Abstract
Background:
At least 10-20% of the patients suffering from depression meet criteria for treatment-resistant depression (TRD). In the last decades, an important role of glutamate in mood modulation has been hypothesized and ketamine, a non noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptors, has been demonstrated to be effective in both MDD and TRD. However, concerns emerged about the optimal dosage, and frequency of administration of this treatment.
Methods:
aiming to systematically review the current literature focusing on the main pharmacological properties and impact of ketamine in TRD, a detailed literature search in PubMed/Medline and ScienceDirect databases was conducted. Twenty-four manuscripts including a total of 416 patients fulfilled inclusion criteria.
Results:
Most studies demonstrated that the NMDA antagonist ketamine has rapid antidepressant effects in TRD patients, confirming the active role of glutamate in the pathophysiology of this complex condition. Ketamine has been demonstrated to be rapidly effective and was associated with a significant clinical improvement in depressive symptoms within hours after administration. Also, ketamine was also found to be effective in reducing suicidality in TRD samples.
Limitations:
The long-term efficacy of ketamine has not been investigated by most studies. The psychotomimetic properties may complicate the application of this pharmacological agent.
Conclusions:
Ketamine may be considered a valid and intriguing antidepressant option for the treatment of TRD. Further studies are needed to evaluate its long-term antidepressant efficacy in patients with TRD.
Keywords: Antidepressant effect, ketamine, NMDA receptors, pharmacological properties, treatment-resistant depression.
INTRODUCTION
Major depressive disorder (MDD) is a disabling illness that is associated with frequent relapses, incomplete recovery between episodes, and persistent psychosocial and functional impairment [1-3]. MDD is considered one of the ten leading causes of disability worldwide and is also associated with an increased risk of suicidal behaviours [4-7]. Although many psychopharmacological agents are currently available for the treatment of MDD [8], approximately 10-20% of patients treated with the common antidepressant medications do not achieve complete recovery and meet the criteria of treatment-resistance [9, 10].
Treatment-resistant depression (TRD) affects more than 1% of individuals in the United States, and approximately 30% of all depressed patients may be classified as affected by refractory depression [11]. TRD is a disabling disorder associated with relevant psychosocial impairment and poor social/occupational outcome [12, 13]. Although many definitions of TRD have been provided in the current literature [11, 14], TRD may be generally defined as a failure to respond to at least two different types of antidepressants for a period longer than 4 weeks at the maximum recommended dose. To date, the pathogenesis of TRD remains quite unclear.
According to the monoamine hypothesis, depressive symptoms are mainly related to a deficit in the synaptic availability of monoamines and most antidepressant drugs are believed to modulate these monoamine neurotransmitter systems (e.g., norepinephrine, dopamine, or serotonin) [15]. As it’s well documented that the therapeutic effect with the common antidepressant medications emerges only after 4-12 weeks of treatment [16], pharmacological agents that exert rapid antidepressant effects may be actually considered a real unmet need in clinical practice [17]. In order to develop more effective drugs, it’s crucial for clinicians to focus on novel molecular targets outside of the monoamine system [5, 7, 8].
Glutamate, the major excitatory amino acid in the central nervous system, has important roles in many normal and abnormal physiological processes. These roles include neurodevelopmental and neurotrophic effects (nerve cell growth, differentiation, function, and maintenance), neurocognitive functions (learning and memory), modulation of other neurotransmitter systems, and neurodegeneration (nerve cell damage or death). Glutamate systems have been directly or indirectly implicated in mood and anxiety disorders, schizophrenia, substance abuse (e.g., alcohol and hallucinogens), and various neurodegenerative disorders (e.g., stroke and other traumatic brain injuries, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), etc.).
Glutamate is released from nerve cells, binds to receptors, and is removed by reuptake transporters. Glutamate receptor systems are complex, and they can be segregated into various distinct receptor subtypes according to their molecular and pharmacological properties. The two main classes of glutamate receptors are divided in “ionotropic” and “metabotropic.” Ionotropic glutamate receptors are classified into three groups: N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA), and kainate. Each group also has multiple subtypes. Similarly, metabotropic glutamate receptors are classified into three groups, with eight subtypes. Drugs can potentially bind to different glutamate receptor subtypes and therefore modulate glutamate function in different ways (with different physiological and clinical effects). Most clinically relevant studies have focused on drugs that modulate glutamate function via NMDA receptors, although there is interest in AMPA and metabotropic receptors as well.
The first use of a glutamate-modulating drug therapy for mental disorders was reported more than 50 years ago [18, 19]. Cycloserine is an antibiotic drug originally developed for treating tuberculosis. When given at high doses to depressed patients with tuberculosis, in 1959 Crane [18] reported that cycloserine had significant antidepressant effects, but it was also associated with serious adverse neuropsychiatric effects. Cycloserine is a chemical analogue of the amino acid alanine. At lower doses, it is a partial receptor agonist (receptor-stimulating) at the NMDA receptor site, but it is an NMDA receptor antagonist (receptor-blocking) at higher doses.
Ketamine, is a high-affinity NMDA receptor antagonist that also binds to opioid µ and sigma receptors. Ketamine has been reported to modulate dopamine transmission [20-22], but whether its antidepressant effect is linked to this dopamine activity is a matter of debate. Ketamine has been associated with antidepressant effects in animal models of depression and with rapid antidepressant effects in human studies of depression. The rapid and sustained antidepressant properties of ketamine have been documented by several case reports/series, prospective open-label, double-blind placebo- or active-controlled studies that will be examined below.
In this paper, we aimed to systematically review the current literature focusing on the main pharmacological effect of ketamine in patients with TRD.
METHODS
Information Sources, Search Strategy and Study Selection
A detailed search strategy, as summarized in Fig. (1), was used to identify relevant studies. In order to provide a new and timely critical review about the role of ketamine in TRD, we performed a systematic PubMed/Medline, Scopus, and Science Direct search to identify all papers and book chapters in the English language during the period between 1980 and November 2013.
Fig. (1).
Flowchart of the search and selection process.
The search used a combination of the following terms: “Ketamine” OR “Ketamine treatment” OR “NMDA Receptor Antagonist Ketamine” AND “Treatment-resistant-depression” OR “Therapy-resistant-depression” OR “Refractory depression” OR “Treatment-resistant major depressive disorder” OR “Treatment-resistant MDD”. When a title or abstract seemed to describe a study eligible for inclusion, the full article was examined to assess its relevance based on the inclusion criteria. Two blinded, independent researchers (GS and FR) conducted a two-step literature search. Any discrepancies between the two reviewers were resolved by consultations with the senior authors (PG, HRH, MA). The reference lists of the articles included in the review were also manually checked for relevant studies while other publications were cross-referenced for any additional published articles. Only English language full-text articles reporting original data about the main topic were included.
Study Design and Eligibility Criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses‘ (PRISMA) guidelines [23]. Studies were included according to the following criteria: (a) being an original paper in a peer-reviewed journal; (b) containing results concerning the efficacy of ketamine in TRD. Fig. (1) summarizes the search strategy used for selecting studies (identification, screening, eligibility, inclusion process) in the present review. Papers that were not written in English, book chapters, conference abstracts, and case studies were not reviewed.
Recorded Variables
We retained the following variables for each article about ketamine in TRD: study design, sample characteristics, main results, response and remission rates (for open-label and randomized double-blind studies), limitations and conclusions (for more details, see Tables).
RESULTS
Number of Selected Studies
The combined search strategy yielded a total of 278 articles, of which 38 full-text articles were screened and 240 excluded. In detail, we excluded from the present study duplicates or articles not published in peer reviewed journals and not in English language; papers without abstracts or manuscripts including abstracts that did not explicitly mention the link between ketamine and TRD; articles published before 1980; articles including unclear data concerning materials and methods or number of patients analyzed. Of the initial 38 papers, 28 were judged eligible. However, four full-text articles were also excluded due to their low relevance for the main theme. This left 25 papers examining a total of 416 patients that fulfilled our inclusion criteria.
SUMMARY OF MAIN RESULTS
Case Reports and Case Series
Some (eight) case reports and case series including a total of seventeen patients reported data regarding the efficacy of ketamine in patients with TRD.
Szymkowicz et al. [24] reported that three patients responded successfully to the ketamine infusions and no significant side-effects have been found after ketamine administration.
After 24 hours following the first dose of ketamine, Murrough et al. [25] showed a significant antidepressant activity (89% change in MADRS scores) of ketamine in a subjects treated with ketamine. A sustained (three months) remission from depression has also been reported.
Segmiller et al. [26] found that 50% of the six TRD patients which were enrolled showed an improvement in depressive symptoms in both the short and longer term. In two patients (33.3%) remission has been reached whereas two other patients experienced dissociative symptoms.
Robust changes in depression symptoms measured by the BDI scores in response to ketamine have also been suggested by Messer et al. [27]. No memory or concentration impairments have been associated with ketamine infusions.
Similarly, Paslakis et al. [28] showed that a significant improvement was obtained with the use of ketamine in two patients whereas no significant side effects were reported.
Subjects experienced a significant improvement of depressive symptoms on the second day post ketamine infusion based on Hamilton Depression Rating Scale (HDRS), and Beck Depression Inventory (BDI) scores [29]. The first improvement has been reported 25 minutes after ketamine infusion but a persistent antidepressant effect has also been observed throughout the subsequent 7 days. Specifically, after the first ketamine infusion, a profound improvement of depressive symptoms was reported the second day post infusion whereas the second infusion was less effective.
Finally, Paul et al. [30] reported that one patient did not respond to both intravenous administration of ketamine and S-ketamine whereas another patient showed a rapid antidepressant effect as assessed by a relevant decrease in HAMD21 and BDI at days 1 and day 3 but not until day 6. Both patients experienced psychomimetic side effects during ketamine infusion which were absent when S-ketamine has been administered. Table 1 summarizes the most relevant case reports reporting the antidepressant efficacy of ketamine in patients with TRD.
Table 1.
Most relevant case reports/series reporting the antidepressant efficacy of ketamine on suicidality in patients with TRD.
| Author(s), Year | Sample Characteristics | Main Results | Number of Infusions Needed for Achieving Antidepressant Effects | Route of Administration | Limitations | Conclusions |
|---|---|---|---|---|---|---|
| Szymkowicz et al. 2013 [24] | Three patients were administered ketamine at 0.5 mg/kg for 40 minutes and evaluated with the MADRS. | All three patients responded (after 5 infusions) and remitted after ketamine infusions. No significant side-effects have been reported. | 6.6 infusions | Intravenous infusions | This was an open-label naturalistic study without blinding, randomization, or placebo control. The small sample size did not allow the generalization of the findings. | Low-dose repeated intravenous ketamine has a rapid and safe antidepressant activity in patients with TRD. |
| Segmiller et al. 2013 [26] |
Six patients with TRD were treated with 40-minute ketamine infusion (0.25 mg) and evaluated with HDRS21 before and 120 minutes after each infusion. | Three patients (50%) showed an improvement in depressive symptoms in both short and longer term period. Specifically, patients responded after one ketamine infusion. Remission has been reached in two patients (33.3%); two patients reported dissociative symptoms. | Single infusion | Intravenous infusions | The small sample size did not allow the generalization of the findings. Dissociative symptoms should represent a limitation when interpreting the present findings. Dissociative symptoms could be observed more frequently in patients treated with S-ketamine. | The most relevant antidepressant effect has been reported after the first ketamine administration. Multiple administrations of S-ketamine appear to be well tolerated in most cases. |
| Murrough et al. 2011 [25] |
A 45-year-old women with TRD who took a three-times-weekly intravenous infusions (0.5 mg/kg) of ketamine every two weeks | After 24 hours following the first dose of ketamine, a significant antidepressant activity (89% change in her MADRS scores) of ketamine has been reported. Remission from depression has been reported for the following three months. | Single infusion | Intravenous infusions | This a case report, the results of which may not be generalized to the whole population. | Ketamine has rapid and sustained antidepressant properties as it may enhance neurogenesis and neuroplasticity mechanisms. |
| Messer et al. 2010 [27] | Two adult patients with TRD randomized to six 0.5 mg/kg infusions of ketamine (on days 1, 3, 5, 7, 9 and 11), and four saline infusions (on days 3, 5, 9, and 11), respectively | Patients reported robust changes in depressive symptoms in response to ketamine treatment (after 1.5 infusions) as measured by the BDI scores. No memory or concentration impairments have been associated with ketamine infusions. | 1.5 infusions | Intravenous infusions | This study is a report of two cases and its results did not allow the generalization to other samples. | Multiple treatments of ketamine may have a prolonged benefit for TRD patients. |
| Paslakis et al. 2010 [28] | Two cases in which oral administration of (S)-ketamine (1.25 mg/kg) for 14 days was performed as add-on therapy | A significant improvement was obtained with the use of ketamine. Response and remission rates are achieved in 50% of cases, respectively. No significant side effects were reported. | Not specified | Oral administration | This a case report, the results of which may not be generalized to the whole population. | S-ketamine showed relevant antidepressant effects and was better tolerated than (R)-ketamine. |
| Liebrenz et al. 2009 [29] |
A 55-year-old male subject with a TRD and co-occurring alcohol and benzodiazepines dependence. The same patient received two intrave-nous infusions of 0.5 mg/kg ketamine over the course of 6 weeks. | After the second day of infusion, the subject experienced a significant improvement of his symptoms (-56.6% at the HDRS; -65.4% at the BDI. He continued to improve throughout the subsequent 7 days. The second infusion was less efficacious (HDRS and BDI were reduced by 43 and 35%, respectively). He returned to baseline by day 7. | Single infusion | Intravenous infusions | This is a case report, the results of which may not be generalized to the whole population. The patient was depressed but also affected by alcohol dependence. Doses and administrations of ketamine need to be carefully investigated. | Ketamine has potent antidepressant effects and act very swiftly. Repeated administrations of ketamine produced positive results. |
| Paul et al. 2009 [30] | Two patients with TRD treated with ketamine and S-ketamine; the severity of depression was rated using the HDRS and BDI. |
One patient did not respond to both treatments whereas in the other patient both intravenous administration of ketamine and S-ketamine showed an antidepressant effect as assessed by a decrease in HDRS21 and BDI at days 1 and day 3 but not until day 6 (response rate 50%). Both patients experienced psychomimetic side effects during ketamine infusion which were absent during treatment with S-ketamine. | Single infusion | Intravenous infusions | This a case report, the results of which may not be generalized to the whole depressed population. | S-ketamine could show similar antidepressant effects as ketamine in drug-resistant depression and was better tolerated than ketamine. |
CLINICAL PROSPECTIVE OPEN-LABEL AND RANDOMIZED DOUBLE-BLIND STUDIES
Several (twenty-five) prospective single- or multiple dose open-label and randomized double-blind placebo- or active-controlled studies including a total of 399 patients reported data regarding the efficacy of ketamine in patients with TRD.
SINGLE- OR MULTIPLE-DOSE OPEN-LABEL STUDIES
Five open-label (single- or multiple-dose) studies evaluated the efficacy of ketamine in patients with TRD.
Mathew et al. [31] reported that, in a sample of twenty-six medication-free patients, 65% of patients met response criterion (50% reduction from baseline on the MADRS) after 24 hours of ketamine infusion. Specifically, 54% of patients met response criterion 72 hours after ketamine; however, lamotrigine did not enhance ketamine antidepressant effects.
Ibrahim et al. [32] have previously found, in a comparison between 17 TRD patients previously not responder to electroconvulsive therapy (ECT) and 23 TRD patients who had not previously received ECT, that depressive symptoms as assessed by MADRS resulted significantly improved in the ECT-resistant group after 230 minutes with a moderate effect size whereas a significant improvement with a large effect size has been reported in the non-ECT-resistant group.
In another study, the same authors [33] reported that forty-two TRD patients significantly improved in MADRS scores from baseline after a single intravenous infusion of ketamine (0.5 mg/kg) with initially large and throughout the 28-day study moderate effect sizes of improvement. It has been showed that the mean time to relapse was 13.2 days whereas 27% of ketamine responders had not relapsed by 4 weeks after a single ketamine infusion.
Shiroma et al. [34] reported that 91.6% achieved response criterion and 66.6% remitted in a sample of fourteen subjects with TRD intravenous ketamine (.5 mg/kg) over 40 minutes during a 12-day period. After the first infusion, only three and one subjects responded and remitted, respectively. The authors suggested that 28.6% patients achieved response and 42.8% remitted after three or more infusions whereas 35.7% subjects experienced a prolonged remission during the 4 weeks of follow-up. Also, the mean time for 42.8% of subjects who relapsed was 16 days.
Murrough and colleagues [35] also conducted a prospective open-label study in a sample of 24 TRD individuals who underwent a washout of antidepressant medications followed by up to six infusions of ketamine (.5 mg/kg) three times weekly over a 12-day period. After 2 hours of treatment, a persistent mean reduction in MADRS scores has been reported and the overall response rate was 70.8%. Response at 4 hours was a significant predictor of response at study end and the median time to relapse after the last ketamine infusion was 18 days.
RANDOMIZED DOUBLE-BLIND PLACEBO- OR ACTIVE-CONTROLLED STUDIES
Three randomized double-blind placebo- or active-controlled studies evaluated the efficacy of ketamine in patients with TRD.
A significant improvement has been observed in eighteen depressed subjects after 110 minutes of ketamine administration compared with placebo [36] in a double-blind placebo-controlled study. The authors reported that 71% of those treated with ketamine met response and 29% met remission criteria the next day after the administration of ketamine whereas 35% of these subjects continued to respond for at least 1 week. Those who were treated with ketamine were 1.46 more likely to respond to ketamine after 24 hours and 0.68 more likely to respond after 1 week.
The study of Ibrahim et al. [33] has been first designed to evaluate the time course of antidepressant response to a single intravenous dose of ketamine and then the efficacy and safety of the addition of riluzole to ketamine compared with ketamine alone. However, findings derived by this latter double-blind, randomized, parallel, placebo-controlled, flexible-dose inpatient study were not reported in this section due to their no relevance to the main theme.
Niciu et al. [37] evaluated 22 patients with TRD who received placebo or intravenous ketamine (.5 mg/kg) over 40 minutes and were evaluated with the Young Mania Rating Scale (YMRS). The authors suggested that 18.1% of patients scored greater than 12 on the YMRS. These patients who were randomized to ketamine peaked at the end of the 40-minute infusion but returned to baseline by the following day.
In a randomized double-blind active-control study, Murrough et al. [38] suggested, in a sample of 73 patients randomized to a single infusion of ketamine or midazolam, that patients treated with ketamine had a greater improvement in the Montgomery-Asberg Depression Rating Scale (MADRS) score when compared to those treated with midazolam after 24 hours of treatment, even after adjustment for baseline scores and site. Patients were 2.18 more likely to respond at 24 hours to ketamine than midazolam.
CLINICAL STUDIES INVESTIGATING NEURO-BIOLOGICAL EFFECTS OF KETAMINE AND/OR ITS MECHANISM OF ACTION IN TRD
A significant improvement in MADRS scores after ketamine treatment was also obtained by Machado-Vieira et al. [39] in a sample of twenty-three subjects but no changes in BDNF levels were found after ketamine compared to baseline. No association was found between antidepressant response and BDNF levels.
Phelps et al. [40] reported in a sample of twenty-six subjects that those with a family history of alcohol dependence showed significantly greater improvement in MADRS scores than those who had no family history of alcohol dependence. Responders to a single, open-label intravenous infusion of ketamine hydrochloride (patients with a strong improvements in depressive symptoms 230 minutes after infusion) showed increased cortical excitability but not spontaneous cortical γ-activity changes as suggested by Cornwell et al. [41] in a sample of twenty drug-free TRD patients. Only in responders, stimulus-evoked somatosensory cortical responses increase after infusion of ketamine compared to pretreatment responses.
Murrough et al. [42] have also evaluated neurocognitive functioning in 25 patients with TRD, suggesting poorer baseline neurocognitive performance compared to non-responders (particularly slower processing speed in patients who responded to ketamine 24 hours following treatment). Lower response rate after 24 hours of ketamine infusion was predicted by negative cognitive effects early after ketamine.
Regional metabolism decreased significantly in the habenula, insula, ventrolateral and dorsolateral prefrontal cortices of the right hemisphere whereas metabolism increased in bilateral occipital, right sensorimotor, left parahippocampal, and left inferior parietal cortices as reported by Carlson et al. [43] in a sample of twenty-two unmedicated patients with TRD. The authors conducted a positron emission tomography (PET) to measure regional cerebral glucose metabolism at baseline and following a single dose of intravenous ketamine (.5 mg/kg) over 40 minutes. Metabolism changes in the right superior and middle temporal gyri have been directly correlated with improved depression ratings and inversely with metabolic changes in right parahippocampal gyrus and temporoparietal cortex.
A significant positive correlation has been suggested between baseline delta sleep ratio calculated as SWA(NREM1)/SWA(NREM2) and reduced MADRS scores from baseline to Day 1 in a sample of thirty TRD drug-free by two weeks patients who received a single open-label intravenous infusion of ketamine hydrochloride (.5 mg/kg) over 40 minutes [44].
STUDIES ASSESSING THE EFFICACY OF KETAMINE ON SUICIDALITY IN TRD PATIENTS
Three studies [45-47] reported the efficacy of ketamine on suicidality in TRD patients.
Price et al. [45] reported that MADRS scores were significantly reduced after 24 hours of a single infusion of ketamine (81% of patients received a rating of 0 or 1 post-infusion in twenty-six patients). MADRS reductions were sustained for 12 days by repeated-dose ketamine but, interestingly, suicidal ideation was also reduced following ketamine. Also, ketamine has been reported to have a rapid beneficial effect on suicidal cognition in this sample of TRD patients. The acute improvements on suicidality were sustained even after repeated ketamine infusions.
Also, Diaz Granados et al. [46] suggested that suicidal ideation scores decreased significantly based on the Scale for Suicide Ideation (SSI) 40 minutes after ketamine infusion in thirty-three TRD patients. This reduction remained significant also after 4 hours of ketamine infusion. Ten subjects (30%) had a score of ≥ 4 at baseline; all these scores dropped below 4 (9 dropped by 40 minutes and 1 by 80 minutes). Significant improvements at all time points have been found for depression, anxiety, and hopelessness.
Larkin and Beautrais [47] examined the efficacy of a single IV dose of ketamine (0.2 mg/kg) in 14 depressed emergency department subjects with suicide ideation. Subjects were monitored for four hours and then re-contacted daily for 10 days. Treatment response and suicidality were evaluated using the MADRS. Mean MADRS scores decreased significantly by four hours. Suicide scores on the MADRS also decreased significantly and were sustained during the 10-day follow-up.
Table 5 summarizes the most relevant clinical studies reporting the efficacy of ketamine on suicidality in TRD patients.
Table 5.
Most relevant clinical studies reporting the antidepressant efficacy of ketamine on suicidality in patients with TRD.
| Author(s), Year | Design | Sample Characteristics | Main Results | Limitations | Conclusions |
|---|---|---|---|---|---|
| DiazGranados et al. 2010b [46] | Open-label study | Thirty-three patients with TRD received a single open-label infusion of ketamine (0.5 mg/kg) and were rated at baseline and 40, 80, 120, and 230 minutes post-infusion with the SSI, MADRS, HDRS, and BDI. |
Suicidal ideation scores decreased significantly after 40 minutes following ketamine infusion (MADRS and BDI suicide items: p <.001). This reduction remained significant through the first 4 hours post-infusion. Ten subjects (30%) had an SSI score ≥ 4 at baseline; all these scores dropped below 4 (9 dropped by 40 minutes and 1 by 80 minutes). Significant improvements at all time points have been found for depression, anxiety, and hopelessness. |
The sample size was relatively small but the effect sizes were large. The open-label nature of the study may have biased the reported response. Whether ketamine may also reduce suicidal ideation in patients with a diagnosis other than TRD is unclear. | Suicidal ideation in TRD patients improved after 40 minutes of ketamine infusion and remained significantly improved for up to 4 hours post-infusion. |
| Price et al. 2009 [45] |
Open-label study | Twenty-six patients with TRD were assessed using the suicidality item of the MADRS 2 hours before and 24 hours following a single subanesthetic dose of intravenous ketamine. Ten patients also completed the Implicit Association assessing implicit suicidal associations at comparable time points. In a second study, nine patients received twice-weekly ketamine infusions over a 12-day period. | MADRS scores were significantly reduced after 24 hours of a single infusion of ketamine and 81% of patients received a rating of 0 or 1 post-infusion. Specifically, of the 13 patients with clinically significant suicidal ideation at baseline, 62% reported a clinically significant improvement on suicidal ideation postinfusion. Implicit suicidal associations were also reduced following ketamine. MADRS reductions were sustained for 12 days by repeated-dose ketamine. |
The sample size was relatively small. The sample of patients with TRD may be not representative of the entire population of depressed subjects. | Ketamine has rapid beneficial effects on suicidal cognition. |
| Larkin and Beautrais, 2011 [47] | Open-label study | Fourteen depressed emergency department patients with suicide ideation were treated with a single i.v. bolus of ketamine (0.2 mg/kg) over 1-2 minutes. |
Mean MADRS scores reduced significantly from 40.4 at baseline to 11.5 at 240 minutes. Median time to MADRS score ≤10 was 80 minutes. Suicidal ideation according to the item 10 of MADRS decreased significantly from 3.9 at baseline to 0.6 after 40 min post-administration. Suicidal ideation improvements were reported for the course of 10 days after ketamine infusions. | The open-label and preliminary nature of the study did not allow the generalization of findings. | Intravenous ketamine may rapidly improve suicidal ideation in depressed emergency department patients. |
SAFETY AND TOLERABILITY DATA ON KETAMINE DERIVED BY OPEN-LABEL AND DOUBLE-BLIND STUDIES
Several open-label and double-blind studies reported findings on the safety and tolerability of ketamine in TRD samples.
Segmiller et al. [26] reported that one male patient discontinued the study because a severe dissociative condition emerged after the first administration and a female patient also reported pronounced dissociative symptoms. In addition, psychomimetic side effects were also reported during ketamine infusion whereas they were completely absent during treatment with S-ketamine in the two patients with TRD assessed by Paul et al. [30]. Similarly, ketamine was associated with a small transient but significant increase in psychotomimetic symptoms and a mild transitory increase in dissociative symptoms (that according to the study of Cornwell et al. [41]) may be explained with a spontaneous somatosensory ketamine-related increases in spontaneous cortical γ-band activity during rest) in other studies [32,34,36].
As reported by Ibrahim et al. [33] and Zarate et al. [36], perceptual disturbances, drowsiness, confusion, elevations in blood pressure and pulse, dizziness, and increased libido occurred during ketamine administration, but resolved within 80 minutes after ketamine’s infusion.
Mania induction has not been reported with a single infusion in patients with TRD. Only a transient mood elevation inconsistent with a persistent substance-induced syndrome has been reported by Niciu and colleagues [37]. Transient talkativeness and decreased inhibition have also been reported by Messer et al. [27] in their case report on a 50-year-old man with a history of depressive symptoms and psychoactive treatment since the age of ten years.
Aan het Rot et al. [48] found that ketamine showed only mild positive psychotic symptoms with the exception of three patients who experienced transient dissociative symptoms. 88.9% of patients responded after the first and sixth ketamine infusions in ten medication-free symptomatic patients. The mean reduction in MADRS scores after the sixth infusion was 85% (12%); in line with other studies, 88.9% of patients relapsed, on average, 19 days after the sixth infusion.
Feeling strange or unreal (58.3%), abnormal sensations (54.2%), blurred vision (50.0%), and feeling drowsy or sleepy (45.8%) were the most common side effects reported 4 hours after each infusion of ketamine [35].
Low-dose ketamine has been also associated with minimal acute neurocognitive effects in TRD patients 40 minutes after ketamine’s infusion (selective impairments confined to the Delayed Recall component of the Hopkins Verbal Learning Test (HVLT) while sparing HVLT Learning and Category Fluency) [42].
In another study, the same authors [38] suggested that the most common adverse events in the ketamine group for up to 4 hours after infusion were dizziness, blurred vision, headache, nausea or vomiting, dry mouth, poor coordination, poor concentration, and restlessness. The authors also reported that 17% of patients had significant dissociative symptoms (i.e., feeling outside of one’s body or perceiving that time is moving more slowly or more quickly than normal) immediately after ketamine infusion and resolved by 2 hours postinfusion.
Finally, ketamine has been also associated with dissociation/perceptual disorders, transient cognitive deficits, euphoria, increased blood pressure and libido, and potential misuse [49].
Table 6 summarizes the most relevant clinical side effects associated with ketamine use in patients with TRD according to both open-label and double-blind studies included in the present review.
Table 6.
Most relevant clinical side effects associated with ketamine according to open-label and double-blind studies in patients with TRD.
| Author(s), Year | Dissociative Symptoms (Psychotomimetic and Perceptual Disturbances) | Confusion | Neurocognitive Effects Including Poor Coordination/ Concentration, and Restlessness | Blurred Vision | Drowsiness | Headache, Nausea or Vomiting | Transient Mood Elevation (Talkativeness and Decreased Inhibition) | Elevations in Blood Pressure and Pulse | Increased Libido |
|---|---|---|---|---|---|---|---|---|---|
| Segmiller et al. (2013) [26] | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Cornwell et al. (2012) [41] | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Ibrahim et al. (2012) [33] | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Zarate et al. (2006) [36] | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Aan het Rot et al. (2010) [48] | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Murrough et al. (2013b) [35] | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Paul et al. (2009) [30] | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Ibrahim et al. (2012) [33] | (-) | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Zarate et al. (2006) [36] | (-) | (+) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| Murrough et al. (2013a; 2013c) [38,42] | (-) | (-) | (+) | (-) | (-) | (-) | (-) | (-) | (-) |
| Murrough et al. (2013a; 2013b) [35,38] | (-) | (-) | (-) | (+) | (-) | (-) | (-) | (-) | (-) |
| Ibrahim et al. (2012) [33] | (-) | (-) | (-) | (-) | (+) | (-) | (-) | (-) | (-) |
| Zarate et al. (2006) [36] | (-) | (-) | (-) | (-) | (+) | (-) | (-) | (-) | (-) |
| Murrough et al. (2013a; 2013b) [35,48] | (-) | (-) | (-) | (-) | (+) | (-) | (-) | (-) | (-) |
| Murrough et al. (2013a) [38] | (-) | (-) | (-) | (-) | (-) | (+) | (-) | (-) | (-) |
| Niciu et al. (2013) [37] | (-) | (-) | (-) | (-) | (-) | (-) | (+) | (-) | (-) |
| Messer et al. (2010) [27] | (-) | (-) | (-) | (-) | (-) | (-) | (+) | (-) | (-) |
| Ibrahim et al. (2012) [33] | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (+) | (-) |
| Zarate et al. (2006) [36] | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (+) | (-) |
| Ibrahim et al. (2011) [32] | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (+) |
| Zarate et al. (2012) [52] | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (+) |
| Berman et al. (2000) [49] | (+) | (-) | (+) | (-) | (-) | (-) | (-) | (+) | (+) |
DISCUSSION
Based on most of the included studies, ketamine was found to exert a rapid and sustained antidepressant effect on samples of TRD patients. All case reports/series [24-30], open-label studies [31-35,39-44,48], and randomized double-blind placebo- or active- controlled studies [36-38] included in our review suggested the active and rapid antidepressant properties of this medication in TRD considering that ketamine as a novel and promising pharmacological option to treat both MDD and TRD patients in contrast with the delayed action of the currently available antidepressant medications requiring several weeks before acting [44].
Ketamine may be considered a valid option for TRD based on its advantages in terms of rapid onset of action (e.g. in most patients after a single infusion) on core depressive symptoms, and hopelessness [50]. In the study of Murrough et al. [38], 64% of the patients treated with ketamine responded and approximately one-third responded specifically to ketamine; this may be undoubtedly defined as a large effect size [51].
In addition, based on three additional open-label studies [45-47], ketamine was also found to be effective in reducing suicidality in TRD patients. Specifically, suicidal ideation in TRD patients improved after few minutes of ketamine infusion and remained stable for up to 4 hours post-infusion. These findings have also been replicated in patients with treatment-resistant bipolar depression by Zarate et al. [52]. In this randomized double-blind placebo-controlled crossover study, 79% of subjects was reported to respond to ketamine at some point during the 2-week trial whereas the effects of ketamine waned from days 7-10 and there was no significant difference compared to placebo.
Interestingly, a family history of alcohol dependence seems to predict a rapid initial antidepressant response to an NMDA receptor antagonist serving as a useful marker for response to NMDA antagonists [40,53]. It has been suggested that NMDA receptors are one of the target linked to the action of ethanol and genetic alterations of NMDA receptor subunits have been associated with the emergence of alcohol dependence [54]. Also, alcohol has been demonstrated to modulate the expression of NR2A in the amygdala and hippocampus which are key brain regions involved in neurobiological processes underlying addiction [55].
Petrenko et al. [56] suggested that genetic variations of NMDA receptor subunits may increase susceptibility to alcohol dependence by modifying the sensitivity of the NMDA complex. As ketamine exerts at least partially an NR2A antagonism, the altered sensitivity of the NMDA receptor complex could justify the greater response to ketamine’s in those with a family history of alcohol dependence.
As oral ketamine has poor bioavailability when compared with the intramuscular formulation, limited evidence investigated the antidepressant properties of the intramuscular formulation of ketamine. Recently, Chilukuri et al. [57], reported that in a sample of 27 subjects with major depression divided randomly into three groups (taking intravenous ketamine in the dose of 0.5 mg/kg, intramuscular ketamine in the dose of 0.5 mg/kg, and intramuscular ketamine in the dose of 0.25 mg/kg, respectively), depressive symptoms as assessed by HDRS fell by 58.86%, 60.29% and 57.36% in each group two hours after ketamine injection, and the improvement was observed for three days. The efficacy and tolerability of hetamine has also been confirmed by some case reports. Grott Zanicotti et al. [58] found a consistent improvement in depressive ratings to repeated (at weekly intervals over 10 months) low dose of intramuscular ketamine. The authors also reported that after ketamine interruption there was no recurrence of depression.
The same authors [59] have previously demonstrated that over a course of six treatments a female patient with metastatic ovarian cancer showed rapid and prolonged response to intramuscular ketamine injections (1mg/kg). In particular, a rapid response and remission of her depressives symptoms have been reported, and pain was also improved for a shorter duration. Also, Cusin et al. [60] found that two patients with bipolar II disorder responded to intramuscular ketamine augmentation.
Ketamine is usually a racemic mixture consisting of two enantiomers: (R)- and (S)- and it is mostly metabolized in norketamine, an active metabolite. (S)-ketamine has been reported as approximately 4 times more active having better pharmacokinetic properties and being more tolerable than (R)-enantiomer [30,61]. Also, (S)-ketamine has been found to induce less psychomimetic adverse effects (e.g., derealization and hallucinations).
Specifically, Paul et al. [30] reported that one patient did not respond to both R- and S-ketamine enantiomers, whereas an antidepressant effect as assessed by a decrease in HDRS21 and BDI at days 1 and 3 after infusion has been observed in another patient after intravenous administration of both R- and S-ketamine. Psychomimetic adverse effects emerged during R-ketamine infusion but were absent during S-ketamine treatment. The authors concluded that S-ketamine showed similar antidepressant effects when compared to R-ketamine in resistant depressed patients although S-ketamine may be better tolerated by the patients.
