Abstract
Pseudouridines (Ψs) are the most abundant and highly conserved modified nucleotides found in various stable RNAs of all organisms. Most Ψs are clustered in regions that are functionally important for pre-mRNA splicing. Ψ has an extra hydrogen bond donor that endows RNA molecules with distinct properties that contribute significantly to RNA-mediated cellular processes. Experimental data indicate that spliceosomal snRNA pseudouridylation can be catalyzed by both RNA-dependent and RNA-independent mechanisms. Recent work has also demonstrated that pseudouridylation can be induced at novel positions under stress conditions, suggesting a regulatory role for Ψ.
Keywords: Pre-mRNA splicing, U2 snRNA, Box H/ACA ribonucleoprotein, Pseudouridine, Induced RNA modification
Core tip: Pseudouridines (Ψs) are the most abundant and highly conserved modified nucleotides identified in various stable RNAs of all organisms. Most Ψs are clustered in regions that are functionally important for pre-mRNA splicing. Ψ has an extra hydrogen bond donor that endows RNA molecules with distinct properties that contribute significantly to RNA-mediated cellular processes. Experimental data indicate that spliceosomal snRNA pseudouridylation can be catalyzed by both RNA-dependent and RNA-independent mechanisms. Recent work has also demonstrated that pseudouridylation can be induced at novel positions under stress conditions, suggesting a regulatory role for Ψ.
INTRODUCTION
Many eukaryotic genes consist of blocks of coding sequences (exons) separated by blocks of noncoding sequences, termed introns[1]. Introns are removed from a primary transcript (pre-mRNA) by a process called pre-mRNA splicing. This process is carried out by a huge complex called the spliceosome, which comprises about 300 proteins and 5 small RNAs[2]. The five small RNAs are uridine-rich, and are thus called U small nuclear RNAs (U1, U2, U4, U5 and U6 snRNAs) (Figure 1)[3,4]. In eukaryotic cells, U snRNAs exist as RNA-protein complexes called small nuclear ribonucleoproteins (snRNPs). During spliceosome assembly, snRNPs are sequentially recruited onto a pre-mRNA substrate, resulting in the formation of several short stretches of RNA-RNA duplexes that play key roles in recognizing, specifying and catalyzing the two successive chemical reactions (Figure 2)[5-9].
Figure 1.
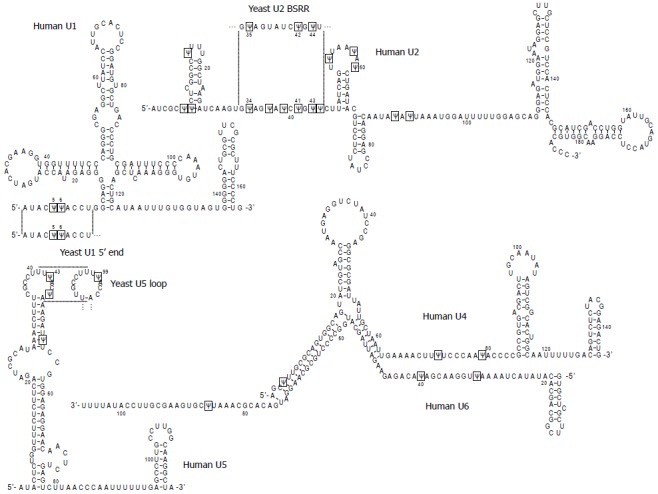
Primary sequences and secondary structures of human spliceosomal snRNAs (U1, U2, U4, U5 and U6). Pseudouridines (Ψ) are boxed. The sequences of yeast snRNAs where the Ψs have their counterparts in human snRNAs (the 5’ end region of U1, branch site recognition region (BSRR) of U2, and loop region of U5) are also shown. The structures are predicted by the “multifold” program and are consistent with the genetic/biochemical mapping data.
Figure 2.
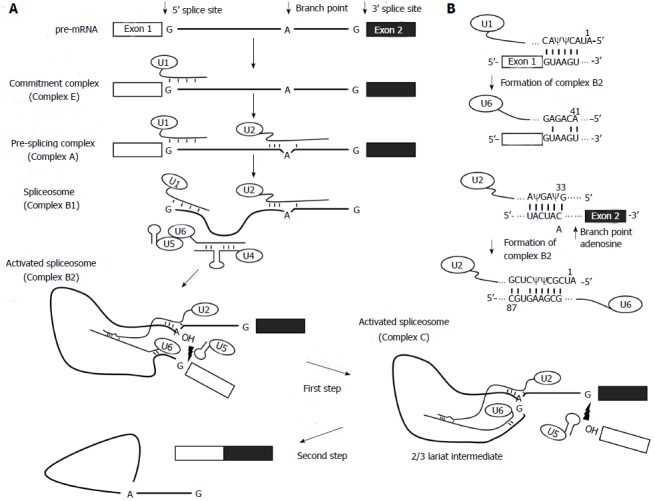
The splicing reaction. A: Steps of the spliceosome-mediated splicing reaction. The thick lines represent the intron and the boxes are exons. The short lines between RNA strands represent watson-crick base-pairing interactions. The 2'-OH groups of branch point adenosine and the cut-off 5' exon are pictured in the activated spliceosomes. The lightning symbols depict a nucleophilic attack that causes a transesterification reaction; B: Putative RNA-RNA hybrids formed during the splicing reaction.
First, the 5’ splice site is recognized by the U1 snRNP through base pairing interactions[10-12], resulting in the formation of the commitment complex or the early (E) complex. Second, U2 snRNP binds, again via base-pairing interactions, to the branch site, and forms a pre-splicing complex, namely complex A[7,13,14]. Base-pairing between the U2 snRNA and the branch site bulges out the branch point nucleotide (typically an adenosine residue), which is thus made available for the first chemical reaction[15-17] (see below). Next, the U4/U6.U5 tri-snRNP particle, in which U4 and U6 are extensively base-paired with each other, joins the A complex, leading to the formation of complex B1[18-21]. Subsequently, a series of RNA-RNA interaction rearrangements occur, resulting in the release of U1 and U4 snRNPs and hence the formation of complex B2 or the spliceosome. In the newly formed spliceosome, U5 contacts the splice sites and U6 base-pairs with both the 5’ splice site and U2[22], thus forming the active site for the first chemical reaction, in which the 2’-OH group of the bulged out branch nucleotide adenosine attacks the 5’ splice site. This generates the 5’ exon and the 2/3 lariat intermediate. Immediately after the first chemical reaction, additional conformational changes occur, leading to the formation of complex C and the initiation of the second chemical reaction, where the liberated 3’-OH of the 5’ exon attacks the phosphate group of the 3’ splice site. The second chemical reaction results in the release of the lariat intron as well as the ligation of the exons (mRNA) (Figure 2)[2,23]. Finally, the mRNA product is released, and the U2, U5, and U6 snRNPs are disassembled and recycled for further rounds of pre-mRNA splicing[24,25].
