Abstract
99mTc-Tetrofosmin (99mTc-TF) and 99mTc-Sestamibi (99mTc-MIBI) are SPECT tracers that have been used for brain tumor imaging. Tumor's multidrug resistance phenotype, namely, P-glycoprotein (p-gp), and the multidrug resistance related proteins (MRPs) expression have been suggested to influence both tracers' uptake. In the present study we set out to compare 99mTc-TF and 99mTc-MIBI uptake in high-grade glioma cell lines and to investigate the influence of gliomas p-gp expression on both tracers' uptake. We used four glioma cell lines (U251MG, A172, U87MG, and T98G). The expression of p-gp protein was evaluated by flow cytometry. Twenty μCi (7.4·105 Bq) of 99mTc-TF and 99mTc-MIBI were used. The radioactivity in the cellular lysate was measured with a dose calibrator. P-gp was significantly expressed only in the U251MG cell line (P < 0.001). In all gliomas cell lines (U251MG, U87MG, A172, and T98G) the 99mTc-TF uptake was significantly higher than 99mTc-sestamibi. The U251MG cell line, in which significant p-gp expression was documented, exhibited the strongest uptake difference. 99mTc-TF uptake was higher than 99mTc-MIBI in all studied high-grade glioma cell lines. Thus, 99mTc-TF may be superior to 99mTc-MIBI for glioma imaging in vivo.
1. Introduction
99mTc-Tetrofosmin (99mTc-TF) and 99mTc-sestamibi (99mTc-MIBI) are tracers that have been used among others for brain tumor imaging [1, 2]. Both agents have been used for the differentiation of glioma recurrence from treatment induced necrosis [3, 4], neoplastic from nonneoplastic intracerebral hemorrhage [5], assessment of glioma and meningioma aggressiveness and patient's prognosis [6, 7]. Nevertheless, several studies proposed that 99mTc-TF might be superior to 99mTc-MIBI for brain tumor imaging, since the latter is influenced to a greater degree by tumor's multidrug resistance phenotype (MDR) [8–10]. MDR phenotype is associated with P-glycoprotein (p-gp) and multidrug resistance related proteins (MRPs) expression. These proteins act as membrane-efflux transporters that pump drugs out of the cancer cells and reduce intracellular concentration [11]. Both 99mTc-TF and 99mTc-MIBI are substrates of these pumps [8, 9]. In the present study we set out to compare 99mTc-TF and 99mTc-MIBI uptake in four high-grade glioma cell lines and the influence of p-gp expression on tracer uptake.
2. Material and Methods
2.1. Cell Lines
The human glioma cell lines U251MG and A172 were obtained from Dr. W. K. Alfred Yung (Department of Neuro-Oncology, M.D. Anderson Cancer Center, Houston, TX). U87MG and T98G were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). They were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Gibco, BRL) and grown at 37°C in a 5% CO2 atmosphere as described in detail elsewhere [12].
2.2. P-Glycoprotein Staining and Detection
P-gp staining was accomplished using a PE-conjugated monoclonal antibody against human p-gp (Anti-P-glycoprotein clone 15D3, Becton Dickinson Immunocytometry Systems, San Jose, USA) in accordance with standard surface staining protocols. In brief, cells were stained for 30 min in the dark at room temperature (20° to 25°C). After completion of staining, cells were washed in PBS and resuspended in 500 μL PBS. Cells were kept on ice until being analyzed by flow cytometry within 1 h. Antibody specificity was controlled by staining of all cell lines with an isotype-matched control antibody (IgG1-PE, isotype control; Becton Dickinson Immunocytometry Systems, San Jose, USA). Flow cytometric analysis of p-gp stained cells was performed on a FACSCalibur (BD Bioscience) equipped with a standard argon laser for 488 nm excitation and with 530/30 nm band pass (FL1), 585/42 nm band pass (FL2), and 670 long pass (FL3) filters. Results were evaluated using CellQuest software (Becton Dickinson, San Jose, USA).
2.3. Radioactive Tracer Experiments
99mTc-Tetrofosmin (Myoview, GE Healthcare, UK) and 99mTc-sestamibi (Stamicis, Cis bio International) were prepared according to the manufacturer instructions. The radiochemical purity of each radiotracer was greater than 95%.
