZQ, Chen ZF. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol Pain. 2014;10(1):4. doi: 10.1186/1744-8069-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, Liu XY, Barry DM, Wan L, Liu ZC, Li H, Turkoz A, Ma K, Cornelius LA, Kopan R, Battey JF, Zhong J, Chen ZF. Chronic itch development in sensory neurons requires BRAF signaling pathways. J. Clin. Invest. 2013;123(11):4769–4780. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139(7):1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 2013;16(2):174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 2007;27(9):2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SJ, Park GH, Kim D, Lee J, Min H, Wall E, Lee CJ, Simon MI, Lee SJ, Han SK. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. U. S. A. 2011;108(8):3371–3376. doi: 10.1073/pnas.1019755108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30(3):582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU. The TGR5 receptor mediates bile acid-induced itch and analgesia. J. Clin. Invest. 2013;123(4):1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340(6135):968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice an atopic dermatitis model. J Invest Dermatol. 2009;129(12):2901–2905. doi: 10.1038/jid.2009.188. [DOI] [PubMed] [Google Scholar]
- 68.Nattkemper LA, Zhao ZQ, Nichols AJ, Papoiu AD, Shively CA, Chen ZF, Yosipovitch G. Overexpression of the gastrin-releasing peptide in cutaneous nerve fibers and its receptor in the spinal cord in primates with chronic itch. J. Invest. Dermatol. 2013;133(10):2489–2492. doi: 10.1038/jid.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kagami S, Sugaya M, Suga H, Morimura S, Kai H, Ohmatsu H, Fujita H, Tsunemi Y, Sato S. Serum gastrin-releasing peptide levels correlate with pruritus in patients with atopic dermatitis. J. Invest. Dermatol. 2013;133(6):1673–1675. doi: 10.1038/jid.2013.38. [DOI] [PubMed] [Google Scholar]
- 70.Timmes TR, Rothbaum R, Kirti Silva CY, Bhawan J, Cummins DL, Wolpowitz D. Gastrin-releasing peptide-expressing nerves comprise subsets of human cutaneous Adelta and C fibers that may sense pruritus. J. Invest. Dermatol. 2013;133(11):2645–2647. doi: 10.1038/jid.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andoh T, Kuwazono T, Lee JB, Kuraishi Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides. 2011;32(10):2098–2103. doi: 10.1016/j.peptides.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J. Neurophysiol. 2010;104(5):2442–2450. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J. Neurophysiol. 2011;106(3):1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J. Neurophysiol. 2013;109(3):742–748. doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain. 2014;155(1):80–92. doi: 10.1016/j.pain.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol. Pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brumovsky P, Watanabe M, Hokfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147(2):469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 78.Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc. Natl. Acad Sci. U.S.A. 2010;107(51):22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68(3):543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65(6):886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]