Abstract
OBJECTIVES:
Describe rates of adherence for sickle cell disease (SCD) medications, identify patient and medication characteristics associated with nonadherence, and determine the effect of nonadherence and moderate adherence (defined as taking 60%–80% of doses) on clinical outcomes.
METHODS:
In February 2012 we systematically searched 6 databases for peer-reviewed articles published after 1940. We identified articles evaluating medication adherence among patients <25 years old with SCD. Two authors reviewed each article to determine whether it should be included. Two authors extracted data, including medication studied, adherence measures used, rates of adherence, and barriers to adherence.
RESULTS:
Of 24 articles in the final review, 23 focused on 1 medication type: antibiotic prophylaxis (13 articles), iron chelation (5 articles), or hydroxyurea (5 articles). Adherence rates ranged from 16% to 89%; most reported moderate adherence. Medication factors contributed to adherence. For example, prophylactic antibiotic adherence was better with intramuscular than oral administration. Barriers included fear of side effects, incorrect dosing, and forgetting. Nonadherence was associated with more vaso-occlusive crises and hospitalizations. The limited data available on moderate adherence to iron chelation and hydroxyurea indicates some clinical benefit.
CONCLUSIONS:
Moderate adherence is typical among pediatric patients with SCD. Multicomponent interventions are needed to optimally deliver life-changing medications to these children and should include routine monitoring of adherence, support to prevent mistakes, and education to improve understanding of medication risks and benefits.
Keywords: medication adherence, sickle cell disease
Background
Sickle cell disease (SCD) is a genetic disorder affecting approximately 100 000 people in the United States.1 SCD is associated with high morbidity and mortality rates. In the past decade, several new medications have become available that have the potential to prolong the duration and improve the quality of life for pediatric patients with SCD.2
Medications shown to be efficacious in research studies may be less effective in clinical practice because of nonadherence.3,4 Adherence has been defined as “the extent to which a patient is taking his medication as prescribed by his healthcare providers.”4 Poor adherence reduces the effectiveness of medications, places patients at risk for serious complications, and significantly increases health care costs.3,4 For example, nonadherence to antibiotic prophylaxis may leave young children with SCD susceptible to overwhelming sepsis and death.5 Nonadherence is estimated to account for $100 to $300 billion in annual US health care costs.6,7
Clinicians report that nonadherence to medications and to monitoring are barriers to treatment in SCD.8 When asked about factors important to adherence to prophylaxis in patients with SCD, clinicians perceptions of important factors did not always agree with factors that actually affect adherence; for example, only 6% included patient fear of side effects and only 20% patient doubts about medication effectiveness.9
Although providers recognize that nonadherence to SCD medications poses a significant barrier to effective disease management, they may have difficulty identifying which patients in their practice exhibit nonadherent behaviors. Approaches to assessing and monitoring adherence by most health care teams may not reliably identify nonadherent patients.10 Compared with more objective methods of estimating adherence (eg, pill count, prescription refill count, serum or urine drug levels), report measures tend to overestimate adherence.4 In addition, adherence to sickle cell medications may vary in other factors such as frequency of use and monitoring required, as seen in other conditions.4,11 Poor adherence to medications in other chronic illnesses has been related to medication, patient, and family characteristics.4,12 To improve clinicians’ understanding of medication adherence in pediatric patients with SCD, we conducted a systematic review of the literature. Among pediatric patients with SCD, our aims were to describe rates of adherence for different SCD medication types, identify patient and medication characteristics associated with nonadherence, and describe the effect of nonadherence and moderate adherence (defined as 60%–80% of doses taken) on clinical outcomes.
Methods
Article Retrieval
We performed a systematic review of PubMed, Cochrane, Embase, Scopus, Web of Science, and the World Health Organization Global Health Library in February 2012 for publications after 1940. Search terms included medication names (eg, hydroxyurea or penicillin prophylaxis), disease names (eg, sickle cell anemia or hemoglobin SS), and adherence terms (eg, patient adherence or medication noncompliance) (see the Appendix). The search was performed by a professional librarian (K.L.) who did not restrict the search in any way; the results of this search were screened by clinician reviewers for relevance to study objectives. In addition, we performed an ad hoc search of bibliographies of articles selected for review to identify additional references not identified in our primary search.
Study Selection
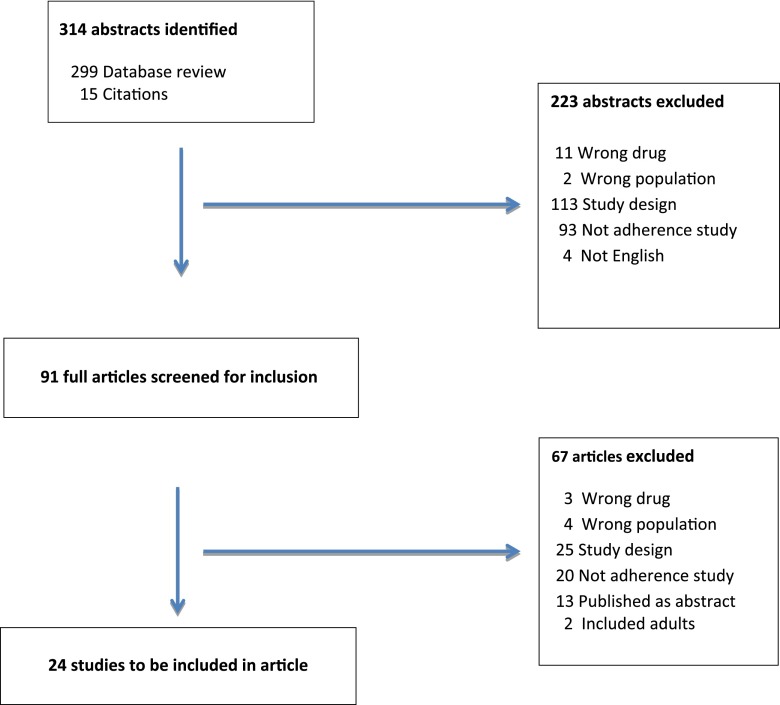
Studies were included if they addressed medication adherence, included patients with SCD, were in English, included pediatric patients <25 years old, and were primary research studies (ie, not a literature review or editorial) (Fig 1). Each abstract was reviewed by two authors (D.G.B., S.L.C., P.K., K.E.W.), who made decisions about whether the full-text article should be reviewed. If ≥1 author felt the article should be reviewed, it was. Each full article was then independently reviewed by 2 authors who made judgments about whether the article should be included in the final review using a standard data form. Reasons for exclusion were recorded. Disagreements were resolved through discussion.
FIGURE 1.
Abstracts identified by search strategy and included in final literature review.
Data Extraction
We adapted a data collection method from those used in our previous research to capture information about study design, medication type, population age and diagnoses, measures of adherence, adherence rates, patient and medication factors associated with nonadherence, and clinical effects of nonadherence or moderate adherence.13–15 As in previous studies, information from each article was entered independently by 2 authors (K.E.W. and C.M.) to minimize bias in data extraction.16 Differences in extraction were reconciled through rereview of the article.
We classified articles based on type of medication studied and, within medications, by the method used to measure adherence, because adherence rates vary depending on the method used.4 Articles included in the review described a range of methods for measuring adherence. These included by report (by parent or adolescent, by clinician, or Morisky scale17–19), direct measurements of unconsumed medication (pill count or measurement of remaining volume for liquid medications), medication monitoring devices (eg, Medication Event Monitoring System [MEMS] caps recording frequency and timing of bottle opening), pharmacy claim data (eg, medication possession ratio [MPR]: days supplied divided by the number of days of observation from the first dispensed dose to the end of a specified follow-up period),20 and drug level (eg, urinary assay).21–23
We assessed reliability of the results for the articles using the Newcastle–Ottawa Quality Assessment Scale; this checklist, which has been used in several studies, is designed to assess the quality of observational studies used in systematic reviews.22–25 Of the 8 domains in the checklist for cohort studies, 4 were relevant to the studies. One author rated all included studies on these 4 items: representativeness of the population, assessment of adherence, adequacy of follow-up, and length of follow-up. For young pediatric patients, studies used parents and other primary caregivers as reporters of adherence; throughout this article we refer to the primary caregiver for the child as “parent.” From each article, we identified risk and protective factors for adherence along each step in the medication use pathway (prescribing, dispensing, administering, monitoring).26 Finally, we defined levels of moderate adherence as taking 60% to 80% of doses.
Results
Our initial search identified 299 abstracts; an additional 15 articles were identified from cited references (Fig 1). Ninety-one articles were identified for full review. Of these, 24 met all inclusion criteria; data were abstracted and tabulated for this review.
Studies enrolled 10 to 519 subjects, but only 5 studies (21%) had more than 100 patients. Seven studies were multisite; 4 were drug trials, in which analysis of medication adherence was a secondary aim. Studies sometimes were missing adherence data for many subjects. For example, 1 study evaluated antibiotic adherence in 50 children using parent report and urine samples, but only 23 urine samples were obtained.27 There were 14 prospective studies; 8 evaluated the clinical impact of nonadherence, and 4 provided information about the effect of moderate adherence on clinical outcomes. Regarding risk for bias in the included studies, according to the Newcastle–Ottawa Quality Assessment Scale, all studies had adequate length of follow-up period, and 92% had adequate assessment of adherence.22,23 Seventy-five percent had adequate representativeness of the sample population; the remaining 25% failed to state how they derived the study cohort. Eighty-eight percent of studies had adequate follow-up information; the 3 studies that did not had missing adherence information on a large portion of the study cohort.
Adherence Rates
Overall rates of adherence were higher for reported measures (48%–89% adherent) than for objective measures such as urinary assays (40%–56% adherent) or pharmacy refill data (12%–60% adherent) (Table 1). Only 1 article compared adherence to different types of medications for SCD and found similar rates of adherence for hydroxyurea (mean MPR 60%), folic acid (mean MPR 61%), and penicillin (mean MPR 55%).28
TABLE 1.
Summary of the Studies Included in Literature Review by Type of Medication
| Author | N | Age | Design | Measures | Mean Adherence | % Patients Adherent |
|---|---|---|---|---|---|---|
| Hydroxyurea | ||||||
| Kinney et al 199929 | 84 | 5–15 y | Multisite RCT (HUG-KIDS) | Pill count: entire 2-wk supply taken | — | 74% |
| Ware et al 200230 | 53a | 5–15 y | Multisite RCT (HUG-KIDS) | Pill count: % of 2-wk supply taken | 94.4% | — |
| Olivieri and Vichinsky 199855 | 10b | 5–18 y | Single-site prospective cohort | MEMS caps: % of pills taken | 96% | — |
| Thornburg et al 201031 | 153c | 9 m–1.5 y | Multisite RCT (Baby HUG) | ≥80% of liquid medication taken by volume remaining at study visit | Consumed 102% of volume prescribed | 89% took >80% doses |
| Thornburg 201032 | 75 | <18 y | Single-site cross-sectional study | Visual analog scale: >75% doses | — | Visual analog: 82% |
| Morisky scale: ≤1 | Morisky: 84% | |||||
| Clinician estimate: “often” or “always” adherent | Clinician report: 85% | |||||
| Refills: ≥5 in 6 mo | Refills: 49% | |||||
| Deferasirox | ||||||
| Alvarez et al 200934 | 21 | 7–21 y | Multisite prospective cohort | Pill count: ≥80% doses taken | Pill count mean 79% | Parent report: 71% |
| Parent report: ≥80% doses taken | Pill count: 43% | |||||
| Raphael et al 200945 | 59 | Pediatric patients | Single-site retrospective cohort | Medical record: missed ≥3 doses a month for ≥2 mo | — | 76% |
| Deferoxamine | ||||||
| Thuret et al 200935 | 70 | ≥6 y | Multisite cross-sectional | Morisky scale: 3 or 4 | — | Morisky scale: 72% |
| Parent or patient report: no missed infusions | Parent report: 43% | |||||
| Treadwell and Weissman 200136 | 31 | 6–21 y | Single-site prospective cohort | Patient report: used in last 2 d | — | 57% |
| Treadwell et al 200542 | 15 | Pediatric patients | Single-site cross-sectional | Refills: % doses | Refill: 60% doses | — |
| Parent report: days since last deferoxamine | Days: 8.7 | |||||
| Morisky scale | Morisky scale: 2.0 | |||||
| Number physical signs of chelation | Physical signs: 2.1 | |||||
| Oral Antibiotic Prophylaxis | ||||||
| Berkovitch et al 199850 | 45 | 9 m–7 y | Single-site prospective cohort | MEMS caps: % doses | 69% | N/A |
| Davis 198956 | 519 | 2 m–5 y | Multistate retrospective claim-based | Low estimate: MPR ≥.33 | — | 69%–84% |
| High estimate: same formula, assumes did not take after expired | ||||||
| Sox et al 200341 | 261 | <4 y | Multistate retrospective claim-based | Refills: days covered by medication fills in 1-y period | 40% | — |
| Buchanan et al 198257 | 62 | 6 m–19 y | Single-site prospective cohort | Urine test + | — | 66% |
| Pejaver et al 199747 | 42 | 11 m–12 y | Single-site prospective cohort | Urine test +; Parent report: never missed dose | — | Urine: 46% |
| Parent report: 62% | ||||||
| Cummins et al 199127 | 50 | ≤16 y | Single-site cross-sectional | Urine test +; Parent report: never missed dose | — | Urine: 47% |
| Parent report: 62% | ||||||
| Teach et al 199858 | 159 | 0.3–24 y | Single-site prospective cohort | Urine test +; Parent or patient report: gave dose in last 15 h | — | Urine: 43% |
| Parent report: 68% | ||||||
| Bitarães et al 200859 | 108 | 3 m–4.5y | Single-site prospective cohort | Urine test +; Parent report: never missing dose | — | Urine: 56% |
| Medical record: record of nonadherence | Parent report: 48% | |||||
| Medical record: 89% | ||||||
| Elliot et al 200140 | 50 | 6 m–5 y | Single-site retrospective cohort | Refill: in past 14 d and average time between 14-d supply | 27 d | Refill: 12% |
| Parent report: of “never late to get refills | Parent report: 60% | |||||
| Witherspoon and Drotar 200639 | 30 | 6 m–6 y | Single-site prospective cohort | Refill: ≤1 uncovered day per month | — | Refill: 33% |
| Parent report: miss <2 d per month | Parent report: 57% | |||||
| Clinician report: very adherent | Clinician report: 50% | |||||
| IM or IV Antibiotic Prophylaxis | ||||||
| King et al 201160 | 78 | 4 m–4 y | Single-site retrospective cohort | Administration record: 80% injections received | — | 89% |
| Multiple Medications | ||||||
| Patel et al 201028 | 93 | 6 m–20 y | Single-site retrospective cohort | Mean MPR | Penicillin MPR: 55% | — |
| Hydroxyurea MPR: 61% | ||||||
| Folic acid MPR: 61% | ||||||
| Babiker 198637 | 40 | 2–5 y | Single-site prospective cohort | Urine test +; Administration record: % indicated injections received | Injections: 92% | Urine (oral antibiotic): 40% |
| Babiker 198638 | 42 | 4–8 y | Single-site prospective cohort | Urine test +; Administration record: % indicated injections received | Injections: 95% | Urine (oral antibiotic): 44% |
RCT, randomized controlled trial.
Only includes patients who received maximum tolerated doses.
17 patients in effectiveness study, only 10 received MEMS caps.
191 patients in study, adherence data available for 153 subjects.
Hydroxyurea
Adherence rates in 3 multicenter drug trials of hydroxyurea ranged from 74% to 94%, as measured using pill counts and MEMS caps, respectively.29–31 In these drug efficacy trials, each lasting ≥6 months, participants had study visits every 2 weeks, where a 2-week supply of pills was dispensed and the pills remaining in the bottle from the previous visit were counted. In the study that was not part of a drug effectiveness study, adherence was 49% (5 of 6 refills in previous 6 months) to 85% (clinician report of “often or always adherent”).32,33
Iron Chelation Therapy
In a study of deferasirox, 43% of patients had good adherence by pill counts and 71% by parent report.34 In studies of deferoxamine, adherence rates were moderate according to the Morisky scale (72%) and patient or parent report of missed doses (43%–57% adherent).35,36
Prophylactic Antibiotics
In the 2 studies that compared different administration routes for prophylactic antibiotics, adherence to injections (>90% injections given) was better than to oral medication (40%–44% positive urine test).37,38 Several studies describe gaps in antibiotic use, also known as “uncovered days.” In 1 study, 33.3% of young children had 14 to 30 uncovered days per month.39 In another, there was an average gap of 27.4 days between fills of a 14-day supply of liquid antibiotics.40 Another found that an average of 60% of a 1-year study period was not covered by antibiotics.41
Factors Associated With Nonadherence
Nonadherence among patients with SCD was often related to beliefs about safety and effectiveness of medications or to mistakes administering medications at home (Table 2). Reported mistakes, such as forgetting to give medicine40 or being too busy to give medicine,32,42,43 were significantly correlated with poor adherence. In addition, parent knowledge was significantly correlated with better adherence.32,36,42 One study estimates that 30% of variance in adherence can be attributed to health beliefs among patients with SCD, such as beliefs about severity of disease or the burden of using medication.40 Risk factors for nonadherence were found to be additive: Patients with more barriers had worse adherence rates.39 On the contrary, preventive clinic visits may be protective; 1 study found that each preventive visit was associated with an additional 12 days of antibiotic prophylaxis use, based on refill data.41
TABLE 2.
Risk and Protective Factors for Barriers to Adherence Among Patients With SCD Along Each Step in Ambulatory Medication Use61
| Step | Barrier | Risk Factor | Protective Factor and Potential Intervention |
|---|---|---|---|
| 1. Prescription given | Physician not prescribing medication | Physician concerns about nonadherence as barrier to prescribing.8 | Education of physicians |
| Difficult for family to come to clinic.32,40 | Transportation to clinic provided62 | ||
| 2. Prescription filled at pharmacy | Prescription not filled | Frequent refills for liquid penicillin due to expiration after 10–14 d.27 | Use of tablets rather than liquids to allow dispensing of higher number of days’ supply |
| Failure to (re)fill prescription.32 | Use of 90-d supply | ||
| Insurance problems.32,45 | Refill reminders | ||
| 3. Remember to give dose | Doses skipped | Parent does not understand could get sick or die without penicillin,27 beliefs about the value and importance of medicine.40 | Caregiver knowledge about indications for and use of medicines32,36,39 |
| Competing demands,32,43 family stress.39,40,42 | Social support for family and child32,36 | ||
| Treatment limited by travel or other change in daily activities. | Parent and child sharing responsibility for medication36,42 | ||
| Child asleep. | |||
| Do not like to use needle.43 | |||
| Forgetting.40 | |||
| Adverse effects of medication.43 | |||
| 4. Measure medication | Medication incorrectly measured or prepared | Parent unsure of dose. | Parent dosing support64,65 |
| Liquid medications harder to measure. | |||
| Dissolve deferasirox in 8 oz. water.63 | |||
| 5. Child takes medication | Medication difficult to take | Child does not like taste.45 | — |
| Adherence worse with home oral penicillin compared with injected in clinic.37,38 | |||
| 6. Monitoring | Inadequate monitoring | Monitoring not completed by patient.8 | |
| Lack of persistence | Difficulty taking, painful to use,43 no obvious benefit. | Each preventive visit associated with 12 more days medication taken41 |
Clinical Impact of Nonadherence and Moderate Adherence
Among hydroxyurea users, nonadherence was associated with reduced fetal hemoglobin levels.32,44 None of the studies of hydroxyurea we reviewed linked moderate adherence to clinical outcomes. Among patients with SCD taking iron chelation, nonadherent patients had less reduction in serum iron than adherent patients (11% vs 44% decline).45 In a study of 15 patients with SCD on deferoxamine, 9 were categorized as moderately adherent; moderately adherent patients had serum ferritin levels that were lower than those of nonadherent patients but not as low as those of adherent patients.42
Three articles described cases of overwhelming sepsis among children with SCD prescribed daily antibiotic prophylaxis.5,46,47 Each described a small number of cases of septicemia; in all but 1, parents reported missing recent antibiotic doses, or patients had a negative urine antibiotic test. It should be noted that adherence rates for those without infections were not reported. There was a significant association between nonadherence to antibiotics and frequency of sickle cell crises and infection in 1 study.46 In another study, patients not adherent to antibiotics had a higher rate of emergency department visits (5.5 per year) than adherent patients (2 per year).47
Discussion
In this systematic review of the medication adherence literature among pediatric patients with SCD, we found that moderate adherence was common. Nonadherence was associated with increased painful crises and increased hospitalizations, yet little information was available about the clinical effects of moderate adherence. Health beliefs (eg, fear of side effects) and factors increasing risk for mistakes (eg, complex medication regimen or more frequent dosing) contributed to nonadherence; barriers to adherence were additive.
We found that medication characteristics, such as route of administration, did influence adherence, consistent with previous literature.4 Interestingly, adherence to injected antibiotics was markedly better than adherence to oral antibiotics in both studies that compared them.37,38 Given that antibiotic prophylaxis is used in children with SCD to prevent life-threatening sepsis, clinicians should consider offering injected antibiotic prophylaxis to families who cannot adhere to oral antibiotics, because the literature indicates that injections ensure fewer unprotected days. Such a decision must be balanced by the consideration that injected antibiotics are more painful than oral antibiotics and that repeated injections may reduce quality of life.
Most studies we reviewed identified a substantial population that was moderately adherent; in studies that measured adherence as a continuous measure, patients took a mean of 40% to 79% of prescribed doses (excluding drug efficacy trials). Such trends can cause physicians to be reluctant to prescribe medications such as hydroxyurea.8 Hydroxyurea toxicity could develop if the physician is unaware of nonadherence and raises the dosage. The 2 studies we found reporting moderate adherence to hydroxyurea and iron chelation indicated that moderate adherence may have some incremental benefit over poor adherence.30,42
If we accept that moderate adherence is ubiquitous and that some patients have poor adherence, then clinician monitoring of adherence is necessary. In 2010 Drotar48 suggested that studies are needed to develop and test routine monitoring of adherence to medications among patients with SCD. We recommend combining parent or adolescent report with more objective measures of adherence to optimize monitoring. Report should be obtained in a way that encourages honest responses, such as self-administered written questions at each visit rather than clinician interview.49 Objective measures for clinicians to routinely monitor adherence should be neither cumbersome nor costly and may include automated collection of pharmacy fill data or pill counts in the office.
To our knowledge, there are few published interventions to improve medication adherence in children with SCD; none are multicenter, and none have significant effect. A randomized trial of a “deferoxamine day camp” for 31 school-age children with SCD did not result in increases in knowledge about deferoxamine or better social support.36 A randomized trial of parent education, regular social worker contact, and a medication calendar among 45 children was associated with a small but not statistically significant improvement in prophylactic antibiotic adherence.50 Literature reviews indicate that, among pediatric patients, multicomponent interventions and those with a behavioral component are more likely to be effective than educational interventions alone.51,52
Multicomponent interventions to improve adherence should target different barriers to adherence. Because barriers to adherence are additive, it stands to reason that as each barrier is overcome, adherence may increase. Many barriers were related to health beliefs, such as beliefs about the value of the medicine, not liking to use a needle, or concerns about the adverse effects of medication. Many other barriers were related to mistakes, such as forgetting, not knowing the correct dosage, or difficulty measuring liquid medications.
The existing literature on adherence to medication among people with SCD has important gaps. Larger, multisite studies of medication adherence are needed in children with SCD. In 2010 Drotar48 highlighted the need for large prospective studies to assess the impact of nonadherence and moderate adherence on biological outcomes. Our review of the literature found few large multisite studies or studies of the impact of moderate adherence. Several studies evaluated adherence as part of an efficacy trial, which does not mimic real-world settings, often using frequent study visits, as in HUG-Kids and BABY HUG, to boost adherence. In some studies, adherence data were not available on the entire population. This limitation introduces potential bias because the more adherent patients may be more likely to participate in adherence measurements. In addition, associations between nonadherence and poor clinical outcomes do not necessarily indicate a causal relationship; prospective studies are needed to distinguish association from causation.
Our systematic review of the literature is subject to several limitations. First, as with any systematic literature review, although our search criteria were designed to be comprehensive, it is possible that we missed relevant articles. Second, we did not include unpublished literature; publication bias tends to result in the availability of more positive associations.53 Third, variation in definitions of adherence limits the comparison of data across studies.54 Finally, variation in population ages, size, and methods to measure adherence prohibits a meta-analysis from being performed and therefore limits interpretation of our findings.54
Conclusions
This review of the literature indicates that many patients with SCD are moderately adherent to medications. Because good adherence is uncommon, we suggest clinicians use routine monitoring of adherence, including parent report and objective measures. Multicomponent interventions should target health beliefs and mistakes in medication administration; educational interventions alone are less likely to be effective. Randomized trials of multicomponent interventions, addressing beliefs and mistakes, are needed to help clinicians optimize outcomes by improving medication adherence.
Supplementary Material
Acknowledgments
We thank Dr Jerry Gurwitz for his support of this project.
Glossary
- MEMS
Medication Event Monitoring System
- MeSH
Medical Subject Headings
- MPR
medication possession ratio
- SCD
sickle cell disease
Footnotes
Dr Walsh conceptualized and designed the study, designed data collection instruments, performed literature review, and drafted the initial manuscript; Drs Cutrona, Kavanaugh, and Bundy conceptualized and designed the study, designed data collection instruments, participated in literature review, and revised the initial manuscript; Dr Crosby participated in literature review, contributed substantially to the interpretation of the review, and iteratively drafted the initial manuscript; Mr Malone designed the data collection instruments, coordinated and supervised literature collection and review, and critically reviewed the manuscript; Ms Lobner designed and implemented the literature search, drafted portions of the initial manuscript, and reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Cutrona is supported by award KL2TR000160 from the National Center for Research Resources (NCRR). Dr Crosby is supported by award 1K07HL108720-03 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–S521 [DOI] [PubMed] [Google Scholar]
- 2.Amrolia PJ, Almeida A, Davies SC, Roberts IAG. Therapeutic challenges in childhood sickle cell disease. Part 2: a problem-orientated approach. Br J Haematol. 2003;120(5):737–743 [DOI] [PubMed] [Google Scholar]
- 3.LeLeiko NS, Lobato D, Hagin S, et al. 6-Thioguanine levels in pediatric IBD patients: adherence is more important than dose. Inflamm Bowel Dis. 2013;19(12):2652–2658 [DOI] [PubMed] [Google Scholar]
- 4.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497 [DOI] [PubMed] [Google Scholar]
- 5.Buchanan GR, Smith SJ. Pneumococcal septicemia despite pneumococcal vaccine and prescription of penicillin prophylaxis in children with sickle cell anemia. Am J Dis Child. 1986;140(5):428–432 [DOI] [PubMed] [Google Scholar]
- 6.Berg JS, Dischler J, Wagner DJ, Raia JJ, Palmer-Shevlin N. Medication compliance: a healthcare problem. Ann Pharmacother. 1993;27(9 Suppl):S1–S24 [PubMed] [Google Scholar]
- 7.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209 [DOI] [PubMed] [Google Scholar]
- 8.Brandow AM, Jirovec DL, Panepinto JA. Hydroxyurea in children with sickle cell disease: practice patterns and barriers to utilization. Am J Hematol. 2010;85(8):611–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurst KE, Sleath BL, Konrad TR. Physicians’ perceptions of factors influencing adherence to antibiotic prophylaxis in children with sickle cell disease. Curr Ther Res Clin Exp. 2003;64(2):116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandow AM, Panepinto JA. Monitoring toxicity, impact, and adherence of hydroxyurea in children with sickle cell disease. Am J Hematol. 2011;86(9):804–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moffet HH, Parker MM, Sarkar U, et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339–344 [PMC free article] [PubMed] [Google Scholar]
- 12.LeLeiko NS, Lobato D, Hagin S, et al. Rates and predictors of oral medication adherence in pediatric patients with IBD. Inflamm Bowel Dis. 2013;19(4):832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutrona SL, Choudhry NK, Fischer MA, et al. Modes of delivery for interventions to improve cardiovascular medication adherence. Am J Manag Care. 2010;16(12):929–942 [PMC free article] [PubMed] [Google Scholar]
- 14.Cutrona SL, Choudhry NK, Stedman M, et al. Physician effectiveness in interventions to improve cardiovascular medication adherence: a systematic review. J Gen Intern Med. 2010;25(10):1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanagh PL, Sprinz PG, Vinci SR, Bauchner H, Wang CJ. Management of children with sickle cell disease: a comprehensive review of the literature. Pediatrics 2011;128(6). Available at: www.pediatrics.org/cgi/content/full/128/6/e1552 [DOI] [PubMed]
- 16.Bundy DG, Byerley JS, Liles EA, Perrin EM, Katznelson J, Rice HE. Does this child have appendicitis? JAMA. 2007;298(4):438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64(3):255–257, discussion 258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–354 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74 [DOI] [PubMed] [Google Scholar]
- 20.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574, discussion 575–577 [DOI] [PubMed] [Google Scholar]
- 21.Bergman AB, Werner RJ. Failure of children to receive penicillin by mouth. N Engl J Med. 1963;268(24):1334–1338 [DOI] [PubMed] [Google Scholar]
- 22.Markowitz M, Gordis L. A mail-in technique for detecting penicillin in urine: application to the study of maintenance of prophylaxis in rheumatic fever patients. Pediatrics. 1968;41(1):151–153 [PubMed] [Google Scholar]
- 23.Charney E, Bynum R, Eldredge D, et al. How well do patients take oral penicillin? A collaborative study in private practice. Pediatrics. 1967;40(2):188–195 [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 10, 2014
- 25.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–676 [DOI] [PubMed] [Google Scholar]
- 26.Bates DW, Cullen DJ, Laird N, et al. ADE Prevention Study Group . Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA. 1995;274(1):29–34 [PubMed] [Google Scholar]
- 27.Cummins D, Heuschkel R, Davies SC. Penicillin prophylaxis in children with sickle cell disease in Brent. BMJ. 1991;302(6783):989–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel NG, Lindsey T, Strunk RC, DeBaun MR. Prevalence of daily medication adherence among children with sickle cell disease: a 1-year retrospective cohort analysis. Pediatr Blood Cancer. 2010;55(3):554–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinney TR, Helms RW, O’Branski EE, et al. Pediatric Hydroxyurea Group . Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Blood. 1999;94(5):1550–1554 [PubMed] [Google Scholar]
- 30.Ware RE, Eggleston B, Redding-Lallinger R, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99(1):10–14 [DOI] [PubMed] [Google Scholar]
- 31.Thornburg CD, Rogers ZR, Jeng MR, et al. Adherence to study medication and visits: data from the BABY HUG trial. Pediatr Blood Cancer. 2010;54(2):260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornburg CD, Calatroni A, Telen M, Kemper AR. Adherence to hydroxyurea therapy in children with sickle cell anemia. J Pediatr. 2010;156(3):415–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Candrilli SD, O’Brien SH, Ware RE, Nahata MC, Seiber EE, Balkrishnan R. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86(3):273–277 [DOI] [PubMed] [Google Scholar]
- 34.Alvarez O, Rodriguez-Cortes H, Robinson N, et al. Adherence to deferasirox in children and adolescents with sickle cell disease during 1-year of therapy. J Pediatr Hematol Oncol. 2009;31(10):739–744 [DOI] [PubMed] [Google Scholar]
- 35.Thuret I, Hacini M, Pégourié-Bandelier B, et al. Socio-psychological impact of infused iron chelation therapy with deferoxamine in metropolitan France: ISOSFER study results. Hematology. 2009;14(6):315–322 [DOI] [PubMed] [Google Scholar]
- 36.Treadwell MJ, Weissman L. Improving adherence with deferoxamine regimens for patients receiving chronic transfusion therapy. Semin Hematol. 2001;38(1 Suppl 1):77–84 [DOI] [PubMed] [Google Scholar]
- 37.Babiker MA. Prophylaxis of pneumococcal infection in sickle-cell disease by the combined use of vaccination and penicillin. Ann Trop Paediatr. 1986;6(3):179–181. Available at: www.mrw.interscience.wiley.com/cochrane/clcentral/articles/321/CN-00045321/frame.html [DOI] [PubMed] [Google Scholar]
- 38.Babiker MA. Compliance with penicillin prophylaxis by children with impaired splenic function. Trop Geogr Med. 1986;38(2):119–122 [PubMed] [Google Scholar]
- 39.Witherspoon D, Drotar D. Correlates of adherence to prophylactic penicillin therapy in children with sickle cell disease. Child Health Care. 2006;35(4):281–296 [Google Scholar]
- 40.Elliott V, Morgan S, Day S, Mollerup LS, Wang W. Parental health beliefs and compliance with prophylactic penicillin administration in children with sickle cell disease. J Pediatr Hematol Oncol. 2001;23(2):112–116 [DOI] [PubMed] [Google Scholar]
- 41.Sox CM, Cooper WO, Koepsell TD, DiGiuseppe DL, Christakis DA. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA. 2003;290(8):1057–1061 [DOI] [PubMed] [Google Scholar]
- 42.Treadwell MJ, Law AW, Sung J, et al. Barriers to adherence of deferoxamine usage in sickle cell disease. Pediatr Blood Cancer. 2005;44(5):500–507 [DOI] [PubMed] [Google Scholar]
- 43.Treadwell M, Sung J, Murray E, et al. Barriers to deferoxamine adherence for adults with sickle cell disease. Blood (ASH Annual Meeting Abstracts). 2004 104: Abstract 3760 [Google Scholar]
- 44.Ware RE, Zimmerman SA, Schultz WH. Hydroxyurea as an alternative to blood transfusions for the prevention of recurrent stroke in children with sickle cell disease. Blood. 1999;94(9):3022–3026 [PubMed] [Google Scholar]
- 45.Raphael JL, Bernhardt MB, Mahoney DH, Mueller BU. Oral iron chelation and the treatment of iron overload in a pediatric hematology center. Pediatr Blood Cancer. 2009;52(5):616–620 [DOI] [PubMed] [Google Scholar]
- 46.Patel AB, Athavale AM. Sickle cell disease in central India. Indian J Pediatr. 2004;71(9):789–793 [DOI] [PubMed] [Google Scholar]
- 47.Pejaver RK, Ahmed FE, Al Hifzi I. Compliance to penicillin prophylaxis amongst Saudi children with sickle cell disease. J Ir Coll Physicians Surg. 1997;26(4):268–270 [Google Scholar]
- 48.Drotar D. Treatment adherence in patients with sickle cell anemia. J Pediatr. 2010;156(3):350–351 [DOI] [PubMed] [Google Scholar]
- 49.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf). 2005;27(3):281–291 [DOI] [PubMed] [Google Scholar]
- 50.Berkovitch M, Papadouris D, Shaw D, Onuaha N, Dias C, Olivieri NF. Trying to improve compliance with prophylactic penicillin therapy in children with sickle cell disease. Br J Clin Pharmacol. 1998;45(6):605–607. Available at: www.mrw.interscience.wiley.com/cochrane/clcentral/articles/218/CN-00683218/frame.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dean AJ, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child. 2010;95(9):717–723 [DOI] [PubMed] [Google Scholar]
- 52.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611 [DOI] [PubMed] [Google Scholar]
- 53.Dubben H-H, Beck-Bornholdt H-P. Systematic review of publication bias in studies on publication bias. BMJ. 2005;331(7514):433–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartolucci AA, Hillegass WB. Overview, strengths, and limitations of systematic reviews and meta-analyses. In: Chiappelli F, Caldeira Brant XM, Neagos N, Oluwadara OO, Ramchandani MH, eds. Evidence-Based Practice: Toward Optimizing Clinical Outcomes. Berlin, Germany: Springer-Verlag; 2010:17–34 [Google Scholar]
- 55.Olivieri NF, Vichinsky EP. Hydroxyurea in children with sickle cell disease: impact on splenic function and compliance with therapy. J Pediatr Hematol Oncol. 1998;20(1):26–31 [DOI] [PubMed] [Google Scholar]
- 56.Davis H. Use of computerized health claims data to monitor compliance with antibiotic prophylaxis in sickle cell disease. Pharmacoepidemiol Drug Saf. 1998;7(2):107–112 [DOI] [PubMed] [Google Scholar]
- 57.Buchanan GR, Siegel JD, Smith SJ, DePasse BM. Oral penicillin prophylaxis in children with impaired splenic function: a study of compliance. Pediatrics. 1982;70(6):926–930 [PubMed] [Google Scholar]
- 58.Teach SJ, Lillis KA, Grossi M. Compliance with penicillin prophylaxis in patients with sickle cell disease. Arch Pediatr Adolesc Med. 1998;152(3):274–278 [DOI] [PubMed] [Google Scholar]
- 59.Bitarães EL, Oliveira BM, Viana MB. Compliance with antibiotic prophylaxis in children with sickle cell anemia: a prospective study. J Pediatr (Rio J). 2008;84(4):316–322 [DOI] [PubMed] [Google Scholar]
- 60.King L, Ali S, Knight-Madden J, MooSang M, Reid M. Compliance with intramuscular penicillin prophylaxis in children with sickle cell disease in Jamaica. West Indian Med J. 2011;60(2):177–180 [PubMed] [Google Scholar]
- 61.Walsh KE, Mazor KM, Roblin D, et al. Multisite parent-centered risk assessment to reduce pediatric oral chemotherapy errors. J Oncol Pract. 2013;9(1):e1–e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC) . Update: newborn screening for sickle cell disease—California, Illinois, and New York, 1998. MMWR Morb Mortal Wkly Rep. 2000;49(32):729–731 [PubMed] [Google Scholar]
- 63.Walsh KE, Mazor KM, Stille CJ, et al. Medication errors in the homes of children with chronic conditions. Arch Dis Child. 2011;96(6):581–586 [DOI] [PubMed] [Google Scholar]
- 64.McMahon SR, Rimsza ME, Bay RC. Parents can dose liquid medication accurately. Pediatrics. 1997;100(3 Pt 1):330–333 [DOI] [PubMed] [Google Scholar]
- 65.Frush KS, Luo X, Hutchinson P, Higgins JN. Evaluation of a method to reduce over-the-counter medication dosing error. Arch Pediatr Adolesc Med. 2004;158(7):620–624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.