Abstract
OBJECTIVE:
The goal of this study was to determine if congenital human herpesvirus-6 (HHV-6) infection influences early neurodevelopment.
METHODS:
We enrolled 57 newborns with HHV-6 congenital infection and 242 control newborns without congenital infection into a prospective, double-blind study with 4 visits between 4 and 30 months of age. Assessments included the Fagan Test of Infant Intelligence, the Visual Expectation Paradigm, and the Mental Development Index (MDI) of the Bayley Scales of Infant Development II. Newborn audiology screening and follow-up audiology examinations were completed at 12 to 24 months.
RESULTS:
No differences were noted in baseline characteristics between infants with HHV-6 congenital infection and control infants. No clinical syndrome due to congenital infection with HHV-6 was evident at birth. No differences were identified on the Fagan Test of Infant Intelligence or the Visual Expectation Paradigm between the two groups. In 39 infants with HHV-6 congenital infection, the mean ± SD Bayley Scale of Infant Development II MDI score was 103.4 ± 8.9 at 12 months of age. The matched control infants had a mean score of 105.4 ± 12.4. After controlling for covariates, HHV-6 congenital infection was associated with lower scores on the Bayley Scale of Infant Development II MDI at 12 months of age (mean difference: 4.3 [95% confidence interval: 0.4 to 8.1]; P = .03) compared with infants without HHV-6 congenital infection.
CONCLUSIONS:
Congenital HHV-6 infection may have a detrimental effect on neurodevelopment at 12 months of age and requires further study given that congenital infection with HHV-6 is present in ∼1 in every 101 births.
Keywords: congenital HHV-6 infection, neurodevelopment
What’s Known on This Subject:
Neurodevelopment can be adversely affected by viral infections. Human herpesvirus-6 (HHV-6) is similar to cytomegalovirus and can cause central nervous system disease. Congenital HHV-6 infection occurs in ∼1% of live births, with unknown neurodevelopmental consequences.
What This Study Adds:
HHV-6 congenital infection is associated with lower scores on the Bayley Scales of Infant Development II Mental Development Index compared with control infants at 12 months of age and may have a detrimental effect on neurodevelopment.
Neurodevelopment is a dynamic process that begins in embryogenesis, continues throughout childhood, and is vulnerable to perturbation from a wide array of exposures. Brain development includes the growth and migration of neurons in prenatal life followed by synapse formation and myelination through early childhood, with subsequent synaptic pruning continuing into adulthood.1 Environmental exposures that can affect neurodevelopment include lead, methylmercury, and ethanol, with outcomes dependent in part on the dose and timing of exposure. Congenital cytomegalovirus (CMV) infection is an environmental exposure that can also cause neurodevelopmental disability, with severe disease manifesting at birth in a small minority of children. Sensorineural hearing loss, a recognized effect of congenital CMV infection, may occur alone or with other disabilities, may not develop until early childhood, and has the potential for progressive impairment over time.2
Human herpesvirus-6 (HHV-6), a β herpesvirus closely related to CMV, infects all children, usually in infancy, and often induces a primary undifferentiated febrile illness, with a minority of children exhibiting the syndrome of roseola infantum.3 After the primary infection, HHV-6 remains latent or persistent in several systems of the body, including the central nervous system.4–6 Two species of HHV-6 have been identified, HHV-6A and HHV-6B, with differences noted in epidemiology and disease associations.7
Although it is believed that most infants acquire HHV-6 infection from the saliva of asymptomatic adults, congenital infection with HHV-6 occurs in an estimated 0.99% (95% confidence interval [CI]: 0.87 to 1.11]) of newborns.8 The mechanisms responsible for this mode of transmission have been recently determined with 86% of congenital infections resulting from chromosomal integration of HHV-6 (ciHHV-6). The remaining 14% may be transplacental infections from ciHHV-6 in the mother.9,10 HHV-6 is unique among the human herpesviruses in that the whole viral genome is integrated into one of several different human chromosomes at the telomere in 0.2% to 0.8% of the population and passed from parent to child in the germline via Mendelian inheritance.11,12 The clinical relevance of congenital infection with HHV-6 is undefined but may be similar to CMV and associated with developmental disability.
To determine if congenital HHV-6 infection has an impact on early neurodevelopmental outcome, we performed a prospective double-blind, controlled study comparing infants with congenital HHV-6 infection versus those without congenital infection. A sensitive set of neurocognitive assessments were used, including a global test of mental development and several specific tests of sensory, perceptual, and cognitive functioning.
Methods
Study Participants
Parents of term (≥36 weeks’ gestation) infants born at Strong Memorial Hospital, Highland Hospital, or Rochester General Hospital in Rochester, New York, were invited by letter to enroll in the study if there was a cord blood sample available for testing. Infants with congenital HHV-6 infection were defined as those who had HHV-6 DNA present in their cord blood mononuclear cells. At least 1 matched control infant without HHV-6 congenital infection was selected and approached for enrollment for each infant with congenital HHV-6 infection. Matching criteria included gender, race/ethnicity, date of birth (within 4 weeks), gestational age (within 2 weeks), and mother’s age (within 10 years). Enrollment visits were conducted at 2 to 4 weeks of age or as soon as possible after the parents expressed an interest in the study. Once enrolled, a urine sample was obtained for CMV testing, and all infants with CMV detected in the urine were excluded. All investigators and staff involved in enrollment and infant developmental testing were unaware of the infants’ HHV-6 status. All parents of enrolled newborns were also unaware of the HHV-6 status of their child. Because of this blinding, multiple control children were enrolled to ensure at least 1 match would be identified for each child with HHV-6 congenital infection. The recognition that congenital infection with HHV-6 was caused by both ciHHV-6 and transplacental infection was defined after the study had begun and, therefore, the infants with congenital infection were not initially categorized as having ciHHV-6 or transplacental infection, and the numbers of each type of subjects were not considered in the original design. The Research Subjects Review Board of the University of Rochester approved the study, and all parents provided written informed consent.
Study Protocol
At the initial study visit, information was obtained regarding the birth history of the child, maternal smoking, and alcohol consumption during pregnancy. Parents also self-reported family demographic information (Table 1). At subsequent visits, parents provided interval histories for the child and information on breastfeeding. Other covariates measured included parental intelligence via the Kaufman Brief Intelligence Test (KBIT) and an observer-rated Caldwell Bradley–based instrument (PROCESS) for maternal caregiving. Although the Parenting Stress Index was also completed, the results were highly skewed, suggesting substantial response bias from the parents; it was therefore not included in the analyses. In addition, we collected information on newborn hearing screening performed for routine care. An audiology evaluation (behavioral hearing testing; visual reinforcement audiometry or conditioned play audiometry) was performed as part of the study between 12 and 24 months of age.
TABLE 1.
Study Assessment Schedule
| Variable | Enrollment | 4 mo | 6 mo | 12–18mo | 24–30 mo |
|---|---|---|---|---|---|
| Prenatal/birth and medical history | X | — | — | — | — |
| Interval history | X | X | X | X | |
| Demographic characteristics | X | — | — | — | — |
| Gender | X | — | — | — | — |
| Race/ethnicity | X | — | — | — | — |
| Parental educationa | — | X | — | — | X |
| Parental occupationb | — | X | — | — | X |
| Marital Status | — | — | — | — | — |
| Breastfeeding history | — | X | X | X | X |
| Maternal KBIT | — | Xc | |||
| Caldwell Bradley Instrument -Observer Rated | — | X | X | X | X |
| Parenting Stress Index | — | — | X | X | X |
| Fagan Test of Infant Intelligence | — | X | X | ||
| Visual Expectation Paradigm | — | X | X | X | X |
| Bayley Scales of Infant Development | — | — | — | X | X |
| Audiology (12-24 mo of age) | — | — | — | X | — |
—, data not collected.
Categorized as less than seventh grade, junior high, some high school, high school graduate, some college, 4-year degree, and graduate or professional training.
Categorized as day laborer, semi-skilled worker, semi-professional, craftsman, clerical, small business owner, administrator, and executive or student.
Maternal KBIT was completed once at any visit after enrollment but generally after the 4-month visit.
