Abstract
BACKGROUND:
Current molecular diagnostic methods have detected rhinovirus RNA in a high proportion of asymptomatic infants and children, raising the question of the clinical significance of these findings. This study investigates the prevalence of prolonged rhinovirus RNA presence in the upper respiratory tract of infants during the first year of life.
METHODS:
In a longitudinal study, infants were followed from birth up to 12 months. Nasopharyngeal specimens were collected monthly (months 1–6 and month 9) and during an upper respiratory infection. Rhinoviruses were detected by quantitative reverse-transcription polymerase chain reaction. Presence of repeated rhinovirus RNA was evaluated by nucleotide sequence analysis.
RESULTS:
A total of 2153 specimens from 362 infants were studied; 341 distinct rhinovirus infections in 216 infants were identified. Follow-up specimens were available within 30 days for 179 infections, creating the sample set to assess prolonged rhinovirus presence. Of the 179 infections, 46 involved the detection of the same rhinovirus strain in repeated specimens, including 8 events of prolonged presence of the same strain (detected in specimens collected >30 days apart), representing 4.5% of the evaluable rhinovirus infections. There were 26 events in which a rhinovirus strain was replaced by a different strain within a 30-day interval, representing 14.5% of the 179 infections.
CONCLUSIONS:
Although rhinovirus infections are common in healthy infants, prolonged presence of rhinovirus RNA in the respiratory tract after an upper respiratory infection was uncommon (<5%). Detection of rhinovirus RNA in an infant most likely represents an infection within a 30-day period.
Keywords: rhinovirus, RNA, PCR, upper respiratory tract infection, persistence
What’s Known on This Subject:
Rhinoviruses are commonly detected in both acutely ill and asymptomatic infants and children. The finding may represent new infection or prolonged presence of rhinovirus RNA in the respiratory tract.
What This Study Adds:
In young, otherwise healthy infants, shedding of RNA from the same rhinovirus strain rarely persisted longer than 30 days.
Rhinoviruses are the most common cause of upper respiratory tract infections (URIs).1 Recent studies have shown an increasing prevalence of asymptomatic rhinovirus infections.2–4 Picornavirus nucleic acids are typically shed from the upper respiratory tract for 1 to 3 weeks after infection.4,5 A study in adult volunteers showed that cultivatable rhinoviruses were routinely recovered for ∼2 weeks after infection.6 Recurrent rhinovirus infection has been described in patients with asthma7 and in those at risk of developing asthma.8 These recurrent infections were more likely due to different rhinovirus strains than to persistence of the same strain. Prolonged duration of rhinovirus infections has been described in immunocompromised patients: 4 months in a pediatric stem cell transplant patient,9 8 to 15 months in adult recipients of lung transplants,10 and up to 4 months in adult patients with primary hypogammaglobulinemia.11 To date, the study of persistent or novel repeated rhinovirus infections in infants during the first year of life when humans are first exposed to these viruses has not been reported.
Molecular diagnostic methods such as polymerase chain reaction (PCR) are being used more frequently in place of viral culture for the detection of respiratory viruses, including rhinoviruses. PCR is more sensitive than viral culture; this characteristic, together with the ability of some respiratory viruses (adenovirus, picornaviruses) to persist after infection or to cause asymptomatic infections, can confound the interpretation of positive PCR results. Application of a cutoff value to quantitative PCR may identify clinically relevant rhinovirus infections.12 Large-scale longitudinal studies involving rhinovirus strain characterization are lacking, particularly in infants and children without underlying conditions. Most studies involve children with underlying illness or strain characterization is absent or limited. We previously showed that sequential specimens from children with URI were frequently positive for rhinovirus.13 The aim of this study was to characterize rhinoviruses that are detected repeatedly in sequential samples, to differentiate new infections from prolonged presence of viral nucleic acids. We herein describe the results of sequence analysis of rhinovirus RNA detected in nasopharyngeal specimens from infants during URI episodes and monthly asymptomatic visits.