2.4. Preliminary Experiments
Initially, time activity curves for all cell lines were constructed to study the optimum time for the calculation of tracer activity. More specifically, about 5 × 103 cells were platted per each 4 cm plate. At the fourth day 10 μCi (3.7·105 Bq) (200 μL) of 99mTc-Tetrofosmin was added to the medium. We used four time points (10, 30, 60, and 90 min) for incubation with the tracer and then the medium was discarded. The cells were rapidly washed three times with phosphate buffered saline (PBS) at 4°C. Cells were then treated with 0.5 mL of trypsin. When the cells had detached from the bottom of the well (within 5 min), 1 mL of DMEM was added to stop the proteolytic action. Cell clumps were removed by repeated (at least 10-fold) pipetting of the trypsin/DMEM mixture. The cells were then harvested. The radioactivity in the cellular lysate was counted ten times with a gamma scintillation counter (Wizard 2, Perkin Elmer, USA). Tracer's uptake was increased practically linearly with incubation times up to 30 min. Previous studies on 99mTc-MIBI have used an incubation period of 30 min [13, 14]. Therefore, an incubation time of 30 min was chosen in all experiments (Figure 1).
Figure 1.
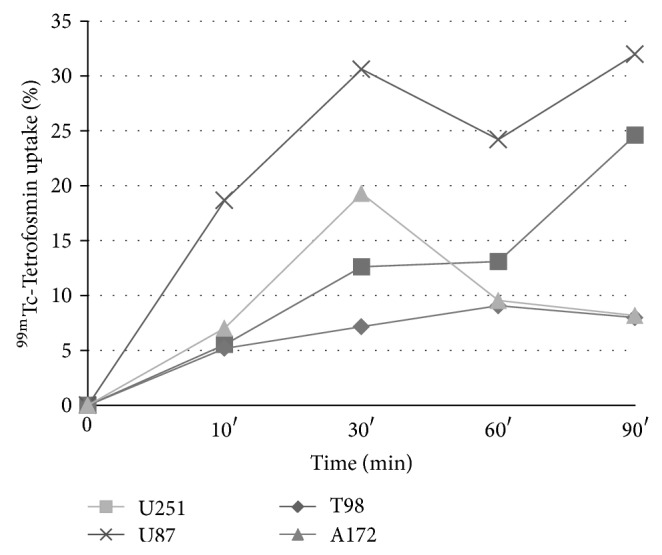
Uptake (%) of 99mTc-TF per cell type. Tracer uptake was practically linear for incubation times up to 30 min in all cell lines (lines were drawn to facilitate the eye).
2.5. Cell Kinetic Studies
About 5 × 105 cells were platted per each 10 cm plate. At the fourth day 20 μCi (7.4·105 Bq) (200 μL) of each tracer was added to the medium. After 30 min of incubation with each tracer, the medium was discarded. The cells were scraped from the dishes. The radioactivity in the cellular lysate was counted with a dose calibrator (VDC 550, Veenstra, The Netherlands) found to have linear response down to 15 kBq of technetium-99 (99mTc), the lowest studied activity The results were expressed as the percentage of the administered activity. All experiments were performed in duplicate and repeated three times.
3. Statistical Analysis
Unless otherwise stated, data are expressed as mean ± SD. The significance of differences between experimental conditions was determined using Mann-Whitney test. The Kolmogorov-Smirnov (KS) statistic, expressed as a D value, was used to compare binding of antibodies and of matched isotype controls. Differences were considered significant at P values less than 0.05.
4. Results
4.1. Study of the P-gp in Glioma Cells
P-gp was significantly expressed in the U251MG cell lines (P < 0.001) (Figure 2). On the contrary, no statistically significant p-gp expression was found in A172, U87MG and T98G cell lines.
Figure 2.

Illustration of flow cytometric and KS aspects. (a) Overlay histogram showing cell-surface P-gp protein expression in U251MG (blue line). The negative control antibody (red line) was mouse IgG1. (b) P-gp protein expression was, also, analyzed using the Kolmogorov-Smirnov (KS) statistic test (D value), which allows the objective and accurate identification of small differences in fluorescence intensity. Samples were considered positive when D ≥ 0.15.
4.2. 99mTc-Tetrofosmin versus99mTc-Sestamibi
The 99mTc-TF uptake, ranging between ~21% and 22% in the four studied cell lines (Table 1), was higher than 99mTc-MIBI in the four studied glioma cell lines (16% to 18%). In U251MG glioma cell the percentage of 99mTc-Tetrofosmin uptake was significantly higher than that of 99mTc-sestamibi (21.0 ± 0.4% versus 16.7 ± 0.9%, P < 0.0001). In U87MG there was also significant higher 99mTc-Tetrofosmin uptake (22.15 ± 1% versus 16.1 ± 1.9%, P = 0.002). In A172 cell line the difference was also statistically significant (21.4 ± 1.3% versus 18.25 ± 0.8%, P = 0.0017). In T98G there was higher 99mTc-Tetrofosmin uptake compared to 99mTc-sestamibi and the difference was also statistically significant (22.1 ± 1.6% versus 17.6 ± 0.95%, P = 0.0002).
Table 1.
Tracer uptake 30 min postincubation in four glioma cell lines.
| Cell line | 99mTc-Tetrofosmin | 99mTc-Sestamibi | P value |
|---|---|---|---|
| A172 | 21.4 ± 1.3% | 18.25 ± 0.8% | 0.0017 |
| U87MG | 22.15 ± 1.0% | 16.1 ± 1.9% | 0.002 |
| U251MG | 21.0 ± 0.4% | 16.7 ± 0.9% | <0.0001 |
| T98G | 22.1 ± 1.6% | 17.6 ± 0.95% | 0.0002 |
5. Discussion
The present study compared the uptake in four high-grade glioma cell lines of the monovalent lipophilic cationic diphosphine TF labeled with 99mTc to that of 99mTc-MIBI. The results showed higher 99mTc-TF uptake, thus suggesting that 99mTc-TF could be superior to 99mTc-MIBI for glioma imaging. Apart from U251MG cell line, in which significant p-gp expression was documented, significant uptake difference was found in the other cell lines suggesting that other mechanisms may be implicated apart from p-gp expression.
99mTc labeled compounds have been proven advantageous in tumor imaging over 201Tl due to higher number of photons of appropriate energy emitted by the human body per administered activity and lower radiation burden to both patient and members of the general public [15]. Regarding the mechanism of tracer uptake, 99mTc-MIBI diffuses passively through the cell membrane and an estimated 95% of intracellular 99mTc-MIBI is localized in mitochondria because of the negatively charged mitochondrial membrane. 99mTc-TF enters viable cells mainly via passive transport, driven by the negative electric potential of the intact cell membrane, and it mostly localizes within the cytosol, with only a small fraction passing into the mitochondria [15].
Chemoresistance is a major obstacle for effective cancer treatment and can be present in a tumor at the time of initial diagnosis or can develop following treatment with chemotherapeutic agents [16]. One mechanism involved is the presence of a multidrug resistance phenotype from the tumor cells. Various genes have been implicated such as MDR1, MRPs, major vault protein (MVP) gene, the MGMT gene, and the Survivin gene [17]. The previous genes can produce resistance to a diverse range of drugs such as vincristine, temozolomide, etoposide, and cisplatin, whereas certain radiotracers are also substrate [11, 16, 17]. The MDR1 gene is the most extensively studied [18]. This gene encodes a transmembrane p-glycoprotein that produces a broad pattern of resistance to several structurally and functionally unrelated drugs by expelling them out of the cells. Consequently, it reduces the intracellular drug concentration. P-gp is an important functional component of the blood-brain barrier [18]. MRPs are members of the ABC superfamily of transmembrane proteins that act as ATP-dependent drug efflux pumps and so far nine MRP members have been identified [3]. MRPs have been reported to confer resistance to various anticancer drugs and, in gliomas, MRP1, MRP3, MRP4, and MRP5 have been reported to be expressed more than the other members [19, 20].
Perek et al. studied the effect of glutathione (GSH) depletion on the chemosensitivity of human malignant glioma cell lines [21]. None of the glioma cell lines used by the authors were p-gp positives but were found to overexpress the MRP1 protein. In glioma cells, the multidrug resistance proteins are usually involved rather than P-gp, which is usually expressed in vessels. The authors found that both Tetrofosmin and MIBI are substrates of p-gp and MRP1; however both tracers did not follow the expected behavior of a MDR in all cases suggesting the presence of other mechanisms. In addition, 99mTc-TF was more lipophilic than MIBI and thus could enter easier than MIBI in glioma cells [21].
In the present study U251MG cell line exhibited increased p-gp expression, in accordance with published data [22]. Similar to Perek et al. [21], we believe that, apart from p-gp, other mechanisms may also exist that influence tracer uptake. These mechanisms might explain the difference in tracer uptake in the remaining glioma cell lines that did not exhibit significant p-gp expression. In a previous study we investigated the MRP5 expression which can be normally found in the astrocytes of the subcortical white matter and in the pyramidal neurons [10]. In glioma patients we found that 99mTc-TF uptake was not related to the MRP5 immunohistochemical expression; consequently, this might be another reason explaining the higher 99mTc-TF uptake in the studied glioma cell lines.
A limitation of the present study was the absence of positive and negative control cells lines in order to define MDR protein expression. Thus, we cannot rule out the existence of an experimental bias regarding the uptake of both radiotracers that could influence their relationship with P-gp protein expression. In conclusion, the present study showed that 99mTc-TF uptake is higher than that of 99mTc-MIBI in all high-grade glioma cell lines studied. Thus, 99mTc-TF is anticipated to be a superior tracer to 99mTc-MIBI for gliomas imaging in vivo. Further studies are needed in order to elucidate the exact mechanisms involved in 99mTc-TF uptake.
Acknowledgment
This work was supported by a grant from the Joseph and Esther Gani Foundation.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Schillaci O., Spanu A., Madeddu G. [99mTc]sestamibi and [99mTc]tetrofosmin in oncology: SPET and fusion imaging in lung cancer, malignant lymphomas and brain tumors. The Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2005;49(2):133–144. [PubMed] [Google Scholar]
- 2.Choi J. Y., Kim S. E., Shin H. J., Kim B.-T., Kim J. H. Brain tumor imaging with 99mTc-tetrofosmin: comparison with 201Tl, 99mTc-MIBI, and 18F-fluorodeoxyglucose. Journal of Neuro-Oncology. 2000;46(1):63–70. doi: 10.1023/A:1006391701818. [DOI] [PubMed] [Google Scholar]
- 3.Le Jeune F. P., Dubois F., Blond S., Steinling M. Sestamibi technetium-99m brain single-photon emission computed tomography to identify recurrent glioma in adults: 201 studies. Journal of Neuro-Oncology. 2006;77(2):177–183. doi: 10.1007/s11060-005-9018-8. [DOI] [PubMed] [Google Scholar]
- 4.Alexiou G. A., Fotopoulos A. D., Papadopoulos A., Kyritsis A. P., Polyzoidis K. S., Tsiouris S. Evaluation of brain tumor recurrence by 99mTc-tetrofosmin SPECT: a prospective pilot study. Annals of Nuclear Medicine. 2007;21(5):293–298. doi: 10.1007/s12149-007-0027-x. [DOI] [PubMed] [Google Scholar]
- 5.Minutoli F., Angileri F. F., Cosentino S., Pecorella G. R., Cardali S., De Divitiis O., Germanò A., Baldari S. 99mTc-MIBI SPECT in distinguishing neoplastic from nonneoplastic intracerebral hematoma. Journal of Nuclear Medicine. 2003;44(10):1566–1573. [PubMed] [Google Scholar]
- 6.Nagamachi S., Jinnouchi S., Nabeshima K., Nishii R., Flores L., II, Kodama T., Kawai K., Tamura S., Yokogami K., Samejima T., Wakisaka S. The correlation between 99mTc-MIBI uptake and MIB-1 as a nuclear proliferation marker in glioma—a comparative study with 201TI. Neuroradiology. 2001;43(12):1023–1030. doi: 10.1007/s002340100629. [DOI] [PubMed] [Google Scholar]
- 7.Alexiou G. A., Tsiouris S., Kyritsis A. P., Fotakopoulos G., Goussia A., Voulgaris S., Fotopoulos A. D. The value of 99mTc-tetrofosmin brain SPECT in predicting survival in patients with glioblastoma multiforme. Journal of Nuclear Medicine. 2010;51(12):1923–1926. doi: 10.2967/jnumed.110.080929. [DOI] [PubMed] [Google Scholar]
- 8.Le Jeune N., Perek N., Denoyer D., Dubois F. Influence of glutathione depletion on plasma membrane cholesterol esterification and on Tc-99m-sestamibi and Tc-99m-tetrofosmin uptakes: a comparative study in sensitive U-87-MG and multidrug-resistant MRP1 human glioma cells. Cancer Biotherapy and Radiopharmaceuticals. 2004;19(4):411–421. doi: 10.1089/cbr.2004.19.411. [DOI] [PubMed] [Google Scholar]
- 9.Le Jeune N., Perek N., Denoyer D., Dubois F. Study of monoglutathionyl conjugates TC-99M-sestamibi and TC-99M-tetrofosmin transport mediated by the multidrug resistance-associated protein isoform 1 in glioma cells. Cancer Biotherapy & Radiopharmaceuticals. 2005;20(3):249–259. doi: 10.1089/cbr.2005.20.249. [DOI] [PubMed] [Google Scholar]