Assessments of Infant Neurodevelopment
The Fagan Test of Infant Intelligence
The primary outcome of the Fagan Test of Infant Intelligence is novelty preference; the proportion of time the infant spends looking at a novel stimulus in relation to time looking at a previously presented stimulus. The secondary outcome is fixation duration, which is the mean duration of each look at a stimulus. Novelty preference is a measure of visual recognition memory, whereas fixation duration measures the speed of information processing and attention.13–16 Novelty preference and fixation duration measured in infants are modest predictors of later IQ and specific cognitive abilities.17–20
The Visual Expectation Paradigm
For the Visual Expectation Paradigm (VEXP), infants view a sequence of animated images that appear and disappear at different locations on a video monitor (typically alternating from left to right to left). The VEXP measures the latency to react to a stimulus with a saccadic eye movement. The saccade reaction time measure of the VEXP is an index of attentiveness and speed of information processing.13,21,22 The VEXP also measures anticipatory behavior (proportion of predictive saccades to a future stimulus location), which reflects memory and attention,13 and off-task behavior, a possible measure of inattention.21 Previous research has shown that infant saccade reaction time normally declines during the first 2 years of life, with the decline following a negative exponential function.21,23 Studies have also shown that both reaction time and anticipatory behavior in infancy are modest predictors of IQ and specific cognitive abilities in childhood.22,24,25
The Bayley Scales of Infant Development II
The Mental Development Index (MDI) of the Bayley Scales of Infant Development II (Bayley-MDI) is a global measure of the achievement of cognitive developmental milestones and was administered at 12 months of age. Due to the greater time and resources required for MDI testing, only children with HHV-6 congenital infection and the subset of matched control children were included in this testing. MDI testing at 24 months of age was planned but not completed in the great majority of subjects because of delayed enrollment and insufficient time for full follow-up.
Laboratory Methods
Nested polymerase chain reaction for HHV-6 was conducted. Cord blood mononuclear cells were isolated and used to detect HHV-6 DNA. Chromosomal integration was confirmed by detecting HHV-6 DNA in hair follicle specimens as described previoulsy.9 The polymerase chain reaction protocol was as published and followed by oligo hybridization with specific probes for HHV-6A and HHV-6B. This assay reliably detects ≤10 genomic copies.3
Statistical Analysis
Demographic and baseline characteristics were compared between the HHV-6 congenital infection and control groups by using 2 sample t tests for continuous variables and χ2 or Fisher’s exact tests for categorical variables. To evaluate the association between the Bayley MDI scale at 12 months of age and HHV-6 congenital infection status, multiple linear regression analysis was conducted, with the Bayley MDI score as the dependent variable and the HHV-6 congenital infection status as the independent variable. Covariates included child gender, gestational age, breastfeeding status at 6 months, maternal KBIT score, quality of maternal caregiving, age at testing, and the Hollingshead Index of Social Status. The final regression model was decided by using best subset model selection with minimum Akaike’s information criterion. Robust linear regressions were fitted if observations with high leverage and influence were identified. Robust linear regressions were also used to compare subgroups of infants with congenital HHV-6 (ciHHV-6 or transplacental infection) versus matched control infants in a post hoc analysis. The primary and secondary outcome measures from the VEXP (saccade reaction time, percentage of pictures anticipated, and percentage of inattention trials) were analyzed by using a linear mixed effects model with an indicator of HHV-6 congenital infection status, age of testing (4, 6, 12, and 24 months), and their interactions as independent variables. The HHV-6 effect was regarded as fixed and the subjects as the random effect, specifying an unstructured variance covariance matrix. The analyses were also controlled for covariates including child age, gender, gestational age, and maternal KBIT score. Fagan outcomes evaluated at 4 and 6 months of age were analyzed in a similar fashion as the VEXP. Audiology results were scored based on air conduction thresholds at 6 dB levels and categorized as normal, bilaterally impaired, or unilateral impaired. All statistical analyses were conducted by using SAS version 9.2 (SAS Institute, Inc, Cary, NC).
Results
Study Population
From June 2003 through 2007, we screened 21 207 cord blood samples and identified 207 neonates with congenital infection with HHV-6. From this group, we enrolled 57 infants with congenital HHV-6 infection. Thirteen children withdrew from the study before the 12-month visit (due to relocation [n = 6], loss of interest or too busy [n = 4], maternal illness [n = 1], or lost to follow-up [n = 2]). An additional 5 subjects were excluded from the analyses at 12 months because 2 were <36 weeks’ gestation at birth and 3 did not complete the 12-month assessment, leaving 39 subjects. Of the 39 children with HHV-6 congenital infection evaluated at 12 months of age, 37 had hair follicle samples available for testing and 32 (86%) had ciHHV-6. Thirty-three infants had congenital infection with HHV-6B and 6 had HHV-6A (Supplemental Table 3). There was no recognizable syndrome due to congenital infection with HHV-6 noted at birth in any of the infants.
A total of 242 control infants without HHV-6 congenital infection were also enrolled. Thirty-nine control infants withdrew from the study (relocation [n = 8], loss of interest or too busy [n = 7], maternal illness [n = 1], lost to follow-up [n = 9], noncompliance [n = 7], or no reason given [n = 7]). Four enrollees in the control group were excluded from analysis: 3 were <36 weeks’ gestation at birth and 1 had congenital CMV infection, leaving 199 children at the 12-month evaluation.
Infant Neurodevelopmental Testing
No significant differences were identified in demographic characteristics at enrollment between the 39 infants with congenital HHV-6 infection and the 63 matched control infants included in the Bayley MDI testing (Table 2). Similarly, the 63 matched control infants and the 136 control children who were not included in the Bayley-MDI testing had the same baseline characteristics except for scores on the Hollingshead Index of Social Status.
TABLE 2.
Characteristics According to Group: HHV-6 Congenitally Infected infants, Control Infants Included in the Analysis of Bayley MDI Testing, and Control Infants Who Did not Undergo Bayley MDI Testing
| Characteristic | HHV-6 infection (N = 39) | Bayley-MDI Control Subjects | Pa | Controls Subjects Without Bayley-MDI Scores | Pb |
|---|---|---|---|---|---|
| (N = 63) | (N = 136) | ||||
| Age at 12-mo test, mo | 13.8 ± 1.4 | 13.9 ± 1.7 | .69 | 13.9 ± 1.6 | .99 |
| Male gender | 20 (51%) | 30 (48%) | .72 | 62 (46%) | .79 |
| Race | — | — | .39 | — | .08 |
| White | 32 (82%) | 55 (887%) | — | 110 (81%) | — |
| Black | 3 (8%) | 2 (3%) | — | 17 (13%) | — |
| Other | 4 (10%) | 6 (9%) | — | 7 (5%) | — |
| Hispanic ethnicity | 2 (5%) | 3 (5%) | >.99 | 11 (8%) | .55 |
| Breastfeeding at 6 mo | 23 (59%) | 36 (57%) | .86 | 68 (50%) | .13 |
| Smoked during pregnancy | 4 (10%) | 3 (5%) | .42 | 12 (9%) | .40 |
| Observer Rated Quality of Maternal Caregiving | 75.9 ± 6.2 | 76.7 ± 4.2 | .47 | 75.4 ± 5.6 | .10 |
| Birth weight, kg | 3.6 ± 0.3 | 3.5 ± 0.5 | .14 | 3.5 ± 0.5 | .49 |
| Gestational age, wk | 39.6 ± 1.1 | 39.2 ± 1.3 | .09 | 39.5 ± 1.13 | .13 |
| Maternal KBIT score | 107.2 ± 9.0 | 109.2 ± 8.5 | .28 | 107.1 ± 12.7 | .23 |
| Hollingshead Index of Social Status | 45.5 ± 12.3 | 48.7 ± 10.9 | .23 | 43.9 ± 12.7 | .02 |
Data are presented as mean ± SD or n (%); —, not applicable.
Infants with HHV-6 congenital infection were compared with the matched control group of Bayley-MDI control subjects via 2 sample t tests for continuous variables and χ2 or Fisher’s exact tests for categorical variables.
The control groups were compared via two sample t tests for continuous variables and χ2 or Fisher exact tests for categorical variables.