Methods
Study Description and Subjects
An analysis was performed by using specimens collected as part of a prospective study to determine the prevalence and risks of URI and acute otitis media (AOM) development in the first year of life.14 Subjects were enrolled between October 2008 and April 2013 at the University of Texas Medical Branch (UTMB), Galveston. Study subjects were recruited from the UTMB newborn nursery or the primary care pediatric clinics; they were healthy and resided in Galveston. Preterm infants and those with major medical problems or anatomic/physiologic defects of the ear or nasopharynx were excluded. Subjects were enrolled from near birth (before 1 month of age) and followed to the first AOM episode, or between 6 and 12 months of age. Subjects completed the study at age 6 months if AOM developed before 6 months or were followed up to 12 months for AOM occurrence. Nasopharyngeal swabs were collected at 1, 2, 3, 4, 5, and 6 months and at 9 months if the subjects still remained in the study. During the follow-up period, parents were instructed to report to the study team as soon as the subject began to develop cold symptoms: nasal stuffiness, runny nose, cough, and sore throat, with or without constitutional symptoms such as fever, decreased appetite, and restless sleep. The Institutional Review Board of UTMB approved the study protocol. Written informed consent was obtained from the parents on behalf of the infants.
Specimens
Trained personnel collected nasopharyngeal swab specimens during the monthly visit by introducing a flocked swab (FLOQSwabs; COPAN Diagnostics, Murrieta, CA) into the nose until resistance was met, and then the swab was rotated gently 180°. The swab was then placed in a 1-mL tube of ESwab transport medium (COPAN Diagnostics) and transported to the laboratory on ice. Aliquots were kept frozen at −80°C until testing. During URI episodes, additional nasopharyngeal secretion was collected by using vacuum suction into a mucus trap as described previously.15
Molecular Virological Studies
A high-throughput, quantitative, real-time, reverse-transcription PCR (qRT-PCR) assay was used to detect rhinovirus in respiratory specimens, as described previously.15 Briefly, nucleic acids were extracted from specimens by using the MagMax Total Nucleic Acid isolation kit (Ambion/Applied Biosystems, Austin, TX) and a Biosprint 96 extraction platform (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized from extracted RNA by using an iScript synthesis kit (Bio-Rad, Hercules, CA). Reverse transcription was completed with a Bio-Rad C1000 thermocycler. The generated cDNA was analyzed immediately and then stored at −20°C. cDNA templates were evaluated by using a quantitative PCR (qPCR) assay with primers amplifying the 5′ untranslated region of rhinovirus.16 A separate qPCR for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to evaluate RNA quality.17 TaqMan probes (Sigma-Aldrich, St Louis, MO; Integrated DNA Technologies, Coralville, IA) were used to quantify specific amplification in each reaction.
Sequence analysis of the 5′ nontranslated region of rhinovirus was performed by pyrosequencing with the use of a seminested assay that used our previously reported rhinovirus real-time qRT-PCR amplimer.15 Sequencing of the rhinovirus 5′ nontranslated region is a suitable approach for evaluation of short-term transmission.18,19 Briefly 12.5 µL of iQ supermix (Bio-Rad) was mixed within a 25-µL PCR reaction containing 200 nM of both a biotinylated forward primer (biotin-TGGACAAGGTGCGAAGAGC) and a reverse primer (GGTTAGCCGCATTCAGGG) and 1 µL of the rhinovirus qPCR amplimer15 and nuclease-free water to volume. Thermocycling was completed by using a Bio-Rad C1000 thermocycler and the following protocol: (1) 1.5 minutes, 95°C, (2) 95°C, 15 seconds, (3) 60°C, 1 minute, repeat 50 times, (4) 72°C, 5 minutes, and (5) indefinite hold at 4°C. The generated biotinylated PCR products were pyrosequenced by using PyroMark Gold reagents on a PyroMark Q96 ID platform according to the manufacturers’ instructions (Qiagen). An amplimer specific sequencing primer (TAGCCGCATTCAGGG) was used at a final concentration 0.3 µM in combination with 20 cyclic dispensations of (GCAT).
To further verify prolonged presence of rhinovirus RNA, samples from cases with persistence of the same strain identified by pyrosequencing were subjected to Sanger-based sequencing of the P1–P3 region within the 5′ nontranslated region.20 PCR was performed by using 12.5 µL REDTaqReadyMix (Sigma-Aldrich), 200 nM of forward and reverse primer, 3 µL of cDNA template, and nuclease-free water. PCR products were cloned by using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA). Representative plasmids were purified from isolated bacterial colonies and were Sanger-sequenced (SeqWright, Houston, TX). Results were analyzed by using Genbank/BLAST and Clustal Omega alignment functions. Phylogenetic analysis was performed by using the Information Genomique et Structuale Web portal (http://www.phylogeny.fr/version2_cgi/simple_phylogeny.cgi).
Results
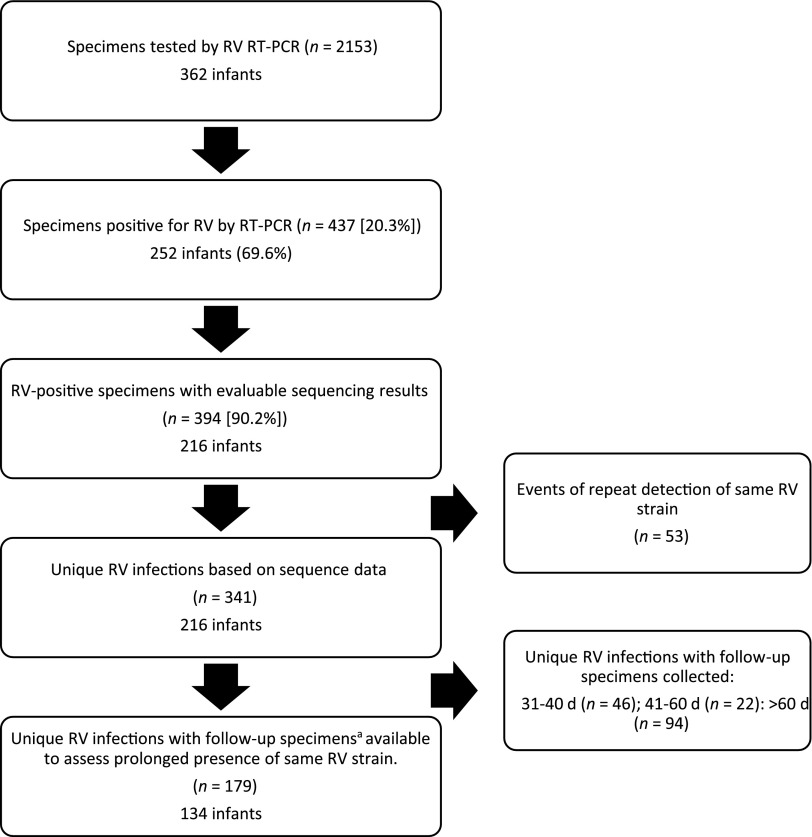
This report included 362 infants studied consecutively over a 5-year period through 5 winter seasons. Of 362 infants, 54% were male, 76% were white, 23% were black, and 1% were Asian. One hundred and eighty (49.7%) infants were Hispanic, and 182 (50.3%) were non-Hispanic. Forty-one (11%) infants were followed for <6 months, 118 (33%) for 6 to <12 months, and 203 (56%) for 12 months. A total of 2153 specimens were collected between November 2008 and October 2013 from 362 infants and were tested for rhinovirus by qRT-PCR (mean: 5.9 specimens per infant). A total of 252 (69.6%) infants tested positive for rhinovirus at least once. Figure 1 shows the flow diagram for the number of subjects and specimens included in the study. Rhinovirus RNA was detected in 437 (20.3%) specimens, for an average of 1.7 rhinovirus isolates per infant. A total of 395 (90%) specimens had evaluable (valid) rhinovirus nucleotide sequencing results; each of these 395 rhinovirus-positive specimens was considered 1 rhinovirus RNA isolate.
FIGURE 1.
Flow diagram for subjects and specimens included in the study. aAfter the detection of a rhinovirus strain, follow-up specimens that were negative for that particular rhinovirus strain were collected within 30 days. Follow-up specimens that were positive for the same rhinovirus strain were collected at any interval (not restricted to 30 days). RT-PCR, reverse-transcription polymerase chain reaction; RV, rhinovirus.
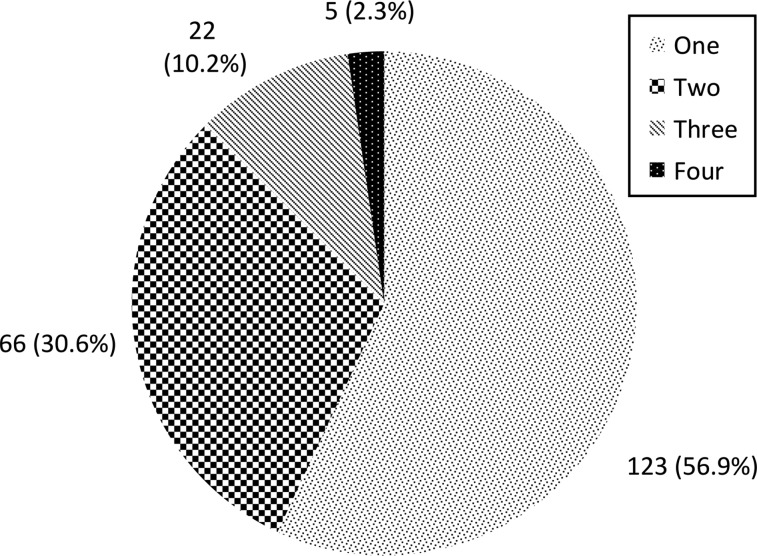
Sequencing results were compared for samples from the same patient over time, and repeat positives of the same rhinovirus strain were identified to represent a prolonged or persistent distinct rhinovirus infection. Figure 2 shows a hypothetical subject and the terminology used for this study. Overall, 341 distinct rhinovirus infections in 216 infants were identified. The number of rhinovirus infections during the study period is shown in Fig 3. There were 26 occurrences in which a rhinovirus strain was replaced by a different strain within a 30-day interval, representing 14.5% of the 179 rhinovirus infections.
FIGURE 2.
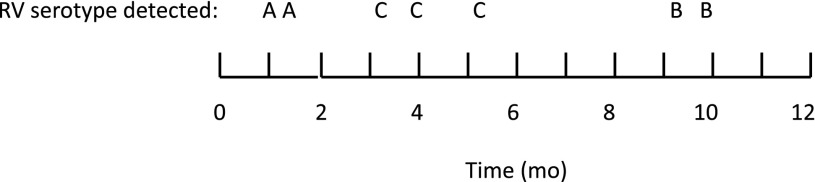
Hypothetical subject and terminology used for study. This subject had 7 rhinovirus RNA isolates (detections) and 3 distinct rhinovirus infections. RV, rhinovirus.
FIGURE 3.
Number of rhinovirus infections in infants (caused by unique strains).
Prolonged presence of rhinovirus RNA was defined as the presence of the same rhinovirus strain for longer than 30 days, with or without intervening negative specimens. To identify cases with prolonged rhinovirus presence, follow-up specimens that were negative for that particular rhinovirus strain must have been collected within 30 days. Follow-up specimens that were positive for the same rhinovirus strain could be collected at any interval (not restricted to 30 days). Of the 341 unique rhinovirus infections, 179 had available follow-up specimens within 30 days or showed prolonged presence of the same rhinovirus strain. Therefore, 179 unique evaluable rhinovirus infections (in 134 infants) served as the sample set to assess prolonged presence of rhinovirus. There were an additional 46 infections that had follow-up specimens collected between 31 and 40 days, 22 that had follow-up specimens collected between 41 and 60 days, and 94 that had either no follow-up specimens or only specimens collected after 60 days. However, these 162 infections were not included in the final analysis. Of the 179 distinct rhinovirus infections analyzed, 148 (83%) had between 2 and 11 follow-up specimens that were negative for the specific rhinovirus strain. Of 134 infants (from whom the 179 rhinovirus infections were identified), 99 had single rhinovirus-positive samples and 35 infants had 2 to 4 rhinovirus isolates.
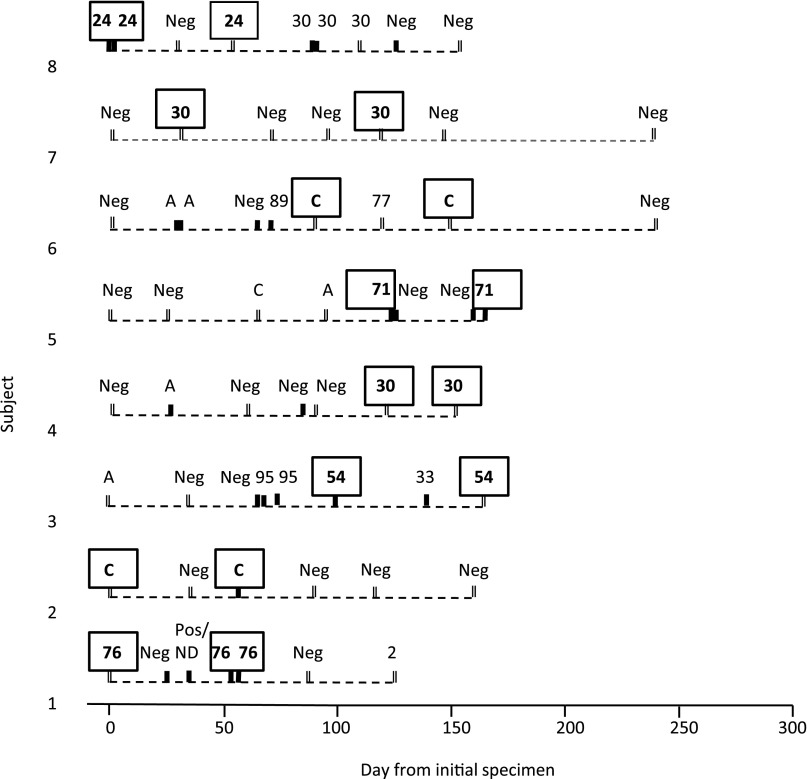
Of 179 distinct rhinovirus infections, 133 were represented by 1 rhinovirus isolate, with follow-up specimens negative for that rhinovirus strain. Another 46 infections (25.7%) consisted of the same rhinovirus strain detected repeatedly. Table 1 displays the time interval between detection of the same strain. The intervals ranged from 1 to 91 days (median: 7.5 days). In 30 (65.2%) of the 46 infections, the same rhinovirus strains were detected from specimens collected <14 days apart. There were 8 infections with prolonged presence of the same rhinovirus strain, ranging from 31 to 91 days, representing 4.5% of 179 evaluable rhinovirus infections. Of these 8 infections, samples were available from 4 for Sanger sequencing to further verify rhinovirus strain persistence identified by pyrosequencing. In all 4 cases, Sanger sequencing identified the same strain in sequential specimens. The 8 individual events of prolonged rhinovirus RNA presence (in 8 different infants) are shown chronologically in Fig 4.
TABLE 1.
Intervals Between Repeated Detections of the Same Strain of Rhinovirus RNA
| Days Between Detection of RNA From Same Strain | Number (% of 179 Rhinovirus Infections) |
|---|---|
| 1–2 | 14 (7.8) |
| 3–7 | 9 (5.0) |
| 8–14 | 7 (3.9) |
| 15–21 | 2 (1.1) |
| 22–30 | 6 (3.4) |
| >30 | 8 (4.5) |
| No repeated detectiona | 133 (74.3) |
| Total | 179 |
Negative on repeat detection within 30 days.
FIGURE 4.
Cases of prolonged presence of rhinovirus RNA in infants. Each specimen is represented by a solid black (I) or double (II) vertical hash marks. Solid marks (I) represent specimens collected during symptomatic URI, and double marks (II) indicate collection during asymptomatic monthly visits. Rhinovirus strains in boxes represent those with repeated presence >30 days apart. Pos/ND, rhinovirus qRT-PCR positive, sequence not determined; Neg, rhinovirus qRT-PCR negative.
We also evaluated the symptom patterns associated with the 8 cases of prolonged rhinovirus presence. The results revealed 4 different symptom patterns: (1) initially symptomatic, with rhinovirus RNA detectable after resolution of symptoms (2 cases; cases 3 and 8); (2) symptomatic throughout (1 case; case 5); (3) asymptomatic throughout (3 cases; cases 4, 6, and 7); and (4) asymptomatic at first detection with later development of symptoms (2 cases; cases 1 and 2). During periods of prolonged rhinovirus RNA presence with either symptoms throughout, or initially asymptomatic and symptomatic later (cases 1, 2, and 5), the viral load increased by 3.79, 3.98, and 4.09 log10 copies/mL over 54, 57, and 41 days, respectively. In contrast, during periods of prolonged rhinovirus RNA presence that were asymptomatic throughout, or initially symptomatic and later resolved (cases 7 and 8), the viral load decreased by 2.51 and 1.28 log10 copies/mL over 91 and 53 days, respectively.
Rhinovirus RNA viral load data and qPCR results for other respiratory viruses were available for some of the cases that showed prolonged presence (Supplemental Table 2). Additional respiratory viruses were frequently detected during the prolonged rhinovirus infections. Coronaviruses were detected in all 8 cases, and their initial detection was most often not associated with respiratory symptoms. A review of medical records of the infants with prolonged presence of rhinovirus RNA found no evidence of underlying conditions that are generally considered to result in persistent RV infections (eg, asthma, hypogammaglobulinemia, or other immunodeficiency disorders).
Discussion
With increased use of molecular diagnostics for respiratory viruses and the associated increase in sensitivity of detection, the biological and clinical significance of detection of viral nucleic acids must be addressed. This issue is complicated by the challenge of differentiating between new infection and prolonged virus presence/persistence. We focused our study on the most common viral infection of the respiratory tract in infants during their first year of life. The data from our large longitudinal study show that frequent infections with different rhinovirus strains are common in infants during the first year of life. Importantly, the results indicated that rhinovirus RNA rarely persisted beyond 30 days after rhinovirus infection.
Of 341 distinct rhinovirus infections documented, we selected for analysis 179 rhinovirus infections that had available follow-up specimens within 30 days or showed prolonged presence of the same rhinovirus strain. Consistent with our earlier study that examined the persistence of adenovirus nucleic acids in nasopharyngeal secretions,13 we used a 30-day cutoff to define prolonged presence of rhinovirus RNA. Other studies have also used a 30-day time limit to distinguish between new and repeated nucleic acid detection.21 Of the 179 rhinovirus infections analyzed, 148 (83%) had between 2 and 11 follow-up specimens that were negative for the specific rhinovirus strain. This finding provided strong evidence that the rhinovirus strain was permanently rather than transiently absent. Clinically, this finding was associated with a lack of symptoms and likely indicated a resolution of infection. Of 179 rhinovirus infections that were evaluated, RNA was detected in 133 (74%) in only a single specimen; 46 (26%) infections showed repeated detection of the same strain. Half of the repeated rhinovirus RNA events were ≤7 days in duration; the majority of repeat positives occurred within a 30-day window. Among the 46 infections with repeated isolates, there were a total of 99 isolates, for an average of 2.2 per infection. The majority of the 46 infections had 2 isolates, several had 3, and none had >3. Only 8 of the 46 repeated rhinovirus RNA isolates were detected >30 days apart, our definition of prolonged presence (4.5% of all evaluable rhinovirus infections).
Of the 216 infants with rhinovirus-positive specimens with sequence data, 93 (43%) had between 2 and 4 different rhinovirus infections during the 6- to 12-month follow-up, whereas 123 (57%) had only a single rhinovirus infection during the study period. Infants can be infected with a variety of rhinovirus strains throughout the first year of life. Indeed, our data show that repeated detection of rhinovirus RNA from nasopharyngeal specimens from infants is more likely to represent new infection than prolonged presence >30 days. Among the 179 rhinovirus infections studied, there were 26 occurrences of a rhinovirus strain being replaced by a different strain within the 30-day follow-up period, compared with only 8 prolonged rhinovirus infections.
Persistent rhinovirus infections (or prolonged presence of rhinovirus RNA) have been described, usually in patients with underlying conditions. Wood et al22 examined rhinovirus RNA persistence in 39 subjects (both children and adults) with acute asthma. Patients were followed up once at 4 to 6 weeks post–hospital admission. Very few positive viral specimens had sequence analysis performed, so it was difficult to assess how frequently persistence of the same rhinovirus strain occurred. Chronic rhinovirus infection and persistence of RNA have also been described in transplant patients9,10 and in those with hypogammaglobulinemia.11 Jartti et al8 studied serial rhinovirus infections in infants at risk of developing asthma. Considering only moderate-to-severe rhinovirus infections, 1 of 150 (0.7%) was associated with the same rhinovirus strain detected at least 2 weeks apart. Because our study included all rhinovirus occurrences regardless of symptoms, our observed higher rate of rhinovirus persistence (8 of 179 [4.5%] >30 days and 16 of 179 [8.9%] >14 days) was not surprising. Unlike other studies, including the Jartti study in infants at risk of asthma, the infants in our study had no underlying conditions.
There is a paucity of information on rhinovirus RNA persistence in otherwise healthy infants with URI. Studies that have examined children without underlying conditions were small, with limited longitudinal sampling, or rhinovirus was not identified to the strain or serotype level, preventing determination of strain persistence. In a study in 58 rhinovirus-infected patients (45 of whom were children) admitted to the hospital, 4 (6.9%; all children) had rhinovirus detected by reverse-transcription PCR in multiple specimens. Only 1 patient had the same rhinovirus strain in multiple specimens, collected 2 months apart.23 Another study followed 26 rhinovirus-infected children hospitalized for acute wheezing. Half of the subjects were reverse-transcription PCR positive at 2 weeks, and 1 (4%) was positive at 5 weeks. Sequence analysis not performed, so the subjects could have had a new infection with a different rhinovirus strain.5
Five of the 8 cases of prolonged rhinovirus RNA presence in this study had intervening negative specimens. These are most likely false negatives due to low rhinovirus RNA viral load or to inadequate sample collection. Reinfection by the same rhinovirus strain is unlikely due to host immunity. Two cases had an intervening sample positive for a different rhinovirus strain, highlighting the number and variety of rhinovirus infections during infancy.
The 8 cases of prolonged rhinovirus RNA presence in our study occurred both in the presence and absence of upper respiratory symptoms. The longest interval between isolates was 91 days (subject 7, who was asymptomatic throughout). In fact, the most common pattern (3 cases) was asymptomatic throughout all isolates. This finding is consistent with reports of asymptomatic rhinovirus infections.2,24 There were 2 cases of rhinovirus RNA detection after resolution of symptoms, suggesting nucleic acid persistence after infection. In only 1 case was the infant symptomatic throughout all rhinovirus RNA isolates (2 specimens collected 41 days apart).
Rhinovirus persistence was found to occur during overlapping infection by other respiratory viruses. Coronavirus infections were most common and usually asymptomatic. Asymptomatic coronavirus infections in children have been reported.24 As expected, the presence or development of symptoms appeared to be associated with an increase in viral load, whereas the absence or resolution of symptoms was associated with a decrease in viral load.
Our study limitations are common to most studies of this type and include the inability to detect rhinovirus RNA present at very low levels, particularly in mixed infections. This inability would result in underreporting of rhinovirus infections, likely including events of prolonged presence. Not all samples had follow-up testing at regular intervals to address the question of virus persistence. However, our criteria for follow-up testing were very strict. If the criteria were relaxed, our number of evaluable rhinovirus infections would have increased substantially, which, in turn, would have decreased the proportion of cases with prolonged rhinovirus presence. On the basis of sample collection, our reported intervals between repeated isolates of rhinovirus RNA may not have always reflected the true duration of persistence, but they provide a more accurate indication than in previous reports. Future studies to provide more precise measurement of rhinovirus persistence should include studies with more frequent, longitudinal sampling with viral load measurement. Future directions for further study might also include multicenter studies for larger numbers of patients.
In summary, we showed that in otherwise healthy infants in the first year of life, rhinovirus URI infrequently resulted in prolonged presence (>30 days) of rhinovirus RNA in the respiratory tract. Infants often have repeated infections with different rhinovirus strains in the first year of life. The detection of rhinovirus RNA by qRT-PCR in this population most likely represents current or recent infection.
Conclusions
Rhinovirus infections in young, otherwise healthy infants rarely result in persistence of RNA beyond 30 days. The detection of rhinovirus RNA most likely represents recent infection. Further studies with frequent follow-up visits will help define the duration of virus shedding in rhinovirus infections with various clinical manifestations.
Supplementary Material
Acknowledgments
We thank Dr Janak Patel and Dr David McCormick for careful review of the manuscript and for clinical assistance. We also thank Dr Alejandro Diego, Dr Stella Kalu, Dr Johanna Nokso-Kiovisto, Dr Tal Marom, Ms Esther Valdivia, Ms Lilia Rodriquez, and Ms Ying Xiong for their assistance with the study subjects and specimens.