- 10.Alexiou G. A., Goussia A., Kyritsis A. P., Tsiouris S., Ntoulia A., Malamou-Mitsi V., Voulgaris S., Fotopoulos A. D. Influence of glioma's multidrug resistance phenotype on 99mTc-tetrofosmin uptake. Molecular Imaging and Biology. 2011;13(2):348–351. doi: 10.1007/s11307-010-0369-y. [DOI] [PubMed] [Google Scholar]
- 11.Stacy A. E., Jansson P. J., Richardson D. R. Molecular pharmacology of ABCG2 and its role in chemoresistance. Molecular Pharmacology. 2013;84(5):655–669. doi: 10.1124/mol.113.088609. [DOI] [PubMed] [Google Scholar]
- 12.Tsamis K. I., Alexiou G. A., Vartholomatos E., Kyritsis A. P. Combination treatment for glioblastoma cells with tumor necrosis factor-related apoptosis-inducing ligand and oncolytic adenovirus delta-24. Cancer Investigation. 2013;31(9):630–638. doi: 10.3109/07357907.2013.849724. [DOI] [PubMed] [Google Scholar]
- 13.Luker G. D., Flagg T. P., Sha Q., Luker K. E., Pica C. M., Nichols C. G., Piwnica-Worms D. MDR1 P-glycoprotein reduces influx of substrates without affecting membrane potential. Journal of Biological Chemistry. 2001;276(52):49053–49060. doi: 10.1074/jbc.M105192200. [DOI] [PubMed] [Google Scholar]
- 14.Kinuya S., Bai J., Shiba K., Yokoyama K., Mori H., Fukuoka M., Watanabe N., Shuke N., Michigishi T., Tonami N. 99mTc-sestamibi to monitor treatment with antisense oligodeoxynucleotide complementary to MRP mRNA in human breast cancer cells. Annals of Nuclear Medicine. 2006;20(1):29–34. doi: 10.1007/BF02985587. [DOI] [PubMed] [Google Scholar]
- 15.Fukumoto M. Single-photon agents for tumor imaging: 201Tl, 99mTc-MIBI, and 99mTc-tetrofosmin. Annals of Nuclear Medicine. 2004;18(2):79–95. doi: 10.1007/BF02985098. [DOI] [PubMed] [Google Scholar]
- 16.Spiegl-Kreinecker S., Buchroithner J., Elbling L., Steiner E., Wurm G., Bodenteich A., Fischer J., Micksche M., Berger W. Expression and functional activity of the ABC-transporter proteins P-glycoprotein and multidrug-resistance protein 1 in human brain tumor cells and astrocytes. Journal of Neuro-Oncology. 2002;57(1):27–36. doi: 10.1023/A:1015735815111. [DOI] [PubMed] [Google Scholar]
- 17.Tews D. S., Fleissner C., Tiziani B., Gaumann A. K. A. Intrinsic expression of drug resistance-associated factors in meningiomas. Applied Immunohistochemistry and Molecular Morphology. 2001;9(3):242–249. doi: 10.1097/00022744-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Demeule M., Régina A., Jodoin J., Laplante A., Dagenais C., Berthelet F., Moghrabi A., Béliveau R. Drug transport to the brain: Key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascular Pharmacology. 2002;38(6):339–348. doi: 10.1016/S1537-1891(02)00201-X. [DOI] [PubMed] [Google Scholar]
- 19.Bronger H., König J., Kopplow K., Steiner H.-H., Ahmadi R., Herold-Mende C., Keppler D., Nies A. T. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Research. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 20.Kuan C.-T., Wakiya K., Herndon J. E., II, Lipp E. S., Pegram C. N., Riggins G. J., Rasheed A., Szafranski S. E., McLendon R. E., Wikstrand C. J., Bigner D. D. MRP3: a molecular target for human glioblastoma multiforme immunotherapy. BMC Cancer. 2010;10, article 468 doi: 10.1186/1471-2407-10-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perek N., Koumanov F., Denoyer D., Boudard D., Dubois F. Modulation of the multidrug resistance of glioma by glutathione levels depletion—interaction with TC-99M-Sestamibi and TC-99M-Tetrofosmin. Cancer Biotherapy and Radiopharmaceuticals. 2002;17(3):291–302. doi: 10.1089/10849780260179251. [DOI] [PubMed] [Google Scholar]
- 22.Kolchinsky A., Roninson I. B. Drug resistance conferred by MDR1 expression in spheroids formed by glioblastoma cell lines. Anticancer Research. 1997;17(5 A):3321–3327. [PubMed] [Google Scholar]


