SUMMARY
Yi et al. (2013) recently reported that angiopoietin-like protein 8 (ANGPTL8) was the long-sought “betatrophin” that could control pancreatic beta cell proliferation. However, studies of Angptl8−/− mice revealed profound reduction of triglyceride levels, but no abnormalities in glucose homeostasis (Wang et al. 2013). We now report that Angptl8−/− mice undergo entirely normal beta cell expansion in response to insulin resistance resulting from either a high fat diet, or from the administration of the insulin receptor antagonist S961. Furthermore, overexpression of ANGPTL8 in livers of mice doubles plasma triglyceride levels, but does not alter beta cell expansion nor glucose metabolism. These data indicate ANGPTL8 does not play a role in controlling beta cell growth, nor can it be given to induce such expansion. The findings that plasma triglyceride levels are reduced by Angptl8 deletion and increased following ANGPTL8 overexpression support the possibility that inhibition of ANGPTL8 represents a therapeutic strategy for hypertriglyceridemia.
INTRODUCTION
The pancreatic beta cell possesses the capacity to self-duplicate (Dor et al., 2004). Insulin resistance elicits a compensatory increase in insulin secretion to maintain normoglycemia. Type 2 diabetes develops when the beta cells cannot compensate for the increase in insulin requirement. After an initial compensatory expansion, beta cell function and mass progressively declines (Butler et al., 2003, Henquin & Rahier, 2011, UKPDS, 1995, Yoon et al., 2003). The factors that expand beta cell mass in response to insulin resistance have not been molecularly defined.
Angiopoietin-like protein 8 (ANGPTL8) is a circulating protein that is expressed primarily in liver and adipose tissue. Hepatic overexpression of ANGPTL8 in mice is associated with hypertriglyceridema, whereas inactivation of Angptl8 causes a reduction in plasma triglyceride levels (Quagliarini et al., 2012; Wang et al., 2013). ANGPTL8 was recently reported to mediate an increase in beta cell proliferation and beta cell mass in mice where insulin resistance was induced by the insulin receptor antagonist S961 (Yi et al., 2013). Overexpression of ANGPTL8, which the authors refer to as betatrophin, induced beta cell proliferation, expansion of beta cell mass, and led to improved glycemic control (Yi et al., 2013).
These findings appear to be in conflict with the observation that Angptl8−/− mice fed either a chow or high fat diet have no alteration in glucose homeostasis (Wang et al., 2013). In this study, we used Angptl8−/− mice to determine if ANGPTL8 is required for beta cell expansion in insulin resistant states as had been previously reported by Yi et al. (2013).
RESULTS
ANGPTL8 Does Not Regulate Beta Cell Function or Growth in Response to High-Fat Diet-Induced Insulin Resistance
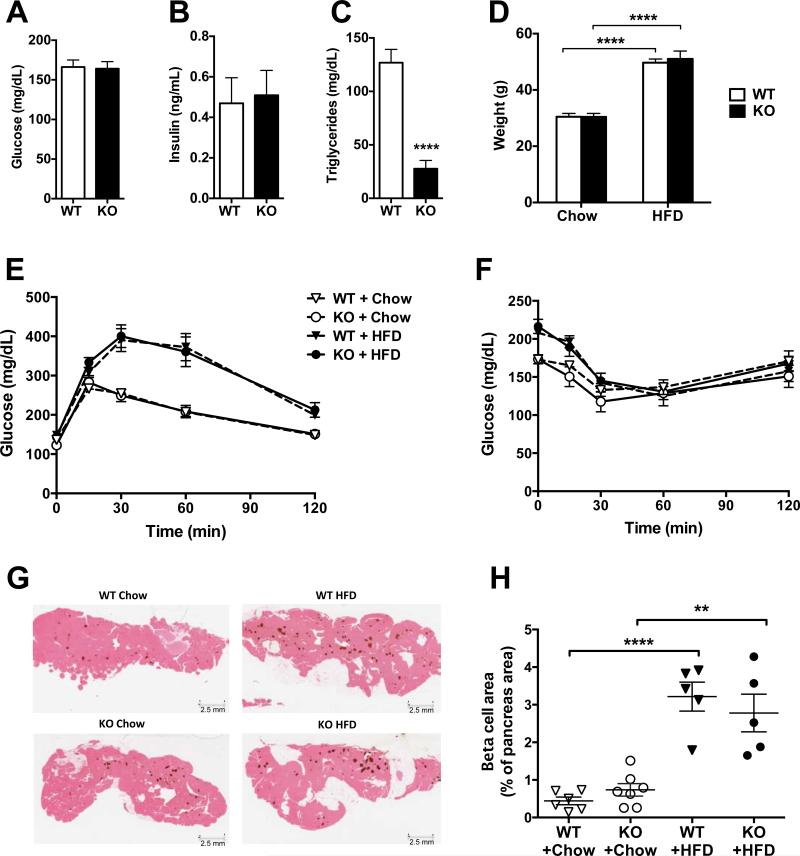
Mice lacking ANGPTL8 had normal non-fasted plasma glucose and insulin levels, but only one-third of the circulating triglycerides of wild-type mice (Fig. 1A-C), as reported previously (Wang et al., 2013). In addition, we confirmed that Angptl8−/− mice had normal glycemic control as revealed by an oral glucose tolerance (Fig. 1E) and insulin tolerance tests (Fig. 1F) (Wang et al., 2013). Angptl8−/− and wild-type mice fed a high-fat diet (60% kCal fat) for 8 weeks had a 40% increase in body weight (Fig. 1D), which was associated with impaired glucose tolerance (Fig. 1E) and reduced insulin sensitivity (Fig. 1F). No differences in the induction of impaired glucose tolerance or insulin resistance were observed between wild-type and knock-out mice. Moreover, the increase in beta cell area (stained for insulin in brown) in fat-fed mice was comparable in Angptl8−/− and wild-type mice (Fig. 1G and H). These data indicate that ANGPTL8 is not essential for beta cell number or function under normal conditions, and is not required for the compensatory beta cell expansion in response to the insulin resistance resulting from high-fat diet.
Figure 1. A High Fat Diet Induces Glucose Intolerance and Beta Cell Growth in Angptl8−/− and Wild-Type Mice.
(A) Non-fasted plasma glucose in male wild-type (WT) and Angptl8−/− (KO) mice on chow diet.
(B) Non-fasted plasma insulin in WT and Angptl8−/− mice on chow diet.
(C) Plasma triglyceride levels in WT and Angptl8−/− mice on chow diet after 18 hr fasting and 6 hr refeeding.
(D) Body weight in WT and Angptl8−/− mice on chow diet and following 8 weeks on high fat diet (HFD).
(E) Glucose tolerance test in WT and Angptl8−/− mice on chow and HFD for 7 weeks.
(F) Insulin tolerance test in WT and Angptl8−/− mice on chow and HFD for 8 weeks.
(G) Insulin immunohistochemistry (brown) of representative pancreas sections from chow and HFD Angptl8−/− and WT mice.
(H) Beta cell area as a percentage of total pancreas area. All groups had 5-7 animals. **p < 0.01; ****p < 0.0001. Values are mean ± SEM. The experiment was repeated and the results were similar.
Overexpression of ANGPTL8 Does Not Change Glucose Tolerance or Beta Cell Area
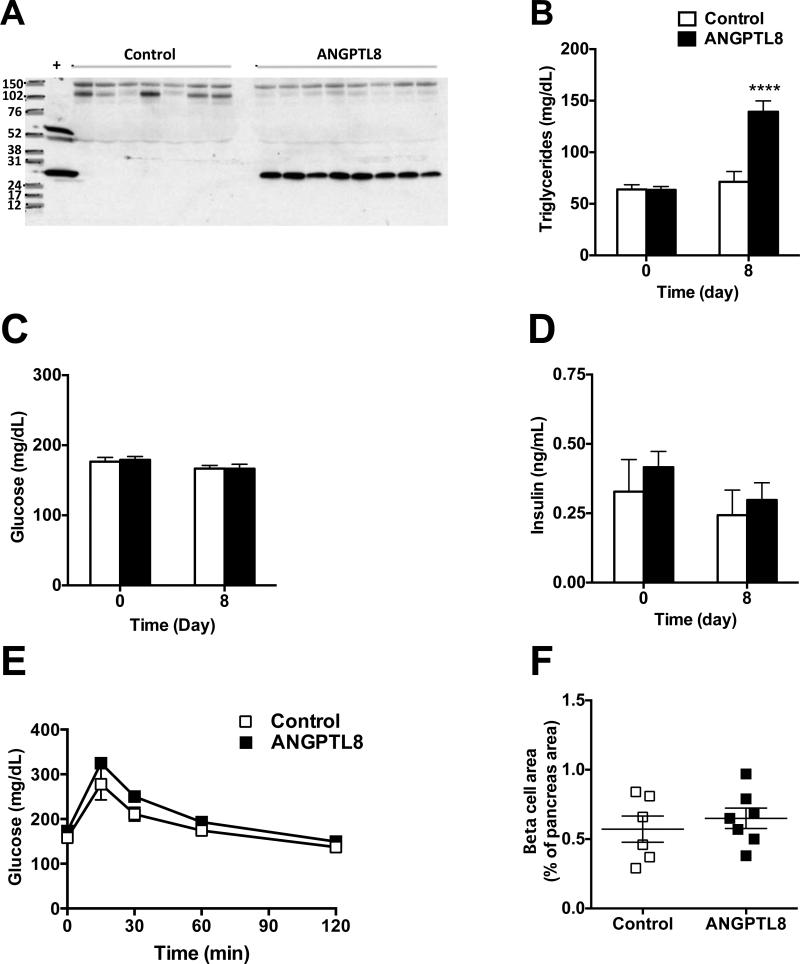
Hydrodynamic delivery of ANGPTL8 expression construct to the livers of chow fed mice resulted in high circulating ANGPTL8 levels (Fig. 2A). Overexpression of ANGPTL8 for 8 days significantly increased plasma triglyceride levels (Fig. 2B) but had no effect on plasma glucose or insulin concentrations (Fig. 2C-D). Oral glucose tolerance tests revealed no difference between control and ANGPTL8 overexpressing mice (Fig. 2E). Beta cell area and mass were similar in control and ANGPTL8 overexpressing mice (Fig. 2F and S1). Consistent with the data in Angptl8−/− mice, these overexpression data do not support a role for ANGPTL8 in the regulation of beta cell function or number but confirm its involvement in triglyceride metabolism.
Figure 2. Overexpression of ANGPTL8 Does Not Alter Glucose Tolerance or Beta Cell Area.
(A) Western blot analysis of plasma following hydrodynamic delivery (HDD) via tail vein injection of cDNA encoding a C-terminal V5 epitope-tagged human ANGPTL8. The control animals were injected with empty vector. An anti-V5 antibody was used for detection of ANGPTL8.
(B) Non-fasted plasma triglyceride levels in mice injected with control or ANGPTL8 DNA before (day 0) and 8 days after HDD.
(C) Non-fasted plasma glucose in mice injected with control or ANGPTL8 DNA before (day 0) and 8 days after HDD.
(D) Non-fasted plasma insulin in mice injected with control or ANGPTL8 DNA before (day 0) and 8 days after HDD.
(E) Oral glucose tolerance test in mice injected with control or ANGPTL8 DNA 6 days after HDD. (F) Beta cell area as a percentage of total pancreas area in mice injected with control or ANGPTL8 DNA 8 days after HDD. See also Figure S1.
All groups had 6-7 animals. ****p < 0.0001. Values are mean ± SEM. The experiment was repeated and the results were similar.
Insulin Receptor Antagonist Promotes Glucose Intolerance and Beta Cell Expansion in Angptl8−/− Mice
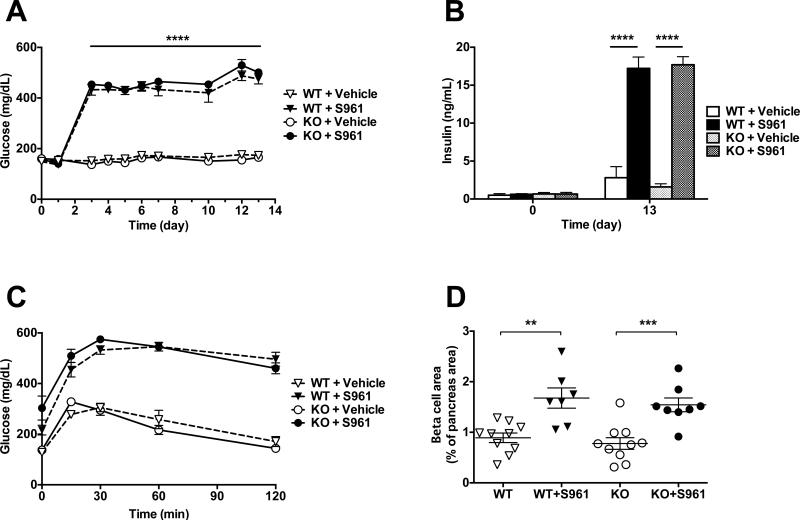
Infusion of the insulin receptor antagonist S961 (20 nMol/week) caused a pronounced and sustained increase in plasma glucose in both Angptl8−/− and wild-type mice (Fig. 3A). Plasma insulin levels were increased >6-fold in both Angptl8−/− and wild-type mice (Fig. 3B). Circulating triglyceride levels were unchanged by S961 in both strains of mice (data not shown). Mice receiving S961 were severely glucose intolerant (Fig. 3C), which was associated with a compensatory doubling of the beta cell area and mass in both Angptl8−/− and wild-type mice (Fig. 3D and S2). These data show that insulin receptor antagonism causes glucose intolerance and beta cell expansion by a mechanism that does not depend upon ANGPTL8.
Figure 3. The Insulin Receptor Antagonist S961 Causes Glucose Intolerance and Beta Cell Growth in Angptl8−/− and Wild-Type Mice.
(A) Non-fasted plasma glucose in Angptl8−/− and WT mice continuously treated with the insulin receptor antagonist S961 (20 nMol/week) or vehicle for 13 days.
(B) Non-fasted plasma insulin on days 0 and 13 in Angptl8−/− and WT mice treated with S961 (20 nMol/week) or vehicle.
(C) Oral glucose tolerance test in Angptl8−/− and WT mice treated with S961 (20 nMol/week) or vehicle at day 11 of the study.
(D) Beta cell area as a percentage of total pancreas area in Angptl8−/− and WT mice treated with S961 (20 nMol/week) or vehicle for 13 days. See also Figure S2.
All groups had 7-10 animals. **p < 0.001; ***p < 0.0005; ****p < 0.0001. Values are mean ± SEM.
DISCUSSION
The main findings of the study are that 1) ANGPTL8 is not required for beta cell function or the compensatory beta cell growth response to insulin resistance; 2) overexpression of ANGPTL8 does not increase beta cell area nor improve glycemic control and 3) ANGPTL8 regulates plasma triglyceride levels.
Yi et al. (2013) reported that ANGPTL8 is a betatrophin that controls beta cell proliferation in mice treated with the insulin receptor antagonist S961. A subsequent publication (Jiao et al., 2014) and our data confirm the findings that administration of S961 causes severe glucose intolerance and beta cell expansion in mice. However, the beta cell growth response was similar in Angptl8−/− and wild-type mice. Thus, ANGPTL8 is not required for the expansion of the beta cell area in response to insulin resistance induced by the insulin receptor antagonist S961.
It had previously been reported that wild-type and Angptl8−/− mice show no difference in glucose homeostasis (Wang et al., 2013). In the current study, we demonstrate that both wild-type and Angptl8−/− mice can mount a significant and similar expansion of their beta cell area in response to high-fat diet-induced insulin resistance. The lack of involvement of ANGPTL8 in the regulation of beta cell function and growth is supported by our hydrodynamic overexpression studies. Specifically, high circulating levels of ANGPTL8 in this study was associated with an increase in plasma triglycerides but caused no change in glycemic control or increase in beta cell area. The lack of expansion of the beta cell area could theoretically be due to simultaneous increases in replication and apoptosis frequencies. However, even if this were the case, it would not change our observation that Angptl8 overexpression did not increase beta cell area, contrary to what was reported by Yi et al. (2014).
The factor(s) controlling beta cell growth in this study have not been identified. Both the insulin receptor antagonist S961 and the high fat diet caused hyperglycemia. Persistent high glucose levels may contribute to the expansion of the beta cell area. This is supported by accumulating evidence that glucose signaling plays an important role for expansion of the beta cell mass. In particular, glucose infusion in rodents stimulates beta cell proliferation (Alonso et al., 2007; Bernard et al., 1998; Bonner-Weir et al., 1989). Furthermore, mice haploinsufficient for beta cell glucokinase are unable to increase their beta cell mass in response to insulin resistance produced by a high fat diet (Terauchi et al., 2007), whereas pharmacological activation of glucokinase stimulates beta cell proliferation (Porat et al., 2011). Insulin signaling is unlikely to regulate the beta cell growth since expansion was observed in the presence of the insulin receptor antagonist S961. Other factors may also contribute to stimulation of beta cell proliferation. For example, hepatic insulin resistance was shown to promote a robust increase in beta cell proliferation, which seems to be mediated by a circulating factor that acts independent of insulin and glucose (El Quaamari et al., 2012; Michael et al., 2000). The identification of the factor(s) causing the expansion of the beta cell area is subject to active investigation. However, those factors may differ in mice and humans. Jiao et al. (2014) showed that S691 administration increases mouse but not human beta cell proliferation.
Studies in humans suggest that ANGPTL8 levels are increased in patients with type 1 diabetes. However, ANGPTL8 levels do not correlate with residual C-peptide levels, glucose control or insulin requirement, suggesting that ANGPTL8 does not protect against beta cell destruction (Espes et al., 2014). The role of ANGPTL8 in type 2 diabetes and obesity remains controversial. ANGPTL8 levels were found in one study to correlate with type 2 diabetes and body mass index (Fu et al., 2014), whereas another study reported no difference in ANGPTL8 levels between lean and morbidly obese or between non-diabetic or type 2 diabetic individuals. In the latter study, ANGPTL8 levels were associated with an atherogenic lipid profile (Fenzl et al., 2014). Genetic sequencing of 56,000 individuals revealed a low frequency coding DNA sequence variant in ANGPTL8 that was associated with higher plasma HDL-cholesterol and lower triglycerides but not with fasting glucose levels (Peloso et al., 2014), once again confirming the role of ANGPTL8 in lipid metabolism rather than in regulation of human beta cell function.
In conclusion, the present data do not support a role for ANGPTL8 in the control of beta cell function or growth. The findings that plasma triglyceride levels are markedly reduced by Angptl8 deletion and increased following ANGPTL8 overexpression support the possibility that inhibition of ANGPTL8 represents a therapeutic strategy for hypertriglyceridemia.
EXPERIMENTAL PROCEDURES
Construct
A cDNA coding for a C-terminal V5 epitope-tagged human ANGPTL8 was generated by PCR using the following primers: 5’-GATATCACTCGAGCGAGGTCT CCACCATGCCAG-3’ and 5’-CAATTAGCGGCCGCTCACGTAGAATCGAGACCGAGG AGAGGGTTAGGGATAGGCTTACCGGCTGGGAGCGCCGCTGTGTGGAG-3’ and a DNA plasmid clone harboring untagged human ANGPTL8 (reference sequence NM_018687.6) as the template. The resulting cDNA was cloned into a mammalian expression vector pRG977. The clone was confirmed by DNA sequencing. The expression and secretion was evaluated by western blot analysis of CHO cell culture medium using an anti-V5 antibody (Sigma, polyclonal rabbit).
Animals
Angptl8−/− mice (75% C57BL/6NTac and 25% 129/SvEvTac background) were generated by homologous recombination using Regeneron's VelociGene® technology as previously described (Wang et al., 2013). Mice were housed (male; 1-5 per cage) in a controlled environment (12-h light/dark cycle, 22±1 °C, 60–70% humidity) and fed ad libitum with standard chow (Purina Laboratory Rodent Diet 5001, LabDiet). Some mice were fed a high-fat diet (Research Diets, D12492; 60% fat by calories). For all studies reported in this paper, comparisons were made between wild-type and knock-out littermates. C57Bl/6 mice (males, 24-28 g; Taconic) were used in the hydrodynamic tail vein overexpression study. All animal procedures were conducted in compliance with protocols approved by the Regeneron Pharmaceuticals Institutional Animal Care and Use Committee.
Hydrodynamic DNA Delivery
To establish a baseline, plasma samples were collected from all mice 7 days prior to hydrodynamic DNA delivery (HDD). On study day 0, mice were sorted into treatment groups (n=6-7 per group) based on body weights and their plasma triglyceride and glucose levels. The mice were anesthetized with isoflurane and injected with plasmid at 50 μg of DNA in sterile saline via tail vein at 10% of their body weight. Plasma was collected during the study to assess lipid levels. Blood glucose was measured from tail tip using Accu-Chek glucometer (Roche). hANGPTL8 expression in plasma was confirmed by western blotting.
S961 Administration
S961 is an inhibitory peptide (43 aa) with high affinity to the insulin receptor, was synthesized by Celtek Peptides (Franklin, TN) using published sequence (Schäffer et al., 2008). S961 or control (PBS) was infused at 20 nMol/week using Alzet osmotic pump (2002 model, Durect Corporation, Cupertino, CA) for 13 days. Blood glucose was measured daily from tail tip using Accu-Chek glucometer (Roche). Plasma was collected during the study to assess insulin and triglyceride levels. Glucose tolerance test was performed on day 11 of the study.
Glucose Tolerance Test
Mice were fasted overnight (16 hr) followed by oral gavage of glucose at 2 g/kg body weight. Blood glucose level was evaluated at 0, 15, 30, 60 and 120 min post-injection.
Insulin Tolerance Test
Mice were fasted for 4 hr followed by intraperitoneal injection of 0.75 U/kg (chow-fed) or 2 U/Kg (high-fat fed) of human insulin (Eli Lilly). Blood glucose level was evaluated at 0, 15, 30, 60 and 120 min post-injection.
Serum Chemistry
Serum triglyceride levels were determined using an ADVIA® 1800 blood chemistry analyzer (Bayer). Circulating insulin level was determined from plasma samples using a Luminex assay (Millipore).
Analysis of Beta Cell Mass and Area
Pancreas was cassetted in a spread anatomical orientation, embedded in paraffin, and sectioned to obtain a full footprint. Sections were stained for insulin using polyclonal guinea pig anti-insulin antibody (DaKo; dilution 1:3000) using an overnight incubation at 4°C. Biotin-conjugated goat anti-guinea pig IgG (Ja ckson ImmunoResearch; dilution 1:1000) was used as secondary antibody and incubated for one hour at room temperature followed by detection with the ABC kit (Vector Laboratories; dilution 1:500). After diaminobenzidine (DAB) / peroxidase brown visualized reaction (0.4 mg/ml DAB, 0.0003% H2O2) sections were counterstained with eosin. Beta cell area was measured using Spectrum software (Indica Labs; Corrales, NM) and expressed as a percentage of the total pancreas area of the section. For the insulin receptor antagonist and HDD studies, we also assessed beta cell mass using point counting morphometry as described previously (Montana et al., 1993). The data obtained by the two methods were similar.
Western Blot Analysis
1.5 μl of plasma from each animal was resolved by SDS-PAGE using Criterion™ TGX™ 4-20% precast gel (Bio-Rad) under reducing conditions and transferred to nitrocellulose membranes. The membranes were probed with an anti-V5 antibody (Sigma, polyclonal rabbit) and detected with donkey anti-rabbit horseradish peroxidase-coupled antibody (Jackson ImmunoResearch) using an enhanced chemiluminescent detection system.
Data analysis
Data are expressed as mean standard error of the mean □ Mean values were compared using unpaired t-tests or 2-way ANOVA as implemented in the Graphpad Prism 6.0 software (Graphpad Software, Inc.).
Supplementary Material
HIGHLIGHTS.
ANGPTL8 regulates triglyceride metabolism in mice
ANGPTL8 does not control glucose homeostasis in mice
Insulin resistance causes normal beta cell expansion in ANGPTL8 deficient mice
Targeted ANGPTL8 overexpression in mice does not induce beta cell growth
ACKNOWLEDGMENTS
We wish to thank Lisa Shihanian, Kristen Tramaglini and Lawrence Miloscio for excellent technical assistance. We would also like to acknowledge support from the NIH (PO1 HL20948).
Abbreviations
- ANGPTL8
Angiopoietin-like protein 8
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
V.G., A.J.M., G.D.Y. and J.G. provided experimental design and coordination, V.G., C.A.A., E.N., S. B.-W., Y.X. and J.G. performed research and analysis of the data, P.E.S. generated new reagents, V.G., J.G., J.C.C, H.H.H. and G.D.Y. provided an intellectual input and wrote the paper.
REFERENCES
- Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocana A. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Thibault C, Berthault MF, Magnan C, Saulnier C, Portha B, Pralong WF, Penicaud L, Ktorza A. Pancreatic beta-cell regeneration after 48-h glucose infusion in mildly diabetic rats is not correlated with functional improvement. Diabetes. 1998;47:1058–1065. doi: 10.2337/diabetes.47.7.1058. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- El Quaamari A, Kawamori D, Dirice E, Liew CW, Shadrach JL, Hu J, Katsuta H, Hollister-Lock J, Qian W-J, Wagers AJ, Kulkarni RN. Liver-derived systemic factors drive β cell hyperplasia in insulin-resistant states. Cell Reports. 2013;3:401–410. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espes D, Lau J, Carlsson P-O. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia. 2014;57:50–53. doi: 10.1007/s00125-013-3071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenzl A, Itariu BK, Kosi L, Fritzer-Szekeers M, Kautzky-Willer A, Stulnig TM, Kiefer FW. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia. 2014;57:1204–1208. doi: 10.1007/s00125-014-3208-x. [DOI] [PubMed] [Google Scholar]
- Fu Z, Berhane F, Fite A, Seyoum B, Abou-Samra AB, Zhang R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Scientific Reports. 2014;4:5013. doi: 10.1038/srep05013. doi: 10.1038/srep05013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720–1725. doi: 10.1007/s00125-011-2118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Lay JL, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes. 2014;63:1283–1288. doi: 10.2337/db13-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnu-son MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Montaña E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J. Clin. Invest. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloso GM, Auer PL, Bis JC, Voorman A, Morrison AC, Stitziel NO, Brofy JA, et al. Am. J. Hum. Gen. 2014;94:223–232. doi: 10.1016/j.ajhg.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliarini F, Wang Y, Kozlitina J, Grishin NV, Hyde R, Boerwinke E, Valenzuela D, Murphy AJ, Cohen JC, Hobbs HH. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 2012;109:19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer L, Brand CL, Hansen BF, Ribel U, Shaw AC, Slaaby R, Sturis J. A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 2008;376:380–383. doi: 10.1016/j.bbrc.2008.08.151. [DOI] [PubMed] [Google Scholar]
- Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J. Clin. Invest. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group UK Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- Yi P, Park J-S, Melton DA. Betatrophin: A hormone that controls pancreatic cell proliferation. Cell. 2013;153:747–759. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J. Clin. Endocrinol. Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM, Cohen JC, Hobbs HH. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA. 2013;110:16109–16114. doi: 10.1073/pnas.1315292110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.