It is notable that all five spliceosomal snRNAs are extensively posttranscriptionally modified[26-29]. Pseudouridine (Ψ), the C5-glycoside isomer of uridine, is the most abundant in these RNAs. For example, there are 14 Ψs out of 189 nucleotides of vertebrate U2 snRNA, accounting for approximately 60% of the total modifications and approximately 7% of the total nucleotides[30-32]. Strikingly, the majority of the Ψs are present in regions that are functionally important for pre-mRNA splicing, including the regions involved in RNA-RNA interactions in the splicing complexes/spliceosome (Figure 1).
Because of its unique structural and chemical properties and its proven biological importance, Ψ has begun to receive increasing research attention. However, due to the difficulty of developing effective assays and experimental systems, there had been little progress in research on RNA pseudouridylation until fairly recently. In the past approximately 17 years, however, several laboratories have made several remarkable discoveries[33-38] underscoring the notion that Ψs in U snRNAs are not just bystanders in the process of pre-mRNA splicing, but that they are active participants in spliceosome assembly and splicing. This review discusses the mechanisms and functions of spliceosomal snRNA pseudouridylation, focusing on the most extensively studied U2 snRNA.
ΨS ARE IMPORTANT FOR RNA FUNCTION
Ψs are abundant, conserved, and reside in important regions of snRNAs
Ψ was first detected as an unknown nucleotide more than 60 years ago[39] and soon afterward it was identified as 5-ribosyluracil, an isomer of uridine (1-ribosyluracil)[40]. Since its discovery, Ψ has been found in various stable RNAs (including rRNAs[41-45], tRNAs[46-49], and snRNAs[26-31,50]) of all organisms, and now it has been known as the most abundant modified nucleotide. Besides being abundant, Ψs are highly conserved across species especially in functionally important regions of snRNAs. For example, both vertebrate and yeast U1 snRNAs contain two Ψs at the 5’ end region (Ψ5 and Ψ6) known to recognize and base-pair with the 5’ splice site during spliceosome assembly (Figure 1 and Table 1). Three of the six Ψs (Ψ34, Ψ41 and Ψ43) in the vertebrate U2 branch site recognition region (BSRR), which is involved in base-pairing with the pre-mRNA branch site, have their counterparts in yeast U2 (corresponding to Ψ35, Ψ42, and Ψ44, respectively). Likewise, one of the Ψs (Ψ43) in the conserved loop of vertebrate U5, which participates in interacting with the 5’ and 3’ exon sequences, is also present in yeast U5 snRNA at the equivalent site (Ψ99). Ψs are also found in the U4-U6 duplex regions as well as in other regions of U6 that are important for function. In other instances, some of these Ψs are conserved in plant snRNAs[51] and in minor-class snRNAs (U4atac and U12)[52]. The phylogenetic conservation as well as the strategic location of Ψs clearly suggests that they play functionally important roles in pre-mRNA spllicing.
Table 1.
Pseudouridylation sites within yeast and human spliceosomal snRNAs
| Organism | snRNA | Position | Catalyst | Ref. |
| Yeast | U1 | Ψ5 | Cbf5 | [52], Unpublished data Yu Lab |
| Ψ6 | Cbf5 | [52], Unpublished data Yu Lab | ||
| U2 | Ψ35 | Pus7 | [52,70] | |
| Ψ42 | H/ACA RNP (snR81) | [52,83] | ||
| Ψ44 | Pus1 | [52] | ||
| (Ψ56; induced) | Pus7 | [87] | ||
| (Ψ93; induced) | H/ACA RNP (snR81) | [87] | ||
| U5 | Ψ99 | NR | [52] | |
| Human | U1 | Ψ5 | H/ACA RNP (ACA47) | [81, 88] |
| Ψ6 | H/ACA RNP (U109) | [88,89] | ||
| U2 | Ψ6 | NR | ||
| Ψ7 | H/ACA RNP (U100) | [43,79] | ||
| Ψ15 | NR | |||
| Ψ34 | H/ACA RNP (U92) | [90, 91] | ||
| Ψ37 | H/ACA RNP (ACA45) | [81,91] | ||
| Ψ39 | H/ACA RNP (ACA26) | [81,91] | ||
| Ψ41 | H/ACA RNP (ACA45) | [81,91] | ||
| Ψ43 | NR | |||
| Ψ44 | H/ACA RNP (U92) | [90,91] | ||
| Ψ54 | H/ACA RNP (U93) | [30,43,91,92] | ||
| Ψ58 | NR | |||
| Ψ60 | NR | [93] | ||
| Ψ89 | H/ACA RNP (ACA35) | [81,91] | ||
| Ψ91 | NR | |||
| U4 | Ψ4 | NR | ||
| Ψ72 | NR | |||
| Ψ79 | NR | |||
| U5 | Ψ43 | H/ACA RNP (ACA57) | [81,94] | |
| Ψ46 | H/ACA RNP (U85) | [80,94] | ||
| Ψ53 | H/ACA RNP (U93) | [30,91,92] | ||
| U6 | Ψ31 | H/ACA RNP (ACA65) | [43] | |
| Ψ40 | H/ACA RNP (ACA12) | [79,81,95] | ||
| Ψ86 | H/ACA RNP (ACA65) | [79] | ||
| U12 | Ψ19 | H/ACA RNP (ACA68) | [43,96] | |
| Ψ28 | H/ACA RNP (ACA66) | [43,96] | ||
| U4atac | Ψ12 | NR | [96] | |
| U6atac | Ψ83 | NR | [96] |
Characteristics of Ψ
Ψ is converted from its isomer, uridine (U) (Figure 3). First, the glycosidic bond of U (N1-C1’) is broken. The uracil base then rotates 180° along the N3-C6 axis, allowing the formation of a new carbon-carbon (C5-C1’) bond between the base and the sugar[53,54]. As a result, the modified uridine, or Ψ, has an extra hydrogen bond donor at its non Watson-Crick edge, which can interact with its own phosphate backbone to form a rigid RNA structure[55-61]. Ψ can also contribute to stabilization of an RNA chain or an RNA-RNA interaction through alteration of RNA local structure or through enhancement of base stacking[62]. In this regard, it is reported that the Ψ-A pair is more stable than the U-A pair[63,64]. Thus, U-to-Ψ conversion endows the modified uridine (Ψ) with chemical properties that are distinct from those of uridine and all other known nucleotides.
Figure 3.
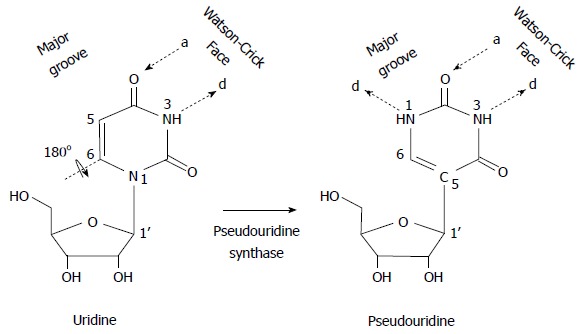
Schematic representation of uridine-to- pseudouridine isomerization. Pseudouridine is a rotational isomer of uridine, in which the N-C glycosidic bond is broken to form the C-C bond. This results in the creation of an extra hydrogen bond donor (d), while the number of hydrogen bond acceptors (a) is unchanged.
Given that they are phylogenetically conserved, that they are clustered in functional regions, and that they have distinct chemical properties, Ψs are expected to affect the function of the RNA in which they reside. Extensive researches carried out over the past 15 years have indeed demonstrated that Ψs have the potential to impact numerous aspects of RNA biology, including structure, thermal stability, and biochemical interactions. Below, we discuss the function of U2 snRNA pseudouridylation.
FUNCTIONS OF U2 SNRNA PSEUDOURIDYLATION
U2 snRNA contains the most Ψs among all known snRNAs (e.g., human U2 snRNA contains 13 Ψs), and for this reason, U2 snRNA pseudouridylation has been the most extensively studied. Three experimental systems have been fairly extensively used to study the function of U2 pseudouridylation, and they are discussed below.
The mammalian cell-free system
Over 20 years ago, Jeffery Patton carried out the first functional analysis of U2 snRNA modification[65,66]. He found that in vitro synthesized U2 snRNA could be efficiently pseudouridylated in HeLa cell S100 extracts[66]. He also demonstrated that the incorporation of 5-fluorouridine (5FU) into U2 snRNA site-specifically blocked U2 snRNA pseudouridylation and that the 5FU-containing U2 snRNP (free of Ψs) is more prone to salt-induced dissociation when compared with U2 snRNP containing regular nucleotides (pseudouridylated)[66]. These results suggested that U2 snRNA lacking Ψs was disadvantaged in snRNP assembly, implying that Ψs contribute to snRNP biogenesis.
A decade later (in 2004), the Lührmann group provided direct experimental evidence for the functional importance of U2 snRNA pseudouridylation in pre-mRNA splicing[67]. In this study, they depleted endogenous U2 snRNP from splicing extracts derived from HeLa cells using affinity selection with oligonucleotides complementary to U2 snRNA. Then they reconstituted the U2 snRNP in vitro using synthesized U2 snRNA. The reconstituted U2 snRNP was added to the U2-depleted extracts, and its ability to support pre-mRNA splicing was then assayed. Their results indicated that the three Ψs located within the 5’ end region (Ψ6, Ψ7 and Ψ15) exhibited cumulative effects on U2 function. Specifically, they are required for the E complex formation. Together, the data obtained from mammalian in vitro systems have clearly suggested that Ψs in U2 snRNA play important roles in snRNP biogenesis and pre-mRNA splicing.
The Xenopus oocyte reconstitution system
A more detailed and systematic analysis of the effects of U2 snRNA pseudouridylation on pre-mRNA splicing was conducted in Xenopus oocytes[35,68,69]. In this experimental system, an endogenous snRNA can be specifically and nearly completely depleted upon injection of an antisense DNA oligonucleotide. Specifically, the DNA oligonucleotide, once injected, forms a duplex with its target snRNA, thus triggering an endogenous RNase H activity, which degrades the snRNA (the RNA strand of the RNA-DNA hybrid). Four hours later, the injected DNA oligonucleotide itself is degraded by an endogenous DNase activity. Following the depletion of the endogenous snRNA, exogenously-derived snRNAs can then be injected, allowing an accurate measurement of capabilities of these injected snRNAs in restoring functional snRNP and pre-mRNA splicing activity.
To examine U2 pseudouridylation, an antisense U2 DNA oligonucleotide is injected. After the endogenous U2 snRNA is depleted, in vitro transcribed U2 (unmodified), cellular U2 (completely modified), or chimeric U2 snRNAs (partially modified) are injected into the U2 snRNA-depeleted Xenopus oocytes. After a short period of reconstitution (approximately 3.5 h), snRNP biogenesis and pre-mRNA splicing activity are analyzed.
Using this system, Yu et al[35] demonstrated that while unmodified in vitro transcribed U2 snRNA was unable to rescue splicing in U2 snRNA-depleted oocytes, cellularly-derived (modified) U2 effectively restored splicing activity, suggesting that U2 modifications, including many pseudouridines, are functionally important for splicing. Using chimeric U2 snRNAs derived from the combination of cellular (modified) and in vitro transcribed U2 (unmodified), Yu et al[35] further dissected U2 modifications and identified the Ψs that reside within the 5’ end region of U2 to be important for splicing. Using anti-snRNP immunoprecipitation and glycerol gradient sedimentation, they also demonstrated that unmodified U2 snRNA was unable to form functional 17S snRNP, and that, consequently, U2 snRNA lacking Ψ was unable to participate in spliceosome assembly.
Surprisingly, however, while modifications in the 5’ end region of U2 snRNA were shown to be required for both snRNP biogenesis and pre-mRNA splicing, the six Ψs in the BSRR (nucleotides 33-46) were not identified as functionally significant under these conditions[35]. Zhao et al[68] later found that Ψ formation occurred much faster in the BSRR than in the 5’ region of U2 snRNA. Indeed, soon after it was injected into the nuclei of Xenopus oocytes, in vitro transcribed U2 became pseudouridylated in the BSRR although pseudouridylation had not yet occurred in the 5’ end region, thus suggesting that the functionality of the Ψs in the U2 BSRR cannot be analyzed under these conditions. To overcome this problem, Zhao et al[69] employed 5FU-containing U2 to site-specifically inhibit Ψ formation in the U2 BSRR. Somewhat expectedly, U2 snRNAs lacking Ψs only in the BSRR failed to support pre-mRNA splicing. Taken together, these results indicate that virtually all Ψs tested in the Xenopus oocyte system are required for snRNP biogenesis and pre-mRNA splicing.
The yeast genetic system
The yeast system has also been used to study U2 pseudouridylation. There are a total of three Ψs (Ψ35, Ψ42 and Ψ44) in Saccharomyces cerevisiae (S. cerevisiae) U2, all of which are located in the BSRR (Figure 1). About a decade ago, all three pseudouridylases responsible for the formation of the three Ψs in yeast U2 were identified (see below), making it possible to carry out genetic experiments to analyze the function of yeast U2 pseudouridylation.
Pus7 catalyzes the formation of Ψ35, which interacts with the nucleotide next to the pre-mRNA branch point adenosine during pre-mRNA splicing. Interestingly, the pus7 deletion strain, although still viable, displayed reduced growth rates under conditions of high salt or when grown in competition with wild-type yeast strains[70] (unpublished data). To examine the functional role of Ψ35 in more detail, Yang et al[37] used a synthetic lethal screen, and found that, interestingly, a combination of pus7 deletion (loss of Ψ35) and a U2 point mutation at position 40 (U40G or U40∆) resulted in a temperature-sensitive growth defect phenotype. They further demonstrated that pre-mRNA accumulated in the mutant strain under restrictive conditions, indicating that Ψ35 in the U2 BSRR contributes to pre-mRNA splicing in S. cerevisiae.
Recently, Pus1 and snR81 (pseudouridylases responsible for the formation of Ψ44 and Ψ42, respectively) were also deleted, either individually or in combination, from the yeast genome. The resulting strains were tested for their ability to support pre-mRNA splicing. These mutant strains exhibited splicing-deficient phenotype (Wu and Yu, unpublished data). Taken together, the data generated thus far strongly suggest that all three Ψs within yeast U2 snRNA play a role in pre-mRNA splicing. These results are consistent with the results obtained from the Xenopus oocyte microinjection system (see above).
Structural analyses
In recent years, various biophysical techniques have been used to investigate the structural aspects of U2 snRNA pseudouridylation. Specifically, in 2001, Berglund et al[71] solved, at 2.18-Å resolution, a crystal structure of a self-complementary RNA modeled after the yeast U2 snRNA-branch site duplex in the absence of Ψ. Surprisingly, the adenosine adjacent to the expected branch point adenosine was bulged out, despite the fact that, in mammalian cell extracts, either of these adenosines was able to serve as the nucleophile that attacks the 5’ splice site during pre-mRNA splicing. Subsequently, Newby and Greenbaum determined solution NMR structures of the yeast U2 snRNA-branch site duplex with or without Ψ35 in the U2 strand[60,62]. They showed that the presence of the Ψ35 in the U2 strand induced a structural change where the branch point adenine base bulged out of the duplex and the nucleophile (the 2’-OH of the adenosine) was placed in an accessible position for the first step of splicing[60].
More recently, Lin et al[72] reported the 1.57-Å resolution crystal structure of the U2 snRNA-branch site duplex in the presence of Ψ35 in the U2 strand. They observed an extra-helical branch point adenosine in which its 2’-OH was prominently exposed and available for attack on the 5’ splice site. Thus, biophysical data have provided detailed structural information indicating that Ψ35 is somehow capable of altering the structure of the duplex, thereby making the 2’-OH group of the branch-point adenosine available for the first step of splicing.
MECHANISMS OF SPLICEOSOMAL SNRNA PSEUROURIDYLATION
Box H/ACA RNP-catalyzed (RNA-dependent) mechanism
In 1997, the Ni et al[73] and Ganot et al[74] demonstrated that box H/ACA RNAs, one of the two major families of small nucleolar RNAs, function as guide RNAs that direct site-specific synthesis of Ψ in rRNA. Box H/ACA RNAs exist in the cell as RNA-protein complexes (box H/ACA snRNPs). Each of the complexes consists of one unique box H/ACA RNA and four common core proteins, Cbf5 (NAP57 or Dyskerin in mammals/humans), Nhp2, Gar1, and Nop10. Each box H/ACA RNA forms a conserved hairpin-hinge-hairpin-tail structure, including a conserved H box in the hinge region and a conserved ACA box in the tail region (Figure 4). Each of the two hairpins in the box H/ACA RNA contains an internal loop (pseudouridylation pocket), which serves as a guide that base pairs with the target RNA to place the target uridine precisely at the base of the upper stem where Cbf5, a catalytic component of box H/ACA RNP, catalyzes the U-to-Ψ conversion[75,76] (Figure 4).
Figure 4.
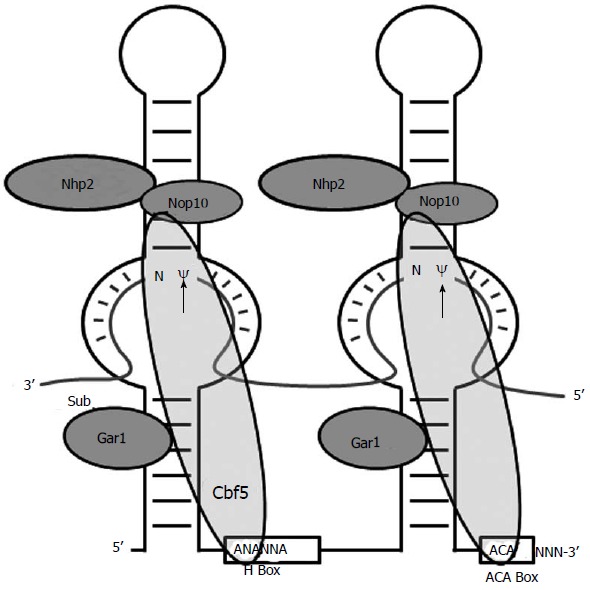
Schematic depiction of box H/ACA RNA. The core components of a box H/ACA RNP, a box H/ACA RNA and four proteins (Nhp2, Nop 10, Gar1 and Cbf5), are shown. An RNA substrate paired with the two internal loops of the box H/ACA RNA is also shown. The arrows indicate the target nucleotides for pseudouridylation. The H box (5'-ANANNA-3') and ACA box (5'-ACA-3') are indicated.
The discovery of the mechanism of box H/ACA RNA-guided rRNA pseudouridylation generated great interest in searching for additional box H/ACA RNAs. Both computational methods and experimental approaches were developed, resulting in the discovery of hundreds of new box H/ACA RNAs in several different organisms[43,77-79]. Interestingly, a number of guide sequences exhibited complementarity with spliceosomal snRNAs, suggesting that the box H/ACA RNAs may also guide pseudouridylation of snRNAs[80,81]. To experimentally verify this hypothesis, several laboratories tested the guide activity of newly identified snRNA-specific box H/ACA RNAs using several independent systems. For example, Zhao et al[82] demonstrated that a Xenopus box H/ACA RNA containing two putative pseudouridylation pockets was indeed able to direct U2 snRNA pseudouridylation at two different sites (positions 34 and 44 by the 5’ pocket and the 3’ pocket, respectively) in Xenopus oocytes. Jády et al[80] showed that U85, a special type of mammalian box H/ACA small nucleolar RNP, specifically directed pseudouridylation of U5 snRNA at position 46. Ma et al[83] reported that one of the yeast box H/ACA RNAs, snR81 RNA, guided Ψ42 formation in yeast U2 snRNA.
The fact that box H/ACA RNAs are able to direct spliceosomal snRNA pseudoruidylation in various organisms strongly suggests that RNA-dependent pseudouridylation is a major (if not the only) mechanism for Ψ formation in spliceosomal snRNAs. In this regard, a large number of box H/ACA RNAs have been identified, and upon inspection of their guide sequences, many of them are predicted to be specific for spliceosomal snRNAs.
Stand-alone protein-catalyzed (RNA-independent) mechanism
At a time when it was widely believed that box H/ACA RNA-dependent mechanism was responsible for Ψ formation in spliceosomal snRNAs, the Branlant lab reported that Pus1, a stand-alone protein pseudouridylase known to catalyze tRNA pseudouridylation, was also responsible for the formation of Ψ44 in S. cerevisiae U2 snRNA[52]. By using purified Pus1 and in vitro synthesized U2 snRNA, they showed that Pus1 catalyzed Ψ44 formation in yeast U2 snRNA. They also showed that deletion of PUS1 resulted in the loss of Ψ44 in yeast U2 snRNA.
This was the first report demonstrating that spliceosomal snRNA pseudorudylation is catalyzed by an RNA-independent mechanism. Here, a stand-alone protein enzyme is responsible for both substrate recognition and catalysis. This mechanism is remarkably different from the RNA-dependent mechanism, in which a guide RNA is used to recognize the substrate and a catalytic protein component Cbf5 catalyzes the isomerization reaction.
Using a singly radiolabled U2 snRNA substrate to screen a yeast GST-ORF fusion library[84], Ma et al[70] subsequently identified YOR243c, a previously uncharacterized ORF, as a stand-alone pseudouridylase responsible for Ψ35 formation in yeast U2 snRNA. YOR243c was subsequently renamed Pseudouridine Synthase 7, PUS7. A BLAST search identified the Pus7 homologs in many organisms, including Schizosaccharomyces pombe, Caenorhabditis elegans, Drosophila melanogaster, and humans. Surprisingly, however, these homologs have no significant sequence or domain similarities to any known members of Ψ synthase families (TruA, TruB, RluA and RsuA families)[70]. Thus, Pus7 represented a novel family of Ψ synthases present in many different organisms. Shortly after the identification of Pus7, its Escherichia coli homolog, TruD, was identified[85]. Thus, yeast Pus7 and its homologs in other organisms have been classified as members of the TruD Ψ synthase family.
From an evolutionary point of view, it is interesting that yeast U2 pseudouridylation is catalyzed by both RNA-dependent (snR81 box H/ACA RNA for Ψ42) and RNA-independent (Pus7 for Ψ35 and Pus1 for Ψ44) mechanisms, whereas pseudouridylation of higher eukaryotic snRNAs is (or at least is widely believed to be) catalyzed exclusively by RNA-dependent mechanism. If it is true that the RNA-dependent mechanism evolved from the RNA-independent mechanism[86], the co-existence of the two mechanisms in yeast would suggest that snR81 box H/ACA RNP responsible for Ψ42 formation has evolved. However, Pus7 and Pus1 responsible for Ψ35 and Ψ44, respectively, might never have evolved (or evolved but were subsequently lost from the genome) in yeast. All box H/ACA RNPs responsible for spliceosomal snRNA pseudouridylation have evolved in higher eukaryotes.
Inducible snRNA pseudouridylation
Until recently all Ψs identified in RNAs have been considered constitutive modifications. In 2011, Wu et al[87] demonstrated for the first time that changes in growth conditions induce U2 pseudouridylation at novel sites, which, in turn, influences splicing. In this study, they exposed yeast cells to a widely used stress-nutrient deprivation (growing cells to saturation or using nutrient-depleted media), and subsequently isolated RNAs from stressed cells for pseudouridylation assays. Remarkably, they detected two novel Ψs (at positions 56 and 93) in U2 snRNA isolated from stressed cells. When the cells were exposed to another widely used stress-heat shock, they also detected Ψ56 (but not Ψ93). These two positions, 56 and 93, had previously been identified as unmodified uridines in yeast U2 snRNA.
Further analyses showed that the stand-alone protein Pus7, which is responsible for Ψ35 formation in U2, catalyzes Ψ56 formation, and that the box H/ACA RNA snR81, which directs pseudouridylation of U2 at position 42 and of 25S rRNA at position 1051[77], guides Ψ93 formation; in the latter case, position 1051 (constitutive) of 25S rRNA and position 93 (inducible) of U2 share a common pseudouridylation guide-the 3’ pseudouridylation pocket of snR81 (the 5’ pocket of snR81 is responsible for Ψ42 formation) (Figure 5). Interestingly, the sequences surrounding U56 and U93 are similar but not identical to the sequences surrounding the constitutively pseudouridylated target sites, Ψ35 of yeast U2 and Ψ1051 of 25S rRNA, respectively[87], suggesting that the inducibility of U2 pseudouridylation at positions 56 and 93 can be attributed to their imperfect substrate sequences or imperfect enzyme-substrate interactions. Indeed, Wu et al[87] subsequently showed that imperfect base-pairing interactions (two mismatches) between the guide sequence of snR81 and the target sequence of U2 (at position 93) were necessary for induced pseudouridylation.
Figure 5.
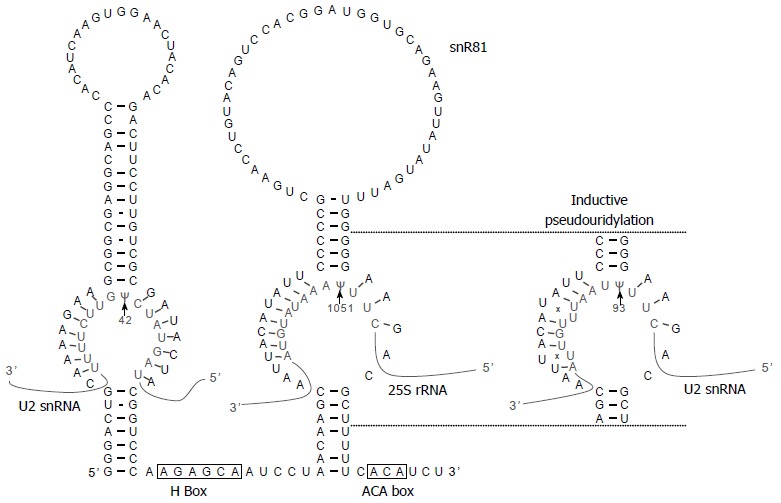
Constitutive and induced pseudouridylation by snR81 box H/ACA ribonucleoprotein. The sequence and structure of snR81 box H/ACA RNA is shown. As arrows indicate, the internal loop (pseudouridylation pocket) within the 5’ hairpin is specific for Ψ42 (constitutive) of U2 snRNA, and the internal loop within the 3’ hairpin is specific for Ψ1051 (constitutive) of 25S rRNA. Under stress coditions, the 3’ pseudouridylation pocket becomes capable of directing the formation of Ψ93 (inducible) of U2 snRNA. As shown by “x”, there are two U-U mismatches between the 3’ pocket and the U2 sequence flanking position 93.
CONCLUSION
It has been more than 60 years since Ψ was reported, and more than 15 years since the box H/ACA RNA family was discovered. Over the years (the last 15-20 years in particular), remarkable progress has been made towards elucidating the mechanism and function of spliceosomal snRNA pseudouridylation. However, the detailed molecular mechanisms of how Ψs affect pre-mRNA splicing remain unclear. With regard to the mechanisms of spliceosomal snRNA pseudouridylation, especially induced Ψ formation, there are still a number of unanswered questions. The concept that Ψ formation can be induced challenges the current paradigm that snRNA modifications are constitutive, and therefore further demonstration of the regulatability of spliceosomal snRNA pseudouridylation will significantly advance our understanding of spliceosomal snRNA modification and function. It is anticipated that the pace of snRNA pseudouridylation research (and RNA modification research in general) will quicken.
ACKNOWLEDGEMENTS
We thank the members of the Yu lab for valuable discussions.
Footnotes
Supported by Grants from the National Institute of Health to Yi-Tao Yu, No. GM104077 and AG39559; and by the University of Rochester CTSA award (to Yi-Tao Yu) from the National Center for Advancing Translational Sciences of the National Institute of Health, No. UL1TR000042
P- Reviewer: Yin JY S- Editor: Song XX L- Editor: A E- Editor: Wu HL
References
- 1.Crick F. Split genes and RNA splicing. Science. 1979;204:264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- 2.Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Mol Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 3.Tarn WY, Steitz JA. Pre-mRNA splicing: the discovery of a new spliceosome doubles the challenge. Trends Biochem Sci. 1997;22:132–137. doi: 10.1016/s0968-0004(97)01018-9. [DOI] [PubMed] [Google Scholar]
- 4.Staley JP, Guthrie C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 5.Bindereif A, Green MR. An ordered pathway of snRNP binding during mammalian pre-mRNA splicing complex assembly. EMBO J. 1987;6:2415–2424. doi: 10.1002/j.1460-2075.1987.tb02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konarska MM, Sharp PA. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987;49:763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- 7.Michaud S, Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev. 1991;5:2534–2546. doi: 10.1101/gad.5.12b.2534. [DOI] [PubMed] [Google Scholar]
- 8.Wassarman DA, Steitz JA. Interactions of small nuclear RNA’s with precursor messenger RNA during in vitro splicing. Science. 1992;257:1918–1925. doi: 10.1126/science.1411506. [DOI] [PubMed] [Google Scholar]
- 9.Karijolich J, Yu YT. Spliceosomal snRNA modifications and their function. RNA Biol. 2010;7:192–204. doi: 10.4161/rna.7.2.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruby SW, Abelson J. An early hierarchic role of U1 small nuclear ribonucleoprotein in spliceosome assembly. Science. 1988;242:1028–1035. doi: 10.1126/science.2973660. [DOI] [PubMed] [Google Scholar]
- 11.Seraphin B, Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell. 1989;59:349–358. doi: 10.1016/0092-8674(89)90296-1. [DOI] [PubMed] [Google Scholar]
- 12.Séraphin B, Rosbash M. The yeast branchpoint sequence is not required for the formation of a stable U1 snRNA-pre-mRNA complex and is recognized in the absence of U2 snRNA. EMBO J. 1991;10:1209–1216. doi: 10.1002/j.1460-2075.1991.tb08062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker R, Siliciano PG, Guthrie C. Recognition of the TACTAAC box during mRNA splicing in yeast involves base pairing to the U2-like snRNA. Cell. 1987;49:229–239. doi: 10.1016/0092-8674(87)90564-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang Y, Weiner AM. A compensatory base change in human U2 snRNA can suppress a branch site mutation. Genes Dev. 1989;3:1545–1552. doi: 10.1101/gad.3.10.1545. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang YA, Goldstein AM, Weiner AM. UACUAAC is the preferred branch site for mammalian mRNA splicing. Proc Natl Acad Sci USA. 1989;86:2752–2756. doi: 10.1073/pnas.86.8.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolford JL. Nuclear pre-mRNA splicing in yeast. Yeast. 1989;5:439–457. doi: 10.1002/yea.320050604. [DOI] [PubMed] [Google Scholar]
- 17.Berglund JA, Chua K, Abovich N, Reed R, Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. doi: 10.1016/s0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 18.Ruby SW, Abelson J. Pre-mRNA splicing in yeast. Trends Genet. 1991;7:79–85. doi: 10.1016/0168-9525(91)90276-V. [DOI] [PubMed] [Google Scholar]
- 19.Sawa H, Abelson J. Evidence for a base-pairing interaction between U6 small nuclear RNA and 5’ splice site during the splicing reaction in yeast. Proc Natl Acad Sci USA. 1992;89:11269–11273. doi: 10.1073/pnas.89.23.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesser CF, Guthrie C. Mutations in U6 snRNA that alter splice site specificity: implications for the active site. Science. 1993;262:1982–1988. doi: 10.1126/science.8266093. [DOI] [PubMed] [Google Scholar]
- 21.Stevens SW, Barta I, Ge HY, Moore RE, Young MK, Lee TD, Abelson J. Biochemical and genetic analyses of the U5, U6, and U4/U6 x U5 small nuclear ribonucleoproteins from Saccharomyces cerevisiae. RNA. 2001;7:1543–1553. [PMC free article] [PubMed] [Google Scholar]
- 22.Madhani HD, Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992;71:803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- 23.Turner IA, Norman CM, Churcher MJ, Newman AJ. Roles of the U5 snRNP in spliceosome dynamics and catalysis. Biochem Soc Trans. 2004;32:928–931. doi: 10.1042/BST0320928. [DOI] [PubMed] [Google Scholar]
- 24.Brow DA. Allosteric cascade of spliceosome activation. Annu Rev Genet. 2002;36:333–360. doi: 10.1146/annurev.genet.36.043002.091635. [DOI] [PubMed] [Google Scholar]
- 25.Licht K, Medenbach J, Lührmann R, Kambach C, Bindereif A. 3’-cyclic phosphorylation of U6 snRNA leads to recruitment of recycling factor p110 through LSm proteins. RNA. 2008;14:1532–1538. doi: 10.1261/rna.1129608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Yu AT, Kantartzis A, Yu YT. Functions and mechanisms of spliceosomal small nuclear RNA pseudouridylation. Wiley Interdiscip Rev RNA. 2011;2:571–581. doi: 10.1002/wrna.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu AT, Ge J, Yu YT. Pseudouridines in spliceosomal snRNAs. Protein Cell. 2011;2:712–725. doi: 10.1007/s13238-011-1087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge J, Yu YT. RNA pseudouridylation: new insights into an old modification. Trends Biochem Sci. 2013;38:210–218. doi: 10.1016/j.tibs.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu YT, Terns RM, Terns MP. Mechanisms and functions of RNA-guided RNA modification. In: Fine-Tuning of RNAFunctions by Modification and Editing., editor. Grosjean H, editor. New York: Springer-Verlag Press; 2005. pp. 223–262. [Google Scholar]
- 30.Reddy R, Busch H. Small nuclear RNAs: RNA sequences, structure, and modifications. In: Birnsteil ML, ed , editors. Structure and Function of Major and Minor Small Nuclear Ribonucleoprotein Particles. Heidelberg: Springer-Verlag Press; 1988. pp. 1–37. [Google Scholar]
- 31.Massenet S, Mougin A, Branlant C. Posttranscriptional modifications in the U small nuclear RNAs. In: Grosjean H, ed , editors. Modification and Editingof RNA. Washington, D. C: ASM Press; 1998. pp. 201–208. [Google Scholar]
- 32.Yu YT, Scharl EC, Smith CM, Steitz JA. The growing world of small nuclear ribonucleoproteins. In: Gesteland RF, Cech TR, Atkins JF, eds , editors. The RNA World. New York: Cold Spring Harbor Laboratory Press; 1999. pp. 487–524. [Google Scholar]
- 33.Ségault V, Will CL, Sproat BS, Lührmann R. In vitro reconstitution of mammalian U2 and U5 snRNPs active in splicing: Sm proteins are functionally interchangeable and are essential for the formation of functional U2 and U5 snRNPs. EMBO J. 1995;14:4010–4021. doi: 10.1002/j.1460-2075.1995.tb00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakin AV, Ofengand J. Mapping of pseudouridine residues in RNA to nucleotide resolution. Methods Mol Biol. 1998;77:297–309. doi: 10.1385/0-89603-397-X:297. [DOI] [PubMed] [Google Scholar]
- 35.Yu YT, Shu MD, Steitz JA. Modifications of U2 snRNA are required for snRNP assembly and pre-mRNA splicing. EMBO J. 1998;17:5783–5795. doi: 10.1093/emboj/17.19.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Yu YT. Detection and quantitation of RNA base modifications. RNA. 2004;10:996–1002. doi: 10.1261/rna.7110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C, McPheeters DS, Yu YT. Psi35 in the branch site recognition region of U2 small nuclear RNA is important for pre-mRNA splicing in Saccharomyces cerevisiae. J Biol Chem. 2005;280:6655–6662. doi: 10.1074/jbc.M413288200. [DOI] [PubMed] [Google Scholar]
- 38.Dai Q, Fong R, Saikia M, Stephenson D, Yu YT, Pan T, Piccirilli JA. Identification of recognition residues for ligation-based detection and quantitation of pseudouridine and N6-methyladenosine. Nucleic Acids Res. 2007;35:6322–6329. doi: 10.1093/nar/gkm657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohn WE, Volkin E. Nucleoside-5′-Phosphates from Ribonucleic Acid. Nature. 1951;167:483–484. [Google Scholar]
- 40.Cohn WE. 5-Ribosyl uracil, a carbon-carbon ribofuranosyl nucleoside in ribonucleic acids. Biochim Biophys Acta. 1959;32:569–571. doi: 10.1016/0006-3002(59)90644-4. [DOI] [PubMed] [Google Scholar]
- 41.Branlant C, Krol A, Machatt MA, Pouyet J, Ebel JP, Edwards K, Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981;9:4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maden BE. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241–303. doi: 10.1016/s0079-6603(08)60629-7. [DOI] [PubMed] [Google Scholar]
- 43.Schattner P, Barberan-Soler S, Lowe TM. A computational screen for mammalian pseudouridylation guide H/ACA RNAs. RNA. 2006;12:15–25. doi: 10.1261/rna.2210406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ofengand J, Fournier MJ. The pseudouridine residues of rRNA: number, location, biosynthesis, and function. In: Grosjean H, eds , editors. Modification and Editing of RNA. Washington, D. C: ASM Press;; 1998. pp. 229–253. [Google Scholar]
- 45.Bachellerie JP, Cavaille J. Small nucleolar RNAs guide the ribose methylations of eukaryotic rRNAs. In: Grosjean H, Benne R, eds , editors. Modification and Editing of RNA. Washington, D. C: ASM Press; 1998. pp. 255–272. [Google Scholar]
- 46.Björk GR. Biosynthesis and function of modified nucleosides in tRNA. In: Soll D, RajBhandary U, eds , editors. tRNA: Structure, Biosynthesis, and Function. Washington, D. C: ASM Press; 1995. pp. 165–205. [Google Scholar]
- 47.Grosjean H, Sprinzl M, Steinberg S. Posttranscriptionally modified nucleosides in transfer RNA: their locations and frequencies. Biochimie. 1995;77:139–141. doi: 10.1016/0300-9084(96)88117-x. [DOI] [PubMed] [Google Scholar]
- 48.Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hopper AK, Phizicky EM. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- 50.Stephenson D, Karijolich J, Yu YT. Functional roles of spliceosomal snRNA modifications in pre-mRNA splicing. In: Smith H, ed , editors. RNA and DNA Editing: Molecular Mechanisms and Their Integration into BiologicalSystems. Hoboken, NJ: Wiley-Interscience; 2008. pp. 175–189. [Google Scholar]
- 51.Solymosy F, Pollák T. Uridylate-Rich Small Nuclear RNAs (UsnRNAs), Their Genes and Pseudogenes, and UsnRNPs in Plants: Structure and Function. A Comparative Approach. Crit Rev Plant Sci. 1993;12:275–369. [Google Scholar]
- 52.Massenet S, Motorin Y, Lafontaine DL, Hurt EC, Grosjean H, Branlant C. Pseudouridine mapping in the Saccharomyces cerevisiae spliceosomal U small nuclear RNAs (snRNAs) reveals that pseudouridine synthase pus1p exhibits a dual substrate specificity for U2 snRNA and tRNA. Mol Cell Biol. 1999;19:2142–2154. doi: 10.1128/mcb.19.3.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kammen HO, Marvel CC, Hardy L, Penhoet EE. Purification, structure, and properties of Escherichia coli tRNA pseudouridine synthase I. J Biol Chem. 1988;263:2255–2263. [PubMed] [Google Scholar]
- 54.Gu X, Liu Y, Santi DV. The mechanism of pseudouridine synthase I as deduced from its interaction with 5-fluorouracil-tRNA. Proc Natl Acad Sci USA. 1999;96:14270–14275. doi: 10.1073/pnas.96.25.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westhof E, Dumas P, Moras D. Hydration of transfer RNA molecules: a crystallographic study. Biochimie. 1988;70:145–165. doi: 10.1016/0300-9084(88)90056-9. [DOI] [PubMed] [Google Scholar]
- 56.Arnez JG, Steitz TA. Crystal structure of unmodified tRNA(Gln) complexed with glutaminyl-tRNA synthetase and ATP suggests a possible role for pseudo-uridines in stabilization of RNA structure. Biochemistry. 1994;33:7560–7567. doi: 10.1021/bi00190a008. [DOI] [PubMed] [Google Scholar]
- 57.Davis DR. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 1995;23:5020–5026. doi: 10.1093/nar/23.24.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auffinger P, Westhof E. RNA hydration: three nanoseconds of multiple molecular dynamics simulations of the solvated tRNA(Asp) anticodon hairpin. J Mol Biol. 1997;269:326–341. doi: 10.1006/jmbi.1997.1022. [DOI] [PubMed] [Google Scholar]
- 59.Charette M, Gray MW. Pseudouridine in RNA: what, where, how, and why. IUBMB Life. 2000;49:341–351. doi: 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- 60.Newby MI, Greenbaum NL. Sculpting of the spliceosomal branch site recognition motif by a conserved pseudouridine. Nat Struct Biol. 2002;9:958–965. doi: 10.1038/nsb873. [DOI] [PubMed] [Google Scholar]
- 61.Kolev NG, Steitz JA. In vivo assembly of functional U7 snRNP requires RNA backbone flexibility within the Sm-binding site. Nat Struct Mol Biol. 2006;13:347–353. doi: 10.1038/nsmb1075. [DOI] [PubMed] [Google Scholar]
- 62.Newby MI, Greenbaum NL. A conserved pseudouridine modification in eukaryotic U2 snRNA induces a change in branch-site architecture. RNA. 2001;7:833–845. doi: 10.1017/s1355838201002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall KB, McLaughlin LW. Properties of pseudouridine N1 imino protons located in the major groove of an A-form RNA duplex. Nucleic Acids Res. 1992;20:1883–1889. doi: 10.1093/nar/20.8.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newby MI, Greenbaum NL. Investigation of Overhauser effects between pseudouridine and water protons in RNA helices. Proc Natl Acad Sci USA. 2002;99:12697–12702. doi: 10.1073/pnas.202477199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patton JR. Multiple pseudouridine synthase activities for small nuclear RNAs. Biochem J. 1993;290(Pt 2):595–600. doi: 10.1042/bj2900595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patton JR. Ribonucleoprotein particle assembly and modification of U2 small nuclear RNA containing 5-fluorouridine. Biochemistry. 1993;32:8939–8944. doi: 10.1021/bi00085a027. [DOI] [PubMed] [Google Scholar]
- 67.Dönmez G, Hartmuth K, Lührmann R. Modified nucleotides at the 5’ end of human U2 snRNA are required for spliceosomal E-complex formation. RNA. 2004;10:1925–1933. doi: 10.1261/rna.7186504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao X, Yu YT. Pseudouridines in and near the branch site recognition region of U2 snRNA are required for snRNP biogenesis and pre-mRNA splicing in Xenopus oocytes. RNA. 2004;10:681–690. doi: 10.1261/rna.5159504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X, Yu YT. Incorporation of 5-fluorouracil into U2 snRNA blocks pseudouridylation and pre-mRNA splicing in vivo. Nucleic Acids Res. 2007;35:550–558. doi: 10.1093/nar/gkl1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma X, Zhao X, Yu YT. Pseudouridylation (Psi) of U2 snRNA in S. cerevisiae is catalyzed by an RNA-independent mechanism. EMBO J. 2003;22:1889–1897. doi: 10.1093/emboj/cdg191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berglund JA, Rosbash M, Schultz SC. Crystal structure of a model branchpoint-U2 snRNA duplex containing bulged adenosines. RNA. 2001;7:682–691. doi: 10.1017/s1355838201002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin Y, Kielkopf CL. X-ray structures of U2 snRNA-branchpoint duplexes containing conserved pseudouridines. Biochemistry. 2008;47:5503–5514. doi: 10.1021/bi7022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ni J, Tien AL, Fournier MJ. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 74.Ganot P, Bortolin ML, Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89:799–809. doi: 10.1016/s0092-8674(00)80263-9. [DOI] [PubMed] [Google Scholar]
- 75.Meier UT, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994;127:1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zebarjadian Y, King T, Fournier MJ, Clarke L, Carbon J. Point mutations in yeast CBF5 can abolish in vivo pseudouridylation of rRNA. Mol Cell Biol. 1999;19:7461–7472. doi: 10.1128/mcb.19.11.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schattner P, Decatur WA, Davis CA, Ares M, Fournier MJ, Lowe TM. Genome-wide searching for pseudouridylation guide snoRNAs: analysis of the Saccharomyces cerevisiae genome. Nucleic Acids Res. 2004;32:4281–4296. doi: 10.1093/nar/gkh768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33:W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hüttenhofer A, Kiefmann M, Meier-Ewert S, O’Brien J, Lehrach H, Bachellerie JP, Brosius J. RNomics: an experimental approach that identifies 201 candidates for novel, small, non-messenger RNAs in mouse. EMBO J. 2001;20:2943–2953. doi: 10.1093/emboj/20.11.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jády BE, Kiss T. A small nucleolar guide RNA functions both in 2’-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J. 2001;20:541–551. doi: 10.1093/emboj/20.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiss AM, Jády BE, Bertrand E, Kiss T. Human box H/ACA pseudouridylation guide RNA machinery. Mol Cell Biol. 2004;24:5797–5807. doi: 10.1128/MCB.24.13.5797-5807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao X, Li ZH, Terns RM, Terns MP, Yu YT. An H/ACA guide RNA directs U2 pseudouridylation at two different sites in the branchpoint recognition region in Xenopus oocytes. RNA. 2002;8:1515–1525. [PMC free article] [PubMed] [Google Scholar]
- 83.Ma X, Yang C, Alexandrov A, Grayhack EJ, Behm-Ansmant I, Yu YT. Pseudouridylation of yeast U2 snRNA is catalyzed by either an RNA-guided or RNA-independent mechanism. EMBO J. 2005;24:2403–2413. doi: 10.1038/sj.emboj.7600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phizicky EM, Martzen MR, McCraith SM, Spinelli SL, Xing F, Shull NP, Van Slyke C, Montagne RK, Torres FM, Fields S, et al. Biochemical genomics approach to map activities to genes. Methods Enzymol. 2002;350:546–559. doi: 10.1016/s0076-6879(02)50984-8. [DOI] [PubMed] [Google Scholar]
- 85.Kaya Y, Ofengand J. A novel unanticipated type of pseudouridine synthase with homologs in bacteria, archaea, and eukarya. RNA. 2003;9:711–721. doi: 10.1261/rna.5230603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lafontaine DL, Tollervey D. Birth of the snoRNPs: the evolution of the modification-guide snoRNAs. Trends Biochem Sci. 1998;23:383–388. doi: 10.1016/s0968-0004(98)01260-2. [DOI] [PubMed] [Google Scholar]
- 87.Wu G, Xiao M, Yang C, Yu YT. U2 snRNA is inducibly pseudouridylated at novel sites by Pus7p and snR81 RNP. EMBO J. 2011;30:79–89. doi: 10.1038/emboj.2010.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reddy R, Henning D, Busch H. Pseudouridine residues in the 5’-terminus of uridine-rich nuclear RNA I (U1 RNA) Biochem Biophys Res Commun. 1981;98:1076–1083. doi: 10.1016/0006-291x(81)91221-3. [DOI] [PubMed] [Google Scholar]
- 89.Gu AD, Zhou H, Yu CH, Qu LH. A novel experimental approach for systematic identification of box H/ACA snoRNAs from eukaryotes. Nucleic Acids Res. 2005;33:e194. doi: 10.1093/nar/gni185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darzacq X, Jády BE, Verheggen C, Kiss AM, Bertrand E, Kiss T. Cajal body-specific small nuclear RNAs: a novel class of 2’-O-methylation and pseudouridylation guide RNAs. EMBO J. 2002;21:2746–2756. doi: 10.1093/emboj/21.11.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shibata H, Ro-Choi TS, Reddy R, Choi YC, Henning D, Busch H. The primary nucleotide sequence of nuclear U-2 ribonucleic acid. The 5’-terminal portion of the molecule. J Biol Chem. 1975;250:3909–3920. [PubMed] [Google Scholar]
- 92.Kiss AM, Jády BE, Darzacq X, Verheggen C, Bertrand E, Kiss T. A Cajal body-specific pseudouridylation guide RNