Abstract
The picosecond dynamics of the photoreaction of an artificial bacteriorhodopsin (BR) pigment containing a retinal in which a five-membered ring spans the C-12 to C-14 positions of the polyene chain (BR5.12) is examined by using time-resolved absorption and fluorescence and resonance Raman spectroscopy. The ring within the retinal chromophore of BR5.12 blocks the C-13 = C-14 isomerization proposed to be a primary step in the energy storage/transduction mechanism in the BR photocycle. Relative to the native BR pigment (BR-570), the absorption spectrum of BR5.12 is red-shifted by 8 nm. The fluorescence spectrum of BR5.12 closely resembles that of BR-570 although the relative fluorescence yield is higher (approximately 10-fold). Picosecond transient absorption (4-ps pulses, 568-662 nm) measurements reveal an intermediate absorbing to the red side of BR5.12. Kinetic fits show that the red-absorbing intermediate appears within < 3 ps and decays with a time constant of 17 +/- 1 ps to form only BR5.12. No emission in the 650- to 900-nm region can be attributed to the red-absorbing species. Since rotation around C-12 - C-13 and isomerization around C-13 = C-14 are prevented in BR5.12, these results demonstrate that motion in these regions of the retinal is (i) necessary to form the K-like intermediate observed in the native BR-570 photocycle and (ii) not necessary to form a red-absorbing intermediate that has spectral and kinetic properties analogous to those of J-625 in the native BR photocycle. Discussions of the excited and ground electronic state assignments for the intermediate observed in the BR5.12 photoreaction are presented.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson G. H., Blanchard D., Lemaire H., Brack T. L., Hayashi H. Picosecond time-resolved fluorescence spectroscopy of K-590 in the bacteriorhodopsin photocycle. Biophys J. 1989 Feb;55(2):263–274. doi: 10.1016/S0006-3495(89)82801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Marti T., Otto H., Heyn M. P., Khorana H. G. A bacteriorhodopsin analog reconstituted with a nonisomerizable 13-trans retinal derivative displays light insensitivity. J Biol Chem. 1992 Apr 5;267(10):6757–6762. [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert J., Stefancic V., Doukas A. G., Alfano R. R., Callender R. H., Pande J., Akita H., Balogh-Nair V., Nakanishi K. Picosecond kinetic absorption and fluorescence studies of bovine rhodopsin with a fixed 11-ene. Biophys J. 1983 Sep;43(3):279–283. doi: 10.1016/S0006-3495(83)84351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Govindjee R., Ebrey T., Bagley K. A., Dollinger G., Eisenstein L., Marque J., Roder H., Vittitow J., Fang J. M. Trans/13-cis isomerization is essential for both the photocycle and proton pumping of bacteriorhodopsin. Biophys J. 1985 Apr;47(4):509–512. doi: 10.1016/S0006-3495(85)83944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney J. K., Brack T. L., Atkinson G. H. Time-resolved absorption and fluorescence from the bacteriorhodopsin photocycle in the nanosecond time regime. Biophys J. 1993 May;64(5):1512–1519. doi: 10.1016/S0006-3495(93)81520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
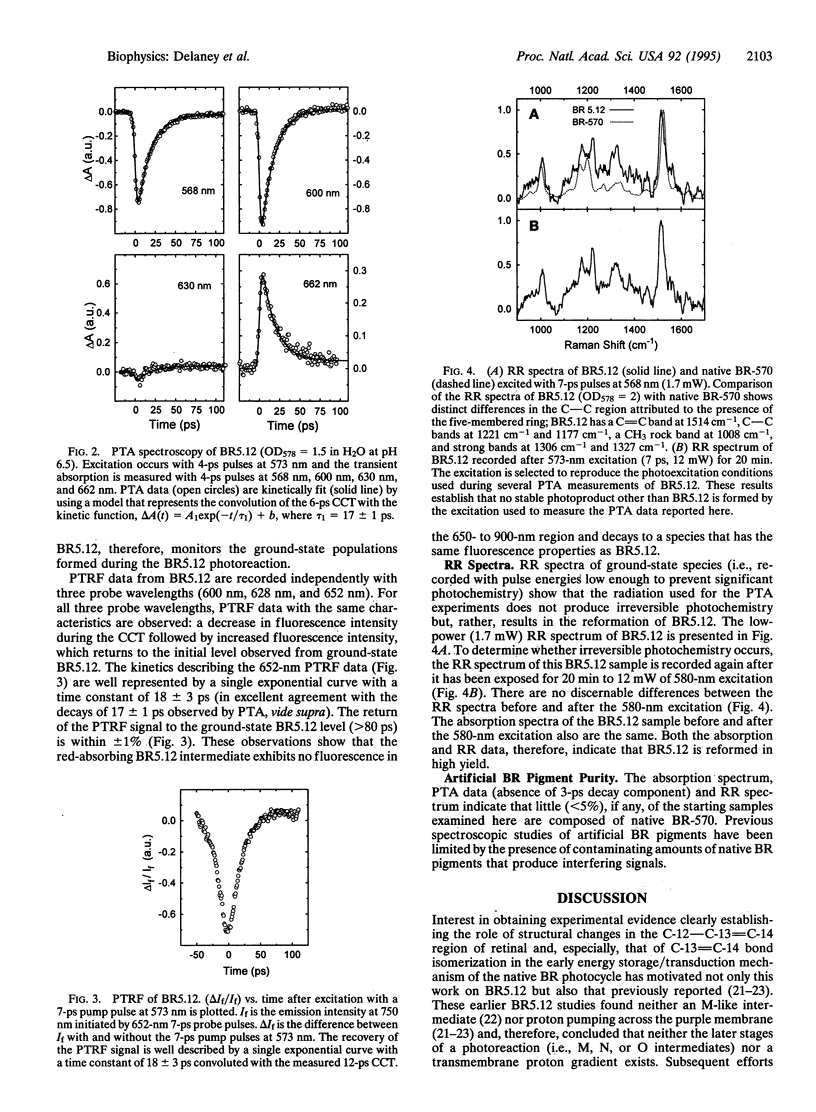
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandori H., Matuoka S., Shichida Y., Yoshizawa T., Ito M., Tsukida K., Balogh-Nair V., Nakanishi K. Mechanism of isomerization of rhodopsin studied by use of 11-cis-locked rhodopsin analogues excited with a picosecond laser pulse. Biochemistry. 1989 Jul 25;28(15):6460–6467. doi: 10.1021/bi00441a045. [DOI] [PubMed] [Google Scholar]
- Katre N. V., Wolber P. K., Stoeckenius W., Stroud R. M. Attachment site(s) of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4068–4072. doi: 10.1073/pnas.78.7.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R. A., Brito Cruz C. H., Pollard W. T., Shank C. V. Direct observation of the femtosecond excited-state cis-trans isomerization in bacteriorhodopsin. Science. 1988 May 6;240(4853):777–779. doi: 10.1126/science.3363359. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Mizukami T., Kandori H., Shichida Y., Chen A. H., Derguini F., Caldwell C. G., Biffe C. F., Nakanishi K., Yoshizawa T. Photoisomerization mechanism of the rhodopsin chromophore: picosecond photolysis of pigment containing 11-cis-locked eight-membered ring retinal. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4072–4076. doi: 10.1073/pnas.90.9.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 1989 Feb;14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Ottolenghi M., Sheves M. Synthetic retinals as probes for the binding site and photoreactions in rhodopsins. J Membr Biol. 1989 Dec;112(3):193–212. doi: 10.1007/BF01870951. [DOI] [PubMed] [Google Scholar]
- Peteanu L. A., Schoenlein R. W., Wang Q., Mathies R. A., Shank C. V. The first step in vision occurs in femtoseconds: complete blue and red spectral studies. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11762–11766. doi: 10.1073/pnas.90.24.11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polland H. J., Franz M. A., Zinth W., Kaiser W., Kölling E., Oesterhelt D. Early picosecond events in the photocycle of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):651–662. doi: 10.1016/S0006-3495(86)83692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg R., Du-Jeon-Jang, Bitting H. C., El-Sayed M. A. Subpicosecond resonance Raman spectra of the early intermediates in the photocycle of bacteriorhodopsin. Biophys J. 1990 Jul;58(1):135–141. doi: 10.1016/S0006-3495(90)82359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