Glossary
- AOM
acute otitis media
- cDNA
complementary DNA
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- qRT-PCR
quantitative, real-time, reverse-transcription polymerase chain reaction
- URI
upper respiratory infection
- UTMB
University of Texas Medical Branch
Footnotes
Dr Loeffelholz co-conceptualized and co-designed the study and wrote the initial manuscript; Dr Trujillo coordinated data collection; Dr Pyles conceptualized and supervised molecular virology studies and reviewed and revised the manuscript; Mr Miller performed molecular virology studies and provided interpretation of the data for inclusion in the manuscript; Dr Alvarez-Fernandez supervised data collection; Dr Pong performed molecular virology studies and performed data collection and analysis; Dr Chonmaitree conceptualized and designed the study, provided overall study supervision, and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by National Institutes of Health grants R01DC005841 and UL1TR000071. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013;26(1):135–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J. 2012;31(12):1221–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154(3):396–400, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. 2006;78(5):644–650 [DOI] [PubMed] [Google Scholar]
- 5.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72(4):695–699 [DOI] [PubMed] [Google Scholar]
- 6.Winther B, Gwaltney JM, Jr, Mygind N, Turner RB, Hendley JO. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256(13):1763–1767 [PubMed] [Google Scholar]
- 7.Linsuwanon P, Payungporn S, Samransamruajkit R, Theamboonlers A, Poovorawan Y. Recurrent human rhinovirus infections in infants with refractory wheezing. Emerg Infect Dis. 2009;15(6):978–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF, Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32(2):314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathak AK, Adams RH, Shah NC, Gustin KE. Persistent human rhinovirus type C infection of the lower respiratory tract in a pediatric cord blood transplant recipient. Bone Marrow Transplant. 2013;48(5):747–748 [DOI] [PubMed] [Google Scholar]
- 10.Kaiser L, Aubert JD, Pache JC, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174(12):1392–1399 [DOI] [PubMed] [Google Scholar]
- 11.Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Osterback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 2010;126(1):120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49(7):2631–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalu SU, Loeffelholz M, Beck E, et al. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J. 2010;29(8):746–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ede LC, Loeffelholz MJ, Alvarez-Fernandez P, et al. Effect of the 2009 influenza A/H1N1 pandemic on viral respiratory infections in the first year of life. Pediatr Infect Dis J. 2012;31(11):1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeffelholz MJ, Pong DL, Pyles RB, et al. Comparison of the FilmArray Respiratory Panel and Prodesse real-time PCR assays for detection of respiratory pathogens. J Clin Microbiol. 2011;49(12):4083–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambarino S, Costa C, Elia M, et al. Development of a RT real-time PCR for the detection and quantification of human rhinoviruses. Mol Biotechnol. 2009;42(3):350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourne N, Pyles RB, Yi M, Veselenak RL, Davis MM, Lemon SM. Screening for hepatitis C virus antiviral activity with a cell-based secreted alkaline phosphatase reporter replicon system. Antiviral Res. 2005;67(2):76–82 [DOI] [PubMed] [Google Scholar]
- 18.Peltola V, Waris M, Osterback R, Susi P, Ruuskanen O, Hyypiä T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197(3):382–389 [DOI] [PubMed] [Google Scholar]
- 19.Savolainen-Kopra C, Blomqvist S, Smura T, et al. 5′ Noncoding region alone does not unequivocally determine genetic type of human rhinovirus strains. J Clin Microbiol. 2009;47(4):1278–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2(10):e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nokso-Koivisto J, Pitkäranta A, Blomqvist S, et al. Viral etiology of frequently recurring respiratory tract infections in children. Clin Infect Dis. 2002;35(5):540–546 [DOI] [PubMed] [Google Scholar]
- 22.Wood LG, Powell H, Grissell TV, et al. Persistence of rhinovirus RNA and IP-10 gene expression after acute asthma. Respirology. 2011;16(2):291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henquell C, Mirand A, Deusebis AL, et al. Prospective genotyping of human rhinoviruses in children and adults during the winter of 2009-2010. J Clin Virol. 2012;53(4):280–284 [DOI] [PubMed] [Google Scholar]
- 24.Nokso-Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkäranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66(3):417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.