Abstract
BACKGROUND AND OBJECTIVE:
Primary ciliary dyskinesia (PCD) is a rare inherited disease affecting motile cilia lining the respiratory tract. Despite neonatal respiratory distress as an early feature, diagnosis is typically delayed until late childhood. Our objective was to identify characteristics that differentiate PCD from common causes of term neonatal respiratory distress.
METHODS:
This was a case-control study. Patients with PCD born after 1994 attending a regional PCD clinic who had a history of neonatal respiratory distress (n = 46) were included. Controls (n = 46), term neonates with respiratory distress requiring a chest radiograph, were randomly selected from hospital birth records and matched on gender, birth month/year, and mode of delivery. Multiple logistic regression was used to determine the association between neonatal characteristics and PCD diagnosis. The diagnostic performance of the best predictive variables was estimated by calculating sensitivity and specificity.
RESULTS:
PCD cases required more oxygen therapy (39 cases, 29 controls, P = .01), longer duration of oxygen therapy (PCD mean = 15.2 days, control mean = 0.80 days, P < .01), had later onset of neonatal respiratory distress (PCD median = 12 hours, control median = 1 hour, P < .001), and higher frequency of lobar collapse and situs inversus (PCD = 70% and 48% respectively, control = 0% for both, P < .001). Situs inversus, lobar collapse, or oxygen need for >2 days had 87% (95% confidence interval: 74–94) sensitivity and 96% (95% confidence interval: 85–99) specificity for PCD.
CONCLUSIONS:
When encountering term neonates with unexplained respiratory distress, clinicians should consider PCD in those with lobar collapse, situs inversus, and/or prolonged oxygen therapy (>2 days).
Keywords: ciliary motility disorders, Kartagener syndrome, PCD, pulmonary atelectasis, respiratory distress syndrome, infant
What’s Known on This Subject:
Primary ciliary dyskinesia presents in infancy with unexplained neonatal respiratory distress, yet diagnosis is often delayed until late childhood. Earlier diagnosis facilitates earlier onset of therapy, which may help to reduce long-term pulmonary morbidity and mortality.
What This Study Adds:
A diagnostic workup for primary ciliary dyskinesia should be considered in a term infant presenting with unexplained respiratory distress and either lobar collapse, situs inversus, or a prolonged oxygen therapy requirement (>2 days).
Primary ciliary dyskinesia (PCD) is a rare inherited disease affecting the structure and function of cilia lining the respiratory tract. Impaired mucociliary clearance leads to bronchiectasis and progressive decline in lung function resulting in end-stage pulmonary disease in early adulthood for some patients.1 The estimated incidence of PCD is ∼1:15 000; however, it is thought to be underdiagnosed and undertreated.2 PCD presents in infancy with unexplained neonatal respiratory distress in the majority of patients (75% to 85% of pediatric patients),1,3,4 yet diagnosis is often delayed until late childhood.5 Diagnosis is delayed as recurrent cough, rhinitis, and otitis media are common childhood presentations, and the diagnostic testing and interpretation requires specialized centers. Earlier age at diagnosis has been shown to be associated with care at a specialized center and thus under referral likely contributes to the delay in diagnosis as well.6 Bronchiectasis7 and lung function decrements8,9 typically start during the preschool years and thus earlier diagnosis may facilitate earlier initiation of disease modifying therapy and possibly reduce long-term pulmonary morbidity.
Neonatal respiratory distress is common in PCD; however, it is common in general and can occur in up to 5% of term infants.10,11 Thus, the aim of this study was to identify clinical manifestations (neonatal characteristics and chest radiograph findings) to differentiate PCD from common causes of term neonatal respiratory distress, such as transient tachypnea of the newborn.
Methods
Study Design
This was a case-control study. The study was approved by the research ethics boards at The Hospital for Sick Children and Mount Sinai Hospital.
Participants
All cases attending the PCD clinic at our institution, a tertiary referral center, born between 1994 and 2012 with a reported history of neonatal respiratory distress were included. PCD was diagnosed by using standard criteria: a defined ciliary ultrastructural defect on electron microscopy, 2 disease causing mutations, or a classic phenotype with low nasal nitric oxide (<77 nL/minute) on at least 2 occasions.12 Controls, term neonates (>36 weeks’ gestation) with respiratory distress requiring a chest radiograph, were randomly selected from health records at a high volume neighboring obstetrical center and were matched 1:1 based on gender, birth month/year, and mode of delivery. Infants born preterm (<36 weeks’ gestation) as well as those with known sepsis, birth asphyxia, neuromuscular disease, congenital lung abnormalities, pulmonary hypoplasia, congenital heart disease, cystic fibrosis, or other genetic/metabolic conditions were excluded.
Variables
Neonatal characteristics of cases and controls were abstracted from health records by using standardized data collection sheets. The variables of interest included gender, birth date, gestational age, mode of delivery, presence of tachypnea and oxygen desaturation, onset of neonatal respiratory distress, documented neonatal diagnosis for respiratory distress (transient tachypnea of the newborn, neonatal pneumonia, meconium aspiration, pneumothorax, or other), oxygen therapy (for at least 1 hour), mechanical ventilation (intubation or continuous positive airway pressure), duration of mechanical ventilation, need for antibiotics, and duration of hospital stay. For the PCD cases, we also included age at diagnosis, situs status (situs solitus versus situs inversus), documented ciliary defect (outer dynein arm, inner dynein arm, central apparatus, or indeterminate), genetic test results, nasal nitric oxide output (nL/minute),13 and the presence of documented bronchiectasis on computed tomography scan.
All available neonatal chest radiographs (within the first 28 days of life) were interpreted and categorized on a standardized data collection sheet by 1 radiologist who was blinded to the case-control status. The diagnostic imaging variables included situs status, cardiomegaly, hyperinflation, increased interstitial markings, fluid in the intralobar fissures, lobar collapse/consolidation, bronchiole wall thickening, pleural effusion, and pneumothorax.
Data Analysis
All statistical analyses were performed by using the statistical software SAS version 9.3 (SAS Institute, Inc, Cary, NC). Frequency of neonatal characteristics and chest radiograph findings were described and then compared in a matched analysis, using conditional logistic analysis for categorical variables and a generalized linear model with stratification of the paired data for continuous variables. When the data were nonnormal, we used the Wilcoxon Signed-Rank test. For the chest radiograph findings where there were only 23 cases, an exact McNemar’s test was used to assess agreement between cases and matched controls. Conditional multiple logistic regression was then used to determine the association between neonatal characteristics and PCD diagnosis. When 2 or more variables were highly correlated, the variable with the best predictive ability was chosen as an input to the multivariable model. Diagnostic performance of the best predictive variables (situs inversus, oxygen >2 days, and lobar collapse) was determined by calculating sensitivity and specificity of these variables to diagnose PCD. Finally, cases were stratified by situs status and analyses were repeated to determine if predictors differed by situs status.
Results
Subject Characteristics
Fifty-five PCD cases were identified from our clinic database, of which 50 (91%) had a reported history of neonatal respiratory distress (Table 1). Four were excluded due to congenital heart disease (1 of these cases was also preterm <35 weeks’ gestation). The remaining 46 (28 boys) PCD cases formed our study population, of which 22 (48%) had situs inversus, 25 (63%) had bronchiectasis, and the median age at diagnosis was 4.3 years (range = 0.1–17 years). Cases with PCD defined by nasal nitric oxide measurements (n = 4) did not differ in neonatal characteristics from cases defined by ciliary or genetic studies. Chest radiographs were available in 23 (50%) of the PCD cases. Of the 46 matched control subjects, all had situs solitus and 31 had chest radiographs available.
TABLE 1.
PCD Cases Included in Study Compared With Total Toronto PCD Clinic Population
| Variable | PCD Study Population, N = 46 (Frequency, %)a | PCD Study Population With Chest Radiograph, N = 23 (Frequency, %)a | Total PCD Clinic Population, N = 55 (Frequency, %)a |
|---|---|---|---|
| Gender, boy | 28 (61) | 16 (70) | 32 (58) |
| Current age, mean (SD) | 10.9 (5.5) | 8.1 (4.8) | 11.0 (5.3) |
| Situs inversus | 22 (48) | 11 (48) | 27 (49) |
| Preterm, <36 wk | 0 | 0 | 1 (2%) |
| Ciliary defect | |||
| ODA | 5 (11) | 2 (9) | 5 (9) |
| ODA/IDA | 22 (48) | 10 (44) | 26 (47) |
| IDA/CA | 7 (15) | 4 (17) | 8 (15) |
| Indeterminate | 3 (6) | 2 (9) | 5 (9) |
| Normal | 5 (11) | 1 (4) | 6 (11) |
| Not available | 4 (9) | 4 (17) | 5 (9) |
| PCD genetics | |||
| DNAH5 | 12 (26) | 7 (30) | 13 (24) |
| DNAH11 | 3 (7) | 2 (9) | 5 (9) |
| CCDC39 | 4 (8) | 2 (9) | 4 (7) |
| CCDC40 | 3 (7) | 2 (9) | 3 (5) |
| LRRC6 | 2 (4) | 0 (0) | 2 (3) |
| Otherb | 4 (9) | 0 (0) | 7 (13) |
| Not available | 12 (26) | 8 (34) | 14 (26) |
| Genetics negative | 6 (13) | 2 (9) | 7 (13) |
| Nasal nitric oxide, nL/min, mean (SD) | 19.9 (26.8) | 18.9 (31.4) | 22.0 (31.1) |
| Bronchiectasis | 25 (63) | 9 (47) | 30 (64) |
| Neonatal respiratory distress (on history) | 46 (100) | 23(100) | 50 (91) |
| Congenital heart disease | 0 | 0 | 4 (7) |
| Age at diagnosis in years, median (range) | 4.3 (0.1–17) | 0.8 (0.1–17) | 5.0 (0.8–17) |
Data are presented as number (%) unless noted otherwise. CA, central apparatus defect; IDA, inner dynein arm defect; ODA, outer dynein arm defect.
Frequencies adjusted for missing values.
Other category includes the following genes: SPAG1, CCDC103, KTU/DNAAF2, CCNO.
Neonatal Variables
PCD cases presented with onset of neonatal respiratory distress later than control subjects (median 12 hours of life; range, 1–168 hours vs 1 hour of life; range, 0–24 hours, P < .001; Table 2). The overall distribution of neonatal diagnosis for respiratory distress differed between cases and controls (P < .001). The most common diagnosis in the control subjects was transient tachypnea of the newborn (65%, n = 28) compared with neonatal pneumonia in the PCD cases (59%, n = 16). The PCD cases required more oxygen therapy (39 cases vs 29 cases, P = .01) and a longer duration of oxygen therapy (mean 15.2 days vs 0.8 days, P < .01). The control subjects required more mechanical ventilation (27 subjects vs 7 cases, P < .01). The mechanical ventilation in the control subjects consisted of continuous positive airway pressure only, whereas 3 of the PCD cases were intubated.
TABLE 2.
Characteristics of Term Neonates With Respiratory Distress
| Variable | PCD Cases, n = 46 (Frequency)a | Control Subjects, n = 46 (Frequency)a | P |
|---|---|---|---|
| Gender, boyb | 28 (61) | 28 (61) | |
| Birth yearb | |||
| 1994–1998 | 17 (37) | 17 (37) | — |
| 1999–2003 | 10 (22) | 10 (22) | — |
| 2004–2008 | 13 (28) | 13 (28) | — |
| 2009–2012 | 6 (13) | 6 (13) | — |
| Caesarian deliveryb | 12 (32) | 16 (35) | — |
| Onset of neonatal respiratory distress, median hours (range) | 12 (1–168) | 1 (0–24) | <.001 |
| Neonatal diagnosis for respiratory distress | <.001 | ||
| Transient tachypnea of newborn | 5 (19) | 28 (65) | — |
| Neonatal pneumonia | 16 (59) | 4 (9) | — |
| Meconium aspiration | 3 (11) | 3 (7) | — |
| Pneumothorax | 0 (0) | 6 (14) | — |
| Other | 3 (11) | 2 (5) | — |
| Therapy | |||
| Oxygen (at least 1 h) | 39 (93) | 29 (63) | .01 |
| Duration of oxygen therapy, mean days (SD) | 15.2 (25.5) | 0.8 (1.9) | .002 |
| Mechanical ventilation (intubation or CPAP) | 7 (18) | 27 (59) | .004 |
| Antibiotics | 29 (91) | 35 (76) | .18 |
| Duration of hospital stay, mean (SD) | 14.9 (13.2) | 3.7 (1.5) | .01 |
Data are presented as number (%) unless noted otherwise. CPAP, continuous positive airway pressure.
Frequencies adjusted for missing values.
Cases and controls matched on these variables.
Diagnostic Imaging Variables
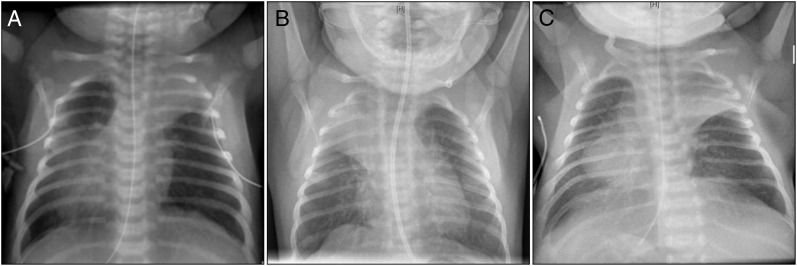
Eleven PCD cases (48%, 95% confidence interval [CI]: 27%–68%) had situs inversus and 16 had lobar collapse (70%, 95% CI: 51%–88%) compared with zero of the control subjects (P < .001 for both variables in matched analysis; Table 3). The lobar collapse was limited to the upper (n = 12, 75%) and middle (n = 4, 25%) lobes and was not associated with intubation (Fig 1).
TABLE 3.
Chest Radiograph Findings in PCD Cases Versus Control Subjects With Neonatal Respiratory Distress
| Variable | PCD Cases (n = 23) | Control Subjects (n = 31) | Matched Control Subjects (n = 23) | Pa | Agreement (n = 23; %) | |||
|---|---|---|---|---|---|---|---|---|
| n | Frequency, % | n | Frequency, % | n | Frequency, % | |||
| Situs inversus | 11 | 48 | 0 | 0 | 0 | 0 | <.001 | 12 (52) |
| Cardiomegaly | 1 | 4 | 2 | 7 | 2 | 9 | .99 | 20 (87) |
| Hyperinflation | 13 | 57 | 10 | 32 | 6 | 26 | .06 | 12 (52) |
| Interstitial markings | 10 | 43 | 5 | 16 | 4 | 17 | .15 | 11 (48) |
| Fluid in intralobar fissures | 0 | 0 | 4 | 13 | 2 | 9 | .50 | 21 (91) |
| Lobar collapse/consolidation | 16 | 70 | 0 | 0 | 0 | 0 | <.001 | 7 (30) |
| Bronchiole wall thickening | 2 | 9 | 0 | 0 | 0 | 0 | .50 | 21(91) |
| Pleural effusion | 0 | 0 | 1 | 3 | 1 | 4 | .99 | 22 (96) |
| Pneumothorax | 1 | 4 | 2 | 7 | 2 | 9 | .99 | 20 (87) |
P value obtained by using McNemar’s exact test to assess agreement between cases and matched controls.
FIGURE 1.
Neonatal chest radiographs demonstrating lobar collapse in 3 different subjects with PCD. A, Situs inversus, left upper and right upper lobe collapse at day of life 2. B, Right upper lobe collapse at day of life 6. C, Situs inversus and left upper lobe collapse on day of life 7.
Furthermore, health record data revealed that 12 out of the 24 cases with missing chest radiographs had reported findings suggestive of lobar collapse; in 9 cases parents reported being told that their children had lung collapse at birth and an additional 3 parents recalled that their children had pneumonias at birth. However, some health records had few details about the nature of the neonatal respiratory illness.
Association Between Neonatal Variables and PCD Diagnosis
With conditional multiple regression modeling, 2 neonatal variables were determined to be independent predictors of PCD: age at onset of neonatal respiratory distress per additional hour of postnatal life (odds ratio [OR], 1.10; 95% CI: 1.01–1.21) and length of oxygen therapy in days (OR, 1.68; 95% CI: 1.26–2.24; Table 4).
TABLE 4.
Association Between Neonatal Characteristics and PCD Diagnosis in Term Infants With Respiratory Distress
| Variable | ORa | 95% CI |
|---|---|---|
| Onset of neonatal respiratory distress, hours of life | 1.10 | 1.01–1.21 |
| Duration of oxygen therapy, d | 1.68 | 1.26–2.24 |
Multivariable model controlling for gender, age, mode of delivery, oxygen requirement, mechanical ventilation, and duration of hospital stay.
Best Predictive Variables for PCD Diagnosis
The majority of PCD cases (63%, n = 24) required >2 days of oxygen, whereas only 2 control subjects (7%) required oxygen for >2 days (Table 5). Thus, we chose to dichotomize oxygen therapy at 2 days for subsequent analysis. The most sensitive prediction for PCD diagnosis (87%) is a combination of situs inversus, oxygen >2 days, and/or lobar collapse on chest radiograph. Of note, the combination of oxygen for >2 days and/or lobar collapse has better sensitivity (83%) for detecting PCD than the combination of oxygen for >2 days and/or situs inversus (sensitivity 78%).
TABLE 5.
Sensitivity and Specificity for the Best Predictive Variables for PCD in Term Neonates With Unexplained Respiratory Distressa
| Variable | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| Situs inversus | 0.48 (0.34–0.62) | 1.00 (0.92–1.00) |
| Lobar collapse | 0.70 (0.49–0.84) | 1.00 (0.89–1.00) |
| Oxygen >2 d | 0.81 (0.65–0.90) | 0.96 (0.85–0.99) |
| Situs inversus and/or lobar collapse | 0.65 (0.51–0.77) | 1.00 (0.92–1.00) |
| Oxygen >2 d and/or situs inversus | 0.78 (0.64–0.88) | 0.96 (0.85–0.99) |
| Oxygen >2 days and/or lobar collapse | 0.83 (0.69–0.91) | 0.96 (0.85–0.99) |
| Oxygen >2 days and/or situs inversus and/or lobar collapse | 0.87 (0.74–0.94) | 0.96 (0.85–0.99) |
Values calculated using the entire sample (n = 46 cases and 46 controls) except for lobar collapse variable (subgroup of 23 cases and 31 controls with CXR).
The data were reanalyzed based on stratifying by situs status, and neonatal predictors of PCD were identical for cases with situs inversus and situs solitus. Cases with situs inversus were diagnosed at an earlier age compared with cases with situs solitus (median age at diagnosis 0.83 years [range = 0.08–14 years] vs 5.0 years [range = 0.08–17 years]; P = .03).
Discussion
To our knowledge, this is the first study to describe neonatal chest radiographs in a cohort of patients with confirmed PCD and the first to demonstrate an association between lobar collapse and PCD in term neonates with respiratory distress. Our results also suggest associations between PCD and prolonged oxygen therapy, as well as later onset of neonatal respiratory distress. For every additional day of oxygen therapy required, the risk of having PCD increases almost twofold. This is in comparison with more common causes of term neonatal respiratory distress, of which transient tachypnea of the newborn is the most common, where neonates typically present within a few hours of life and require oxygen therapy for 1 to 2 days only. Lobar collapse in term neonates is rare yet it was seen in the majority of our PCD cases (70%). This suggests that lobar collapse in the context of unexplained neonatal respiratory distress should lead the clinician to consider PCD in their differential diagnosis. If lobar collapse is seen in addition to situs inversus and/or oxygen for >2 days, the clinician should have a high index of suspicion for PCD. Lobar collapse and oxygen >2 days may be particularly important clinical manifestations in neonates with situs solitus, as situs inversus on its own typically leads clinicians to consider PCD.
The lobar collapse in our patient population was limited to the upper and middle lobes. In contrast, diagnostic studies in older children and adults with PCD have revealed that peribronchial thickening, atelectasis, and air trapping leading to bronchiectasis occur predominantly in the middle and lower lobes.14–16 The pathophysiology to explain our findings remains unknown. One possibility is that PCD is an airway disease with preferential gas trapping and hyperinflation in the lower lobes leading to atelectasis/compression in the upper lobes. An alternative explanation for collapse in the upper/middle lobes could be related to positioning. Because neonates spend the majority of their time supine, it could be that impaired mucociliary clearance leads to mucous build up and lobar collapse preferentially in the dependent lobes (upper/middle when lying supine versus middle/lower in upright children/adults). Intubation errors could be an obvious cause of lobar collapse; however, there was no lobar collapse in the 3 intubated PCD cases.
The results of our study support the existing literature that neonatal respiratory distress is common in PCD1,3,4 because the majority of our PCD clinic population (91%) had a reported history of neonatal respiratory distress. A few case reports exist in the literature documenting atelectasis on chest radiograph in neonates with PCD. Nichamin17 was the first to document the onset of Kartagener syndrome in a neonate at 33 hours of life with cyanosis, retractions, nasal discharge, and fluctuating atelectasis on serial chest radiographs. This was followed by Whitelaw et al18 who report 6 cases of PCD presenting in the first 24 hours of life with tachypnea, retraction, rales, and dextrocardia. Two cases had atelectasis on chest radiograph (1 within 24 hours of life and the second at 5 days of life). Furthermore, a case presentation by Hossain et al4 describes 2 additional cases of PCD in term neonates with unexplained respiratory distress and situs inversus. The first case developed atelectasis in the left lower lobe within the first 7 days of life, and the second had fluctuating atelectasis (left upper and right middle lobes) within the first few days of life. However, our study is the first to systematically study chest radiographs in an unselected cohort of neonates with PCD and to document lobar collapse in the majority (70%), as well as to compare their characteristics to a disease control group that would cause diagnostic confusion.
There are a few limitations to our study that warrant further discussion. The first is the small sample size and missing data due to the difficulty of studying a rare disease and acquiring neonatal chest radiographs in a retrospective study. It is important to note that a larger study with less missing data would serve to increase precision but would likely result in the same conclusions, particularly since the availability of chest radiographs was mainly dependent on the subject’s current age and birth hospital. It is noteworthy to point out that in half of the PCD cases with missing chest radiographs, the health record included details suggestive of lobar collapse, which further strengthens our findings. We did not include these data in our analysis, because they were lower quality data and our sample size was adequate to support our main study findings. Early onset of persistent rhinorrhea is a common feature seen in neonates with PCD; however, due to the retrospective nature of this study it could not be reliably assessed in the neonatal period. Secondly, our control subjects came from a high risk obstetrical center; however, one would assume that term infants born to high risk mothers would be at increased risk for sepsis or pneumonia due to factors such as maternal diabetes or prolonged rupture of membranes and thus may be more likely to have lobar collapse. This would bias the results of our study toward the null hypothesis and hence is unlikely to invalidate our results. In addition, we do not have follow-up of our controls and although unlikely it is possible that some of our controls could have PCD. Based on the incidence of PCD (1:15 000) and neonatal respiratory distress syndrome (NRDS) (1:20) and the fact that 91% of patients with PCD present with NRDS, one would expect that 0 to 1 child out of the ∼50 control subjects would have PCD (0.06 of a person to be exact). In fact, we originally sampled 1 control subject who was also a known PCD case and therefore excluded from the control group. Regardless, it is important to note that having a control subject with PCD would reduce our effect size and thus this limitation, similar to the previous 2, would not be expected to affect the validity of our results. Finally, we did not report positive and negative predictive values in our results, due to the case-control design. However, one can calculate an approximate expected positive predictive value of 74.8% (assuming that 0.12 of every hundred patients with neonatal respiratory distress have PCD) and negative predictive value of 98.2%.
Conclusions
When encountering term neonates with unexplained respiratory distress, clinicians need to consider PCD in those with lobar collapse on chest radiograph, situs inversus, and/or prolonged oxygen therapy (>2 days). Any combination of these findings warrants a referral to a pediatric pulmonologist to consider further diagnostic workup. This would allow diagnosis to occur within the first few months of life: a drastic improvement from the current age at diagnosis of 9 to 13 years.5 The hope is that earlier diagnosis will prompt early disease management such as daily airway clearance techniques and antibiotics for bacterial bronchitis and subsequently lead to a reduction in the pulmonary morbidity and mortality associated with PCD. We have suggested a simple clinical decision rule based on 3 easily obtained clinical manifestations. A prospective study in a larger more geographically spread population would be helpful to provide additional validation of our results.
Glossary
- CI
confidence interval
- OR
odds ratio
- PCD
primary ciliary dyskinesia
Footnotes
Dr Mullowney contributed to the conceptualization and design of the study, designed the data collection instruments, collected and interpreted the data, and drafted the initial manuscript; Dr Manson contributed to acquisition and interpretation of radiological data and reviewed the initial manuscript; Dr Kim contributed to the acquisition of data and reviewed the initial manuscript; Mr Stephens contributed to the analysis and interpretation of the data and reviewed the initial manuscript; Dr Shah contributed to the conception and design of the study and reviewing and revising the initial manuscript; Dr Dell conceptualized and designed the study, contributed to the data analysis and interpretation, and reviewed and revised the initial manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Sharon Dell is supported by US National Institutes of Health (NIH) research grant 5 U54 HL096458-06, funded by the Office of the Director, and supported by Office of Rare Diseases Research (ORDR) and National Heart, Lung, and Blood Institute (NHLBI). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169(4):459–467 [DOI] [PubMed] [Google Scholar]
- 2.Zariwala MA, Omran H, Ferkol TW. The emerging genetics of primary ciliary dyskinesia. Proc Am Thorac Soc. 2011;8(5):430–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferkol T, Leigh M. Primary ciliary dyskinesia and newborn respiratory distress. Semin Perinatol. 2006;30(6):335–340 [DOI] [PubMed] [Google Scholar]
- 4.Hossain T, Kappelman MD, Perez-Atayde AR, Young GJ, Huttner KM, Christou H. Primary ciliary dyskinesia as a cause of neonatal respiratory distress: implications for the neonatologist. J Perinatol. 2003;23(8):684–687 [DOI] [PubMed] [Google Scholar]
- 5.McManus IC, Mitchison HM, Chung EM, Stubbings GF, Martin N. Primary ciliary dyskinesia (Siewert’s/Kartagener’s syndrome): respiratory symptoms and psycho-social impact. BMC Pulm Med. 2003;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehni CE, Frischer T, Strippoli MP, et al. ERS Task Force on Primary Ciliary Dyskinesia in Children . Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur Respir J. 2010;36(6):1248–1258 [DOI] [PubMed] [Google Scholar]
- 7.Brown DE, Pittman JE, Leigh MW, Fordham L, Davis SD. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol. 2008;43(5):514–516 [DOI] [PubMed] [Google Scholar]
- 8.Maglione M, Bush A, Nielsen KG, et al. Multicenter analysis of body mass index, lung function, and sputum microbiology in primary ciliary dyskinesia [published online ahead of print January 13, 2014]. Pediatr Pulmonol. doi: 10.1002/ppul.22984 [DOI] [PubMed] [Google Scholar]
- 9.Marthin JK, Petersen N, Skovgaard LT, Nielsen KG. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med. 2010;181(11):1262–1268 [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Bhat BV. Epidemiology of respiratory distress of newborns. Indian J Pediatr. 1996;63(1):93–98 [DOI] [PubMed] [Google Scholar]
- 11.Hermansen CL, Lorah KN. Respiratory distress in the newborn. Am Fam Physician. 2007;76(7):987–994 [PubMed] [Google Scholar]
- 12.Leigh MW, Hazucha MJ, Chawla KK, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10(6):574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateos-Corral D, Coombs R, Grasemann H, Ratjen F, Dell SD. Diagnostic value of nasal nitric oxide measured with non-velum closure techniques for children with primary ciliary dyskinesia. J Pediatr. 2011;159(3):420–424 [DOI] [PubMed] [Google Scholar]
- 14.Santamaria F, Montella S, Tiddens HA, et al. Structural and functional lung disease in primary ciliary dyskinesia. Chest. 2008;134(2):351–357 [DOI] [PubMed] [Google Scholar]
- 15.Jain K, Padley SP, Goldstraw EJ, et al. Primary ciliary dyskinesia in the paediatric population: range and severity of radiological findings in a cohort of patients receiving tertiary care. Clin Radiol. 2007;62(10):986–993 [DOI] [PubMed] [Google Scholar]
- 16.Kennedy MP, Noone PG, Leigh MW, et al. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am J Roentgenol. 2007;188(5):1232–1238 [DOI] [PubMed] [Google Scholar]
- 17.Nichamin SJ. Kartagener’s syndrome in a newborn infant. J Am Med Assoc. 1956;161(10):966–968 [DOI] [PubMed] [Google Scholar]
- 18.Whitelaw A, Evans A, Corrin B. Immotile cilia syndrome: a new cause of neonatal respiratory distress. Arch Dis Child. 1981;56(6):432–435 [DOI] [PMC free article] [PubMed] [Google Scholar]