Ketamine treatment has also been suggested as safe and well tolerated in TRD patients although some authors [49] suggested that its psychotomimetic and euphoric properties may preclude its utilization in treating depression in clinical practice. Based on recent evidence [37,38], ketamine treatment did not significantly increase the risk of emergent psychotic or manic symptoms over the follow-up period. A poor bioavailability (17%–20%) has been reported after administration of oral ketamine when compared with its higher (93%) bioavailability in the intramuscular administration. Intramuscular ketamine has been instead demonstrated to be rapidly effective on depressive symptoms within hours after administration of a single subanesthetic dose inducing a significant clinical improvement of depressive conditions [62].
Few transient psychoactive and hemodynamic effects adverse effects such as moderate dissociative feelings, blurred vision, dizziness, anxiety, irritability, and headaches have also been described with the use of ketamine. However, most of the mentioned side effcts have been reported to resolve within some hours after ketamine’s infusion. ketamine has been associated with abuse liability and this should be carefully taken into account by clinicians [63,64]. In addition, the possible emergence of hemodynamic changes should suggest cardiorespiratory monitoring as a fundamental component of risk management when ketamine was administered.
Taken together, these evidence suggested that ketamine is safe and well-tolerated in the short term period for nonpsychotic depressed patients when administered at a subanesthetic dose of 0.5 mg/kg over 40 minutes. However, it will be of great importance for clinicians to test both safety and tolerability of ketamine even beyond the first infusion of ketamine.
Ketamine acts by blocking one of the targets for the excitatory neurotransmitter glutamate in the brain, the NMDA glutamate receptors, the antagonism of which may be considered an adequate target for developing new rapid-acting antidepressant therapeutic agents [8]. Robust data allow researchers to hypothesize that NMDA receptor modulation may help to achieve a clinical improvement in patients with severe and chronic forms of depression [38]. NMDA receptor activity plays a fundamental role in the pathophysiology of depression, and the modulation of its functioning may undoubtedly contribute to restore affective disorders [65]. Based on preclinical studies in animal models and neurobiological human studies, specific mechanisms of action have been proposed to explain the antidepressant effects of ketamine.
The antidepressant effects of ketamine have been at least partially reported by inducing neuroplasticity-related processes [66]. The importance of promoting neuroplasticity mechanisms to exert an antidepressant activity is well documented.
Ketamine may represent a rapid neuroplastic modulator drug able to induce enhanced dendritic branching and synaptic receptor number and density [67]. As suggested by Hayley and Litteljohn [68], based on recent evidence ketamine could modify the connectivity of diverse cortical circuitry playing a critical role in determining consciousness, sense of self, and key depressive symptoms such as rumination. In particular, Scheidegger et al. [69] reported that ketamine reduced default mode network metabolic activity by reducing the connectivity within the cingulate and prefrontal cortices in a sample of healthy non-depressed subjects. Also, ketamine has been reported to strengthen appropriate emotional neural connections enhancing synaptogenesis in those brain areas affected by stress-related processes and associated with negative thinking in depressed individuals.
The rapid antidepressant activity reported by convergent evidence is consistent with existing preclinical observations indicating that ketamine rapidly (within hours) increases not only the number but even the functioning of synaptic connections involving cortical or hippocampal neurons [67,70,71]. The additional potential to rapidly reverse both behavioral and neuronal changes associated with chronic stress presumably due to the stimulation of brain-derived neurotrophic factor (BDNF) signaling needs to be also mentioned [72].
To further confirm the neurotrophic effect of BDNF, Laye et al. [73] suggested that some patients who do not respond to ketamine are carriers of a Val66Met (rs6265) single-nucleotide polymorphism (SNP) that is associated with an attenuation of BDNF functioning. Also, Autry et al. [74] reported that ketamine rapidly induced antidepressant-like behavioural effects through the inhibition of spontaneous miniature NMDA-receptor mediated currents leading to decreased eEF2 kinase activity and allowing an increased BDNF translation. In mouse models, the antidepressant effects of ketamine were found to be fast-acting and closely related to the rapid production of BDNF. Ketamine-mediated NMDA receptors blockade at rest may inhibit CaMKIII kinase determining a reduced eukaryotic elongation factor 2 (eEF2) phosphorylation desuppressing BDNF translation.
However, not all studies showed that the rapid initial antidepressant effects of ketamine were mediated by BDNF. Machado-Vieira et al. [39] suggested that inhibitors of eEF2 kinase may induce fast-acting behavioural antidepressant-like effects. Both BDNF and VGLUT1 may presumably be state markers of depression although not necessarily involved in its aetiology [75-77]. Similarly, Lindholm et al. [78], suggested that BDNF signaling does not play a pivotal role in the antidepressant effects of glutamate-based medications. The authors found that neither ketamine nor the AMPA-potentiator LY 451656 activate BDNF signaling although producing a characteristic antidepressant-like response.
LIMITATIONS
The present study should be interpreted in the light of the following limitations. First, most of the available findings suggested that ketamine is effective when administered at a subanesthetic dose of 0.5 mg/kg over 40 minutes in short-term designs, but this is not necessarily associated with high remission rates. As stated by Cusin et al. [60], one of the most important challenge is currently to understand whether ketamine may be also considered effective after the first infusions. Overall, the antidepressant effect of ketamine may be observed after few hours of a single intravenous infusion but the long-lasting sustained antidepressant-like effects of ketamine need to be further investigated. Unless better remission rates have been suggested over time, ketamine should be currently investigated as an augmentation therapy (together with other antidepressant drugs) rather than as a monotherapy (replacing other antidepressant drugs). To date, there are only evidence [52,60,79-81] suggesting the antidepressant effect of ketamine infusion as add-on treatment to mood-stabilizing drugs in bipolar depression and in those severe forms of bipolar depression that are treatment-resistant to common antidepressant medications.
Furthermore, it is quite actually unknown which patients may be considered as responders to ketamine and who does not nor it’s actually documented whether repeated ketamine infusions may be effective in those who are initially non-responders [51]. Biomarkers may provide a better understanding of the pathophysiology of complex and heterogeneous psychiatric disorders like MDD. Salvadore et al. [82] recently reported that rostral anterior cingulated cortex activation may be a reliable biomarker identifying a subgroup of patients who will favorably respond to ketamine's antidepressant effects. Specifically, an increased anterior cingulate cortical activity was positively correlated with a subsequent rapid antidepressant response to ketamine in drug-free depressed patients.
Another limitation that should be taken into account is whether repeated dose therapy with ketamine leads to antidepressant tolerance or habituation (loss of benefit). Along with this, what is the best dosing strategy (e.g., weekly, biweekly, or monthly) concerning ketamine administration is currently quite unclear.
In addition, all MDD subjects examined in this systematic review are treatment-resistant, therefore the present results may not be generalized to other samples of depressed subjects. Based on the current knowledge in the field, we are not able to preliminary know whether ketamine may be also considered effective and safe in patients with other non-treatment-resistant forms of depression.
In addition, the neurobiology underlying MDD is quite complex and both an hyperfunction and hypofunction of the NMDA receptors may be involved in TRD [65]. A recent study suggested that the enhancement, instead of blocking, of the NMDA glutamate receptors may also induce antidepressant-like effects [83]. Also, ketamine has been reported to act not only on the glutamate receptors but also on muscarinic receptors, and voltage-gated calcium channels, as well as inhibiting serotonin and norepinephrine reuptake. The possible antidepressant properties related to the mentioned mechanism of action remains quite actually unclear.
Also, most studies included in the present review are open-label or case-reports/series in nature and did not allow to investigate the antidepressant effects of ketamine in the long-term period. The open-label design needs to be considered as a major limitation as most studies have not been primarily designed to test the antidepressant effect of ketamine per se. In these studies, other unmeasured variables may have influenced the antidepressant activity of ketamine in open-label naturalistic studies. The majority of studies have also a very limited duration to test the sustained antidepressant activity of ketamine after the initial/rapid clinical response to the first infusions. Furthermore, patients with histories of psychotic symptoms or substance abuse are usually excluded by these studies. Moreover, the small sample sizes and lack of a placebo group did not allow the generalization of the findings in most studies (e.g., the power to detect small effects is quite limited).
CONCLUSION
Ketamine has been demonstrated to play a fundamental role in the treatment of TRD patients, elucidating new and intriguing insights into the neurobiology of this complex condition. Although limited to initial observations, ketamine may be effective not only in TRD patients but also on suicidality. However, future studies are recommended in order to test the efficacy of ketamine when compared with other active comparators such as electroconvulsive therapy or antidepressant-antipsychotic drugs combinations.
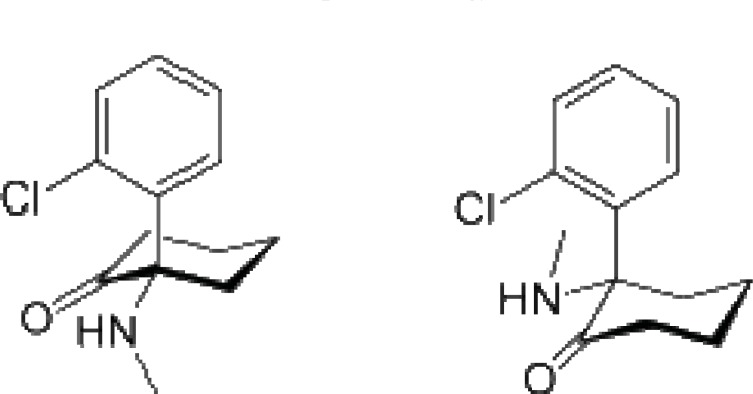
Fig. (2).
Ketamine: a competitive, open-channel NMDA receptor antagonist.
Table 2.
Most relevant open-label single or multiple dose studies reporting the antidepressant efficacy of ketamine in patients with TRD
| Author(s), Year | Design | Sample Characteristics | Main Results | Response and Remission Rates | Limitations | Conclusions |
|---|---|---|---|---|---|---|
| Murrough et al. 2013b [35] |
Prospective open-label study | Twenty-four TRD patients underwent a washout of antidepressant medication followed by a series of up to six infusions of ketamine (.5 mg/kg) three times weekly over a 12-day period. |
The overall response rate at study end was 70.8%. After 2 hours, a large and persistent mean reduction in MADRS scores has been reported with ketamine. The median time to relapse after the last infusion was 18 days. Response at 4 hours was a significant predictor of response at study end. | Response rate at study end was 70.8%. | The open-label design is a major limitation. The study was not designed to test the antidepressant effect of ketamine per se. Finally, the small sample size did not allow the generalization of the findings. | Ketamine administration was associated with a rapid and prolonged antidepressant effect in TRD patients. |
| Shiroma et al. 2013 [34] |
Open-label study | Fourteen subjects with TRD were recruited and completed six infusions of 0.5mg/kg ketamine over 40 minutes during a 12-day period. | Eleven subjects achieved response criterion whereas eight remitted. After the first infusion, only three and one subjects responded and remitted, respectively. Four patients achieved response and six remitted after 3 or more infusions. Five subjects experienced a sustained remission during the 4 weeks of follow-up. The mean time for six subjects who relapsed was 16 days. | Response rate is 91.6% and remission rate is 66.6%. | The small sample and lack of a placebo group did not allow the generalization of the present findings. | The study confirmed the efficacy and safety of repeated ketamine infusions. Repeated ketamine infusions showed superior antidepressant properties as compared to a single infusion in terms of both response and remission. |
| Ibrahim et al. 2012 [33] |
Open-label study | Forty-two subjects with TRD and a MADRS score ≥22 received a single intravenous infusion of ketamine (0.5 mg/kg). Four to six hours post-infusion, subjects were randomized to double-blind treatment with either riluzole (100–200 mg/day) or placebo for 4 weeks. |
Patients significantly improved in MADRS scores from baseline. The effect size of improvement with ketamine was initially large and remained moderate throughout the 28-day trial. The mean time to relapse was 13.2 days. Overall, 27% did not relapse throughout the 4-week study. 38% did not relapse for at least 2 weeks, and 58% did not relapse for at least a week. |
Response rate is 62%. | The relatively small sample size and the treatment-resistance of the patient sample did not allow the generalization of the main findings. TRD patients with a history of many years of illness may have different neurobiological and pharmacological response profiles when compared to those at their first episode of depression or with those with low years of illness. | A single 40-minute infusion of ketamine was associated with a rapid and persistent antidepressant response for up to 4 weeks. |
| Ibrahim et al. 2011 [32] |
Open-label study | Comparison between 17 patients with TRD previously not responder to ECT and 23 patients with TRD who had not previously received ECT, all treated with ketamine (0.5 mg/kg) | Depressive symptoms significantly improved based on MADRS total scores in the ECT-resistant group at 230 minutes with a moderate effect size whereas at 230 minutes the non-ECT exposed group demonstrated a significant improvement with a large effect size. | Response rate is 100% and remission rate is 0%. | This is an open-label study and the reported effects could have been biased. | Ketamine improved depressive symptoms in MDD patients who had previously not responded to ECT. |
| aan het Rot et al. 2010 [48] |
Open-label study | Six infusions of ketamine over 12 days in ten medication-free symptomatic patients with TRD | 88.9% of patients responded after the first infusion as well as after the sixth infusion. The mean reduction in MADRS scores after the sixth infusion was 85% (12%) but 88.9% of patients relapsed after the study, on average, 19 days after the sixth infusion. Ketamine showed mild positive psychotic symptoms with the exception of three patients who experienced transient dissociative symptoms. | Response rate is 100% and remission rate is 72%. |
Patients were not tested with cognitive measures. Given the small sample size, the open-label design, and the inclusion of participants who previously responded to a single ketamine dose, type I errors may be not excluded. | Repeated doses of ketamine may be useful for the acute treatment of TRD. |
| Mathew et al. 2010 [31] |
Open-label study | Twenty-six medication-free patients received open-label intravenous ketamine (0.5 mg/kg) over 40 minutes. Two hours prior to infusion, patients were randomized to lamotrigine (300 mg) or placebo. | Seventeen patients (65%) met response criterion (MADRS scores were reduced of 50%) 24 hour after ketamine administration. Lamotrigine failed to attenuate the mild, transient side-effects associated with ketamine and did not enhance its antidepressant effects. 54% of patients met response criterion 72 hours after ketamine and proceeded to participate. | Response rate is 65%. | This is a pilot study, the results of which may be not generalized to the whole depressed population. | Ketamine is well-tolerated in patients with TRD and may have rapid and prolonged antidepressant properties. |
Table 3.
Randomized double-blind placebo-controlled or active-controlled studies evaluating the efficacy of ketamine in patients with TRD.
| Author(s), Year | Design | Sample Characteristics | Main Results | Response and Remission Rates | Limitations | Conclusions |
|---|---|---|---|---|---|---|
| Murrough et al. 2013a [38] | Two-site, parallel-arm, randomized controlled placebo- control study | Seventy-three patients were randomized to a single infusion of ketamine or to midazolam assessed by the MADRS. | The ketamine group had greater improvement on the MADRS score than the midazolam group 24 hours after treatment. After adjustment for baseline scores and site, the MADRS score was lower in the ketamine group than in the midazolam group by 7.95 points (95% confidence interval [CI], 3.20 to 12.71). At 24 hours, the likelihood of response was greater with ketamine than midazolam (odds ratio, 2.18; 95% CI, 1.21 to 4.14), with response rates of 64% and 28%, respectively. | Response rate is 64%. |
The rigid recruitment criteria are a major limitation. Patients with histories of psychotic symptoms or substance abuse were excluded. Also, a significant percentage (17.2%) of screened patients refused or were unable to tolerate psychotropic medication washout prior to randomization. Finally, only the efficacy of a single infusion of ketamine over a brief follow-up period has been investigated. | Ketamine showed rapid antidepressant effects after a single infusion in TRD patients. |
| Niciu et al. 2013 [37] |
Randomized, controlled, crossover study | Twenty-two patients with TRD who received placebo or 40-minute ketamine infusion were evaluated with the YMRS. | Overall, 4 of 22 patients scored greater than 12 on the YMRS. These patients who were randomized to ketamine peaked at the end of the 40-minute infusion and returned to baseline by the following day. | Response and remission rates are not reported. | The unipolar subjects were unmedicated for at least 2 weeks before and during the whole study. The small sample size did not allow the generalization of the findings. | This transient mood elevation is inconsistent with a persistent substance-induced syndrome. The study did not support mania induction with a single dose of ketamine in TRD patients. |
| Zarate et al. 2006 [36] |
Randomized placebo-controlled, double-blind crossover study | Eighteen subjects with DSM-IV TRD were randomized in a placebo-controlled, double-blind crossover study. | A significant improvement was observed in depressed subjects treated with ketamine (110 minutes after administration) compared with subjects treated with placebo. The effect size for the drug difference was 1.46 [0.91-2.01] after 24 hours, and 0.68 [0.13-1.23] after 1 week. 71% of those treated with ketamine met response and 29% met remission criteria the next day after the administration of ketamine. 35% of these subjects continued to respond for at least 1 week. |
Response rate is 71% and remission rate is 29% the day following ketamine infusion. | The sample size was relatively small to allow the generalization of the present findings. Limitations in preserving study blind may have biased patient reporting by reducing placebo effects, potentially confounding results. | Ketamine was associated with robust and fast antidepressant effects when administered within 2 hours post-infusion. Also, a significant improvement was observed for at least 1 week. |
Table 4.
Clinical studies investigating neurobiological effects and/or mechanism of action of ketamine in TRD samples.
| Author(s), Year | Design | Sample Characteristics | Main Results | Response and Remission Rates | Limitations | Conclusions |
|---|---|---|---|---|---|---|
| Duncan et al. 2013 [44] | Open-label study | Thirty TRD patients who had been drug-free for two weeks received a single open-label infusion of ketamine hydrochloride (.5mg/kg) over 40 minutes and assessed with the MADRS before and after infusion. | A significant positive correlation was observed between baseline delta sleep ratio calculated as SWA (NREM1)/ SWA (NREM2) and reduced MADRS scores from baseline to Day 1. | Response rate is 40%. | The small sample size did not allow the generalization of the findings. Also, these results may not be extended to other non-TRD samples. | Delta sleep ratio may be a useful baseline biomarker of response to ketamine. Ketamine may exert a rapid antidepressant effect in TRD patients. |
| Carlson et al. 2013 [43] | Open-label study | Twenty-two unmedicated patients with TRD underwent PET to measure regional cerebral glucose metabolism at baseline and following a single intravenous dose of ketamine (0.5 mg/kg) over 40 minutes. | Regional metabolism decreased significantly in the habenula, insula, ventrolateral/dorsolateral prefrontal cortices of the right hemisphere whereas metabolism increased in bilateral occipital, right sensorimotor, left parahippocampal, and left inferior parietal cortices. Improvement in depression correlated directly with metabolism changes in the right superior and middle temporal gyri and inversely with metabolic changes in right parahippocampal gyrus and temporoparietal cortex. | Response rate is 30%. | Ketamine was administered within an open-label design. Also, patients were not compared with a parallel control sample randomized to placebo. Potential confounding factors (e.g. the placebo effect) may have influenced the main results. The small sample size did not allow for comparisons between responders and non- responders. Finally, the spatial resolution of PET is low relative to the small size of the habenula. | A reduced metabolism in the right habenula, parahippocampal gyrus, and other brain structures of the extended medial and orbital prefrontal networks was associated with rapid anti-depressant effects of ketamine in TRD patients. |
| Murrough et al. 2013c [42] |
Open-label study | Neurocognitive functioning has been evaluated in 25 patients with TRD. | A poorer baseline neurocognitive performance compared to non-responders (particularly slower processing speed) has been found in patients who responded to ketamine 24 hours following treatment. Even after 24 hours of ketamine infusion, negative cognitive effects immediately after ketamine administration predicted lower response rate. | Response rate is 40%. | The limited sample size and the open-label administration of ketamine did not allow the generalization of findings (the power of the study to detect small effects is limited). The neurocognitive battery has been administered only once. Finally, the association between baseline neurocognition and antidepressant activity may be influenced by other unmeasured variables. | An inverse relationship between cognitive effects of ketamine and antidepressant efficacy has been reported. |
| Cornwell et al. 2012 [41] |
Open-label study | Twenty drug-free TRD patients received a single, open-label intravenous infusion of ketamine hydrochloride (.5 mg/kg). Magnetoencephalographic recordings were made approximately 3 days before and 6.5 hours after the infusion. | Responders (patients with a strong improvements in depressive symptoms 230 minutes after infusion) showed increased cortical excitability but not spontaneous cortical Γ-activity changes. Stimulus-evoked somatosensory cortical responses increase after infusion of ketamine compared to pre-treatment responses only in responders. | Response rate is 45%. | Whether NMDAR antagonism is a necessary starting point for modifying cortical circuitry in a way that proves to be clinically beneficial is a matter of debate. The open-label nature of the study represents a major limitation and raises doubts regarding the specificity of ketamine effects. | Enhanced cortical excitability but not spontaneous cortical Γ-activity differentiates responders from non-responders to ketamine. |
| Phelps et al. 2009 [40] | Open-label study | Twenty-six subjects with TRD were treated with an open-label intravenous infusion of ketamine hydrochloride (0.5 mg/kg) and rated with MADRS at baseline, 40, 80, 120, and 230 minutes post-infusion. |
Subjects with a family history of alcohol dependence showed significantly greater improvement in MADRS scores than those who had no family history of alcohol dependence. | Response rate is 43% and remission rate is 26%. | The small sample size limited the generalization of findings. There was no control group. Self-reporting was used to determine the family history. The drug was open-label, the duration of the study was limited to a very rapid clinical response. | A family history of alcohol dependence was a predictor of a rapid initial antidepressant response to ketamine. |
| Machado-Vieira et al. 2009 [39] | Open-label study | Twenty-three subjects with TRD recruited in an open-label intravenous infusion of ketamine hydrochloride (0.5 mg/kg), rated using MADRS at baseline and at 40, 80, 120, and 230 minutes post-infusion. | A significant improvement on MADRS scores after ketamine treatment was obtained but no changes in BDNF levels were found after subjects received ketamine compared to baseline. No association was found between antidepressant response and BDNF levels. | Response rate is 47.8%. | The study evaluated only the initial rapid effects of ketamine, it is possible that BDNF levels might change at later time-points being involved in the prolonged antidepressants’ effects of this drug. | Rapid and initial antidepressant effects of ketamine resulted not mediated by BDNF. |
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Soleimani L, Lapidus KA, Iosifescu DV. Diagnosis and treatment of major depressive disorder. Neurol. Clin. 2011;29:177–179. doi: 10.1016/j.ncl.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Langlieb AM, Guico-Pabia CJ. Beyond symptomatic improvement:assessing real-world outcomes in patients with major depressive disorder. Prim. Care Companion J. Clin. Psychiatry. 2010;12:1–12. doi: 10.4088/PCC.09r00826blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greer TL, Kurian BT, Trivedi MH. Defining and measuring functional recovery from depression. CNS Drugs. 2010;24:267–284. doi: 10.2165/11530230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Pompili M, Iliceto P, Luciano D, Innamorati M, Serafini G, Del Casale A, Tatarelli R, Girardi P, Lester D. Higher hopelessness and suicide risk predict lower self-deception among psychiatric patients and non-clinical individuals. Riv. Psichiatr. 2011;46:24–30. [PubMed] [Google Scholar]
- 5.Serafini G, Pompili M, Innamorati M, Giordano G, Montebovi F, Sher L, Dwivedi Y, Girardi P. The role of microRNAs in synaptic plasticity, major affective disorders and suicidal behavior. Neurosci. Res. 2012;73:179–190. doi: 10.1016/j.neures.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Serafini G, Pompili M, Innamorati M, Fusar-Poli P, Akiskal HS, Rihmer Z, Lester D, Romano A, de Oliveira IR, Strusi L, Ferracuti S, Girardi P, Tatarelli R. Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. J. Affect. Disord. 2011;129:47–55. doi: 10.1016/j.jad.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors 2001 systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 8.Serafini G, Pompili M, Innamorati M, Dwivedi Y, Brahmachari G, Girardi P. Pharmacological properties of glutamatergic drugs targeting NMDA receptors and their application in major depression. Curr. Pharm. Des. 2013;19:1898–1922. doi: 10.2174/13816128113199990293. [DOI] [PubMed] [Google Scholar]
- 9.Fava M, Rush AJ, Wisniewski SR, Nierenberg AA, Alpert JE, McGrath PJ, Thase ME, Warden D, Biggs M, Luther JF, Niederehe G, Ritz L, and Trivedi MH. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients a STAR*D report. Am. J. Psychiatry. 2006;163:1161–1172. doi: 10.1176/ajp.2006.163.7.1161. [DOI] [PubMed] [Google Scholar]
- 10.Petersen T, Gordon JA, Kant A, Fava M, Rosenbaum JF, Nierenberg AA. Treatment resistant depression and axis i co-morbidity. Psychol. Med. 2001;31:1223–1239. doi: 10.1017/s0033291701004305. [DOI] [PubMed] [Google Scholar]
- 11.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry . 006a;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 12.Holtzheimer PE, Mayberg HS. Stuck in a rut rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM. The impact of treatment-resistant depression on health care utilization and costs. J. Clin. Psychiatry. 2002;63:963–971. doi: 10.4088/jcp.v63n1102. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ. STAR*D Study Team Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 15.Murrough JW, Charney DS. Is there anything really novel on the antidepressant horizonκ. Curr. Psychiatry Rep. 2012;14:643–649. doi: 10.1007/s11920-012-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belmaker RH. The future of depression psychopharmacology. CNS Spectr. 2008;13:682–687. doi: 10.1017/s1092852900013766. [DOI] [PubMed] [Google Scholar]
- 17.Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M. STAR*D Study Team Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N. Engl. J. Med. . 006b;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 18.Crane GE. Cyloserine as an antidepressant agent. Am. J. Psychiatry. 1959;115:1025–1056. doi: 10.1176/ajp.115.11.1025. [DOI] [PubMed] [Google Scholar]
- 19.Crane GE. The psychotropic effects of cycloserine a new use for an antibiotic. Compr. Psychiatry. 1961;2:51–59. [Google Scholar]
- 20.Krystal JH, Perry EB, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D'Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch. Gen. Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- 21.Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA, Simkowitz P, Hurley A, Cooper T, Volkow ND, Cancro R. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 22.Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL. Effects of (S)-ketamine on striatal dopamine a [11C] raclopride PETstudy of a model psychosis in humans. J. Psychiatr. Res. 2000;34:35–43. doi: 10.1016/s0022-3956(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Prisma Group.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions explanation and elaboration. B.M.J. 2009;339:B2535. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szymkowicz SM, Finnegan N, and Dale RM. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. J. Affect. Disord. 2013;147:416–420. doi: 10.1016/j.jad.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murrough JW, Perez AM, Mathew SJ, Charney DS. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J. Clin. Psychiatry. 2011;72:414–415. doi: 10.4088/JCP.10l06447blu. [DOI] [PubMed] [Google Scholar]
- 26.Segmiller F, Rüther T, Linhardt A, Padberg F, Berger M, Pogarell O, Möller HJ, Kohler C, Schüle C. Repeated S-ketamine infusions in therapy resistant depression a case series. J. Clin. Pharmacol. 2013;53:996–998. doi: 10.1002/jcph.122. [DOI] [PubMed] [Google Scholar]
- 27.Messer M, Haller IV, Larson P, Pattison-Crisostomo J, Gessert CE. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J. Neuropsychiatry Clin. Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- 28.Paslakis G, Gilles M, Meyer-Lindenberg A, Deuschle M. Oral administration of the NMDA receptor antagonist S-ketamine as add-on therapy of depression a case series. Pharmacopsychiatry. 2010;43:33–35. doi: 10.1055/s-0029-1237375. [DOI] [PubMed] [Google Scholar]
- 29.Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with a treatment-resistant major depression. World J. Biol. Psychiatry. 2009;10:640–643. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- 30.Paul R, Schaaff N, Padberg F, Möller HJ, Frodl T. Comparison of racemic ketamine and S-ketamine in treatment-resistant major depression: report of two cases. World J. Biol. Psychiatry. 2009;10:241–244. doi: 10.1080/15622970701714370. [DOI] [PubMed] [Google Scholar]
- 31.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression a pilot randomized, placebo-controlled continuation trial. Int. J. Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA. Jr Rapid decrease in depressive symptoms with an N-methyl-D-aspartate antagonist in ECT-resistant major depression. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2011;35:1155–1159. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, Lim KO. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J. Affect. Disord. 2013 doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry . 013b;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli N, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 37.Niciu MJ, Luckenbaugh DA, Ionescu DF, Mathews DC, Richards EM, Zarate CA. Subanesthetic dose ketamine does not induce an affective switch in three independent samples of treatment-resistant major depression. Biol. Psychiatry. 2013;74:e23–e24. doi: 10.1016/j.biopsych.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression a two-site randomized controlled trial. Am. J. Psychiatry . 013a;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J. Clin. Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA ., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol. Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol. Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murrough JW, Wan LB, Iacoviello B, Collins KA, Solon C, Glicksberg B, Perez AM, Mathew SJ, Charney DS, Iosifescu DV, Burdick KE. Neurocognitive effects of ketamine in treatment-resistant major depression association with antidepressant response. Psychopharmacology (Berl) . 013c doi: 10.1007/s00213-013-3255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Manji HK, Zarate CA, Drevets WC. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression a preliminary positron emission tomography study. Biol. Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan WC, Selter J, Brutsche N, Sarasso S, Zarate CA. Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J. Affect. Disord. 2013;145:115–119. doi: 10.1016/j.jad.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz GN, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA. Jr Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry . 010b;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int. J. Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 48.Aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 49.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 50.Dowben JS, Grant JS, Keltner NL. Ketamine as an alternative treatment for treatment-resistant depression. Perspect. Psychiatr. Care. 2013;49:2–4. doi: 10.1111/ppc.12006. [DOI] [PubMed] [Google Scholar]
- 51.Rush AJ. Ketamine for treatment-resistant depression ready or not for clinical useκ. Am. J. Psychiatry. 2013;170:1079–1081. doi: 10.1176/appi.ajp.2013.13081034. [DOI] [PubMed] [Google Scholar]
- 52.Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine's antidepressant efficacy in bipolar depression a randomized controlled add-on trial. Biol. Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luckenbaugh DA, Ibrahim L, Brutsche N, Franco-Chaves J, Mathews D, Marquardt CA, Cassarly C, Zarate CA. Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipol. Disord. 2012;14:880–887. doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, Mann K. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch. Gen. Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- 55.Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psycho- pharmacology (Berl.) 2006;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- 56.Petrenko AB, Yamakura T, Fujiwara N, Askalany AR, Baba H, Sakimura K. Reduced sensitivity to ketamine and pentobarbital in mice lacking the N-methyl-D-aspartate receptor GluRepsilon1 subunit. Anesth. Analg. 2004;99:1136–1140. doi: 10.1213/01.ANE.0000131729.54986.30. [DOI] [PubMed] [Google Scholar]
- 57.Chilukuri H, Reddy NP, Pathapati RM, Manu AN, Jollu S, Shaik AB. Acute antidepressant effects of intramuscular versus intravenous ketamine. Indian J. Psychol. Med. 2014;36:71–76. doi: 10.4103/0253-7176.127258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grott Z C, Perez D, Glue P. Case report long-term mood response to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J. Palliat. Med. 2013;16:719–720. doi: 10.1089/jpm.2013.0057. [DOI] [PubMed] [Google Scholar]
- 59.Zanicotti CG, Perez D, Glue P. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J. Palliat. Med. 2012;15:400–403. doi: 10.1089/jpm.2011.0314. [DOI] [PubMed] [Google Scholar]
- 60.Cusin C, Hilton GQ, Nierenberg AA, Fava M. Long-Term Maintenance With Intramuscular Ketamine for Treatment-Resistant Bipolar II Depression. Am. J. Psychiatry. 2012;169:868–869. doi: 10.1176/appi.ajp.2012.12020219. [DOI] [PubMed] [Google Scholar]
- 61.Mion G, Villevieille T. Ketamine pharmacology an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther. 2013;19:370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howland RH. Ketamine for the treatment of depression. J. Psychosoc. Nurs. Ment. Health Serv. 2013;51:11–14. doi: 10.3928/02793695-20121219-01. [DOI] [PubMed] [Google Scholar]
- 63.White JM, Ryan CF. Pharmacological properties of ketamine. Drug Alcohol Rev. 1996;15:145–155. doi: 10.1080/09595239600185801. [DOI] [PubMed] [Google Scholar]
- 64.Morgan CJ, Muetzelfeldt L, Curran HV. Ketamine use, cognition and psychological wellbeing a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104:77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- 65.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants the path to ketamine and beyond. Biol. Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tedesco V, Ravagnani C, Bertoglio D, and Chiamulera C. Acute ketamine-induced neuroplasticity: ribosomal protein S6 phosphorylation expression in drug addiction-related rat brain areas. Neuroreport. 2013;24:388–393. doi: 10.1097/WNR.0b013e32836131ad. [DOI] [PubMed] [Google Scholar]
- 67.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayley S, Litteljohn D. Neuroplasticity and the next wave of antidepressant strategies. Front. Cell. Neurosci. 2013;7:218. doi: 10.3389/fncel.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, and Seifritz E. Ketamine decreases resting state functional network connectivity in healthy subjects implications for antidepressant drug action. PLoSOne. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duman RS, Li N. A neurotrophic hypothesis of depression role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012;367:2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol. Psychiatry. 2012;72:e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, Del Rio J. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1). Eur. J. Neurosci. 2007;25:281–290. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- 76.Farley S, Dumas S, El Mestikawy S, Giros B. Increased expression of the Vesicular Glutamate Transporter-1 (VGLUT1) in the prefrontal cortex correlates with differential vulnerability to chronic stress in various mouse strains effects of fluoxetine and MK-801. Neuropharmacology. 2012;62:503–517. doi: 10.1016/j.neuropharm.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Lindholm JS, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC, Skolnick P, Rantamäki T, Castrén E. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf+/κheterozygous null mice. Neuropharmacology. 2012;62:391–397. doi: 10.1016/j.neuropharm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry . 2010a;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rybakowski JK, Permoda-Osip A, Skibinska M, Adamski R, Bartkowska-Sniatkowska A. Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involvedκ. Hum. Psychopharmacol. 2013;28:87–90. doi: 10.1002/hup.2271. [DOI] [PubMed] [Google Scholar]
- 81.Sienaert P, Lambrichts L, Dols A, De Fruyt J. Evidence-based treatment strategies for treatment-resistant bipolar depression a systematic review. Bipol. Disord. 2013;15:61–69. doi: 10.1111/bdi.12026. [DOI] [PubMed] [Google Scholar]
- 82.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA , Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol. Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P, Tun R, Huang KH, Chang YC, Lane HY, Tsai GE. Inhibition of Glycine Transporter-I as a Novel Mechanism for the Treatment of Depression. Biol. Psychiatry. 2013;74:734–741. doi: 10.1016/j.biopsych.2013.02.020. [DOI] [PubMed] [Google Scholar]