The mean ± SD MDI score at 12 months of age in the infants with HHV-6 congenital infection was 103.4 ± 8.9 (range: 86–122) and in the matched control children, the mean score was 105.4 ± 12.4 (range: 61–126) (Supplemental Tables 3 and 4). One control infant had a Bayley-MDI score of 61, which is >3 SDs below the mean. A linear regression analysis removing this value showed that infants with congenital HHV-6 infection had lower Bayley MDI scores at 12 months of age (mean difference: 4.1 [95% CI: 0.3 to 7.9]; P = .03). Gestational age, type of feeding, and age at test remained in the regression model after model selection procedure by using Akaike’s information criterion. After controlling for the same set of covariates, a robust regression including all subjects with HHV-6 congenital infection and matched control subjects also revealed a lower Bayley-MDI score at 12 months of age in the HHV-6 congenital infection group (mean difference: 4.3 [95% CI: 0.4 to 8.1]; P = .03). The Bayley-MDI score was also significantly associated with a greater gestational age at birth (estimate: 3.1 [95% CI: 1.5 to 4.6]; P < .01), and breastfeeding status at 6 months of age (estimate: 4.6 [95% CI: 0.9 to 8.3]; P = .02). Post hoc analyses using robust regression were also performed with the type of congenital infection as the independent variable with identical covariates, and the results found that children with ciHHV-6 had significantly lower MDI scores than matched control children (mean difference: 4.1 [95% CI: 0.0 to 8.2]; P = .05). There were no differences between children with transplacental infection and matched control children.
Results of the Fagan Test of Infant Intelligence indicated no differences between infants with and without congenital HHV-6 infection for novelty preference, mean fixation duration for familiarization trials, or mean fixation duration for novelty preference trials. However, mean fixation duration reported the expected decline with age for both the familiarization trials (estimate: –0.82 [95% CI: –1.16 to –0.48]) and the novelty preference trials (estimate: –0.56 [95% CI: –0.87 to –0.25]) in a model controlling for the child’s age, gender, gestational age, and maternal KBIT score. No age by congenital infection status interaction was detected.
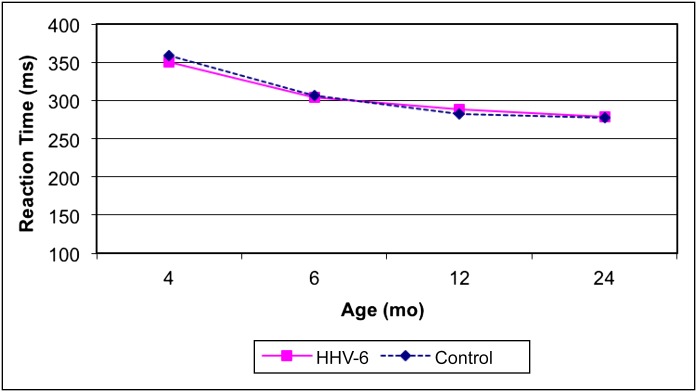
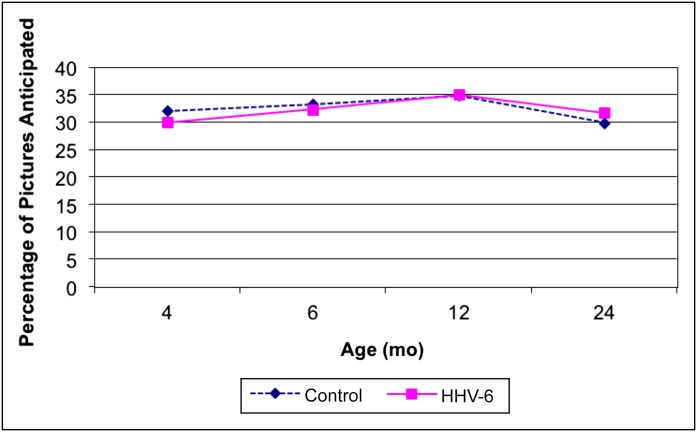
A repeated measures analysis of VEXP also showed the expected decline in saccade reaction time with postnatal age (estimate: –2.72 [95% CI: –4.08 to –1.36]), but reaction time did not differ according to congenital HHV-6 infection status in unadjusted or adjusted models (P = .82). Moreover, there was no suggestion of an age by congenital HHV-6 infection status interaction (Fig 1). Similarly, the analyses of the secondary outcomes (anticipatory behavior and inattentive behavior) revealed no significant differences between the 2 groups (Figs 2 and 3).
FIGURE 1.
Unadjusted mean values of VEXP saccade reaction time by age and group.
FIGURE 2.
Unadjusted mean values of VEXP anticipatory behavior by age and group.
FIGURE 3.
Unadjusted mean values of VEXP inattentive trials by age and group.
No infant had hearing loss identified by results of the newborn screening. Seventeen children with HHV-6 congenital infection (15 with ciHHV-6) and 68 control children underwent audiology evaluations from 12 to 24 months of age. No child had any identifiable hearing loss (95% CI: 0.0 to 17) detected at that time.
Discussion
We prospectively evaluated a group of infants with congenital HHV-6 infection and a control group of infants without congenital infection and found that the children with infection had significantly lower scores at 12 months of age on the Bayley-MDI score. In a post hoc subset analysis, the difference in Bayley-MDI score between the congenital infection group and the control group was also identified in children with ciHHV-6. The number of infants with transplacental infection was too small to draw any conclusions. The association between HHV-6 congenital infection and Bayley-MDI scores is a novel finding but consistent with what is known of the biology of HHV-6 and the clinical characteristics of postnatal infection. Evidence suggests that HHV-6 not only infects the central nervous system but may be pathogenic. HHV-6 B is associated with seizures and febrile status epilepticus; in immunocompromised hosts, reactivation is associated with cognitive dysfunction, delirium, and posttransplant acute limbic encephalitis.3,26–29 Although these links between HHV-6 infection and neurologic disease have been identified, this study is the first to evaluate the early neurodevelopmental impact of congenital HHV-6 infection.
HHV-6 is a β herpesvirus similar to CMV and causes congenital infection in approximately the same percentage of the population. However, unlike congenital CMV infection, we did not detect any clinical manifestations at birth of congenital infection with HHV-6, nor did we identify hearing loss; however, our numbers may have been too small to detect these outcomes. The risk of severe disease due to congenital CMV infection is greatest in women who acquire primary infection during early pregnancy, suggesting the absence of prior immunity is associated with the risk of infection and disease in the fetus.30 Unlike CMV, HHV-6 infection occurs in essentially all children by 3 years of age, and thus all pregnant women have had previous HHV-6 infection. Preexisting immunity in the mother may be one reason why infants with congenital HHV-6 infection have no recognizable illness at birth and are protected against severe disease during the newborn period. However, unlike congenital CMV infection, only a minority of infants with congenital HHV-6 infection have transplacental infection (14%), with the majority of congenital infection due to ciHHV-6 (86%).9 Because ciHHV-6 is due to complete viral integration into the telomere region of a human chromosome, the virus is present in every nucleated cell of the embryo from conception.31 In this setting, the presence of maternal immunity may not play a role during the early stages of development before the transfer of maternal antibody. Our current limited data suggest that although HHV-6 is present in the developing fetus, it does not appear to cause major anomalies but may possibly exert a more subtle effect on the child’s neurodevelopment that might become manifest with time.
The Bayley-MDI is an accepted measure of mental development in infants and toddlers.32,33 Studies have shown a significant correlation between the MDI at 24 months of age and the Wechsler Preschool and Primary Scale of Intelligence–Revised full-scale IQ at 5 years of age, suggesting the MDI is a reliable and accurate measure of cognitive development.34 We found that infants with congenital HHV-6 infection scored >4 points lower on the MDI at 12 months of age than control children after controlling for covariates. Although this difference is small, research on well-recognized neurotoxins, such as lead, have reported similar differences in MDI scores associated with varying levels of exposure during gestation. Hu et al35 found a decrease of 4.1 on the MDI at 24 months of age in infants in association with maternal plasma concentrations of lead in the first trimester of pregnancy. A decrease of 3.8 points on the MDI score was also reported for 12-month-old children in association with maternal cocaine use.36 Our finding needs to be confirmed, but the observed decrease in the Bayley score gives credence to the possibility that congenital infection with HHV-6, and specifically ciHHV-6, may exert significant detrimental effects on infant cognitive development. The clinical significance of this finding deserves further study, especially if these small differences noted in infancy result in progressive effects over time.
Conversely, our study found no differences between the infants with congenital HHV-6 infection and control subjects on the Fagan Test of Infant Intelligence and the VEXP. This finding may be due to the timing or specific types of developmental effects of congenital HHV-6 infection during infancy.
The strengths of the present study include the prospective, double-blind, and longitudinal design with repeated and overlapping neurodevelopmental assessments of the enrolled infants. Despite these strengths, however, there are limitations. Congenital HHV-6 infection occurs in only 1% of the population, making it difficult to study large numbers of infected newborns. In addition, the length of follow-up of the cohort was limited to <2 years of age, a time at which subtle defects in neurodevelopment are difficult to identify and precisely define. Due to the longitudinal design, subject attrition was also a limitation. Finally, we were unable to fully evaluate the effect of ciHHV-6 and transplacental infection separately due to the timing of the study and the much larger number of subjects that would be required for each group. The effect of other factors related to chromosomal integration (eg, the chromosome that is the site of integration and possible differences in pathogenicity between HHV-6A versus HHV-6B) will also require much larger numbers of subjects for study and were beyond the scope of this investigation.
Conclusions
Our data suggest that congenital HHV-6 infection may be associated with cognitive impairment at 12 months of age as noted on the Bayley-MDI, with a magnitude similar in effect to well-known environmental toxins. Because congenital infection with HHV-6 is present in ∼1 in every 101 births, further study of the neurodevelopmental effects of HHV-6 congenital infection during infancy and later childhood are warranted.
Supplementary Material
Acknowledgments
We are indebted to all of the children and families that participated in this study. We are also grateful to Mark Orlando, PhD, MBA, University of Rochester Medical Center, Department of Audiology, for his completion of the audiology testing and to Linda Anderson for her participation in the study.
Glossary
- Bayley-MDI
Mental Development Index of the Bayley Scales of Infant Development II
- CI
confidence interval
- ciHHV-6
chromosomal integration of human herpesvirus-6
- CMV
cytomegalovirus
- HHV-6
human herpesvirus-6
- KBIT
Kaufman Brief Intelligence Test
- VEXP
Visual Expectation Paradigm
Footnotes
Deceased.
Dr Caserta participated in the conceptualization and design of the study, coordinated and supervised data collection, and drafted the initial manuscript; Dr Hall participated in the conceptualization and design of the study, coordinated and supervised data collection, and reviewed and revised the manuscript; Dr Canfield participated in the conceptualization and design of the study, conducted the initial analyses, and reviewed and revised the manuscript; Dr Davidson participated in the conceptualization and design of the study and critically reviewed the manuscript; Dr Lofthus and Mr Schnabel participated in the design of the study, coordinated data collection, and critically reviewed the manuscript; Ms Carnahan and Ms Shelley participated in the design of the study and the acquisition of data, and critically reviewed the manuscript; and Dr Wang participated in the analyses of the data and reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
The data were presented, in part, at the 7th International Conference on HHV-6 and HHV-7; February 27-March 2, 2011; Reston, Virginia.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: All phases of this study were supported by grants from the National Institute of Child Health and Human Development (grant RO1 HD 44430-01), and the National Center for Research Resources, National Institutes of Health (General Clinical Research Center grant 5 MO1 RR00044). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Buka I. Children and Neurodevelopmental Behavioural Intellectual Disorders (NDBID). Training for the Health Sector. Published 2011. Updated October 2011. Available at: www.who.int/ceh/capacity/neurodevelopmental.pdf. Accessed May 2013
- 2.Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56(9):1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall CB, Long CE, Schnabel KC, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331(7):432–438 [DOI] [PubMed] [Google Scholar]
- 4.Norton RA, Caserta MT, Hall CB, Schnabel K, Hocknell P, Dewhurst S. Detection of human herpesvirus 6 by reverse transcription-PCR. J Clin Microbiol. 1999;37(11):3672–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun DK, Dominguez G, Pellett PE. Human herpesvirus 6. Clin Microbiol Rev. 1997;10(3):521–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caserta MT, Hall CB, Schnabel K, Lofthus G, McDermott MP. Human herpesvirus (HHV)-6 and HHV-7 infections in pregnant women. J Infect Dis. 2007;196(9):1296–1303 [DOI] [PubMed] [Google Scholar]
- 7.Ablashi D, Agut H, Alvarez-Lafuente R, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014;159(5):863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145(4):472–477 [DOI] [PubMed] [Google Scholar]
- 9.Hall CB, Caserta MT, Schnabel K, et al. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics. 2008;122(3):513–520 [DOI] [PubMed] [Google Scholar]
- 10.Hall CB, Caserta MT, Schnabel KC, et al. Transplacental congenital human herpesvirus 6 infection caused by maternal chromosomally integrated virus. J Infect Dis. 2010;201(4):505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbuckle JH, Medveczky MM, Luka J, et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107(12):5563–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nacheva EP, Ward KN, Brazma D, et al. Human herpesvirus 6 integrates within telomeric regions as evidenced by five different chromosomal sites. J Med Virol. 2008;80(11):1952–1958 [DOI] [PubMed] [Google Scholar]
- 13.Jacobson SW, Jacobson JL, O’Neill JM, Padgett RJ, Frankowski JJ, Bihun JT. Visual expectation and dimensions of infant information processing. Child Dev. 1992;63(3):711–724 [PubMed] [Google Scholar]
- 14.Rose SA, Feldman JF. Prediction of IQ and specific cognitive-abilities at 11 years from infancy measures. Dev Psychol. 1995;31(4):685–696 [Google Scholar]
- 15.Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory: independent contributions of speed and attention. Dev Psychol. 2003;39(3):563–571 [DOI] [PubMed] [Google Scholar]
- 16.Colombo J. Infant Intelligence Tests: Past and Prologue. London, United Kingdom: Sage; 1993 [Google Scholar]
- 17.Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Dev. 1986;57(2):251–274 [DOI] [PubMed] [Google Scholar]
- 18.Fagan JF, McGrath SK. Infant recognition memory and later intelligence. Intelligence. 1981;5(2):121–130 [Google Scholar]
- 19.Fagan JF, III, Singer LT, Montie JE, Shepherd PA. Selective screening device for the early detection of normal or delayed cognitive development in infants at risk for later mental retardation. Pediatrics. 1986;78(6):1021–1026 [PubMed] [Google Scholar]
- 20.Kavsek M. Predicting later IQ from infant visual habituation and dishabituation: a meta-analysis. J Appl Dev Psychol. 2004;25(3):369–393 [Google Scholar]
- 21.Canfield RL, Smith EG, Brezsnyak MP, Snow KL. Information processing through the first year of life: a longitudinal study using the visual expectation paradigm. Monogr Soc Res Child Dev. 1997;62(2):1–145 [PubMed] [Google Scholar]
- 22.Dougherty TM, Haith MM. Infant expectations and reaction time as predictors of childhood speed of processing and IQ. Dev Psychol. 1997;33(1):146–155 [DOI] [PubMed] [Google Scholar]
- 23.Canfield RL, Wilken J, Schmerl L, Smith EG. Age-related change and stability of individual—differences in infant saccade reaction-time. Infant Behav Dev. 1995;18(3):351–358 [Google Scholar]
- 24.Benson JB, Cherny SS, Haith MM, Fulker DW. Rapid assessment of infant predictors of adult IQ–Midtwin Midparent Analyses. Dev Psychol. 1993;29(3):434–447 [Google Scholar]
- 25.Dilalla LF, Plomin R, Fagan JF, et al. Infant predictors of preschool and adult IQ—a study of infant twins and their parents. Dev Psychol. 1990;26(5):759–769 [Google Scholar]
- 26.Epstein LG, Shinnar S, Hesdorffer DC, et al. FEBSTAT study team . Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study. Epilepsia. 2012;53(9):1481–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JA, Koo S, Guzman Suarez BB, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18(11):1638–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward KN, Andrews NJ, Verity CM, Miller E, Ross EM. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child. 2005;90(6):619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zerr DM, Fann JR, Breiger D, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117(19):5243–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nigro G, Adler SP. Cytomegalovirus infections during pregnancy. Curr Opin Obstet Gynecol. 2011;23(2):123–128 [DOI] [PubMed] [Google Scholar]
- 31.Morissette G, Flamand L. Herpesviruses and chromosomal integration. J Virol. 2010;84(23):12100–12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris SR, Megens AM, Backman CL, Hayes VE. Stability of the Bayley II Scales of Infant Development in a sample of low-risk and high-risk infants. Dev Med Child Neurol. 2005;47(12):820–823 [DOI] [PubMed] [Google Scholar]
- 33.Lowe JR, Erickson SJ, Schrader R, Duncan AF. Comparison of the Bayley II Mental Developmental Index and the Bayley III Cognitive Scale: are we measuring the same thing? Acta Paediatr. 2012;101(2):e55–e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munck P, Niemi P, Lapinleimu H, Lehtonen L, Haataja L, PIPARI Study Group . Stability of cognitive outcome from 2 to 5 years of age in very low birth weight children. Pediatrics. 2012;129(3):503–508 [DOI] [PubMed] [Google Scholar]
- 35.Hu H, Téllez-Rojo MM, Bellinger D, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114(11):1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer LT, Arendt R, Minnes S, et al. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002;287(15):1952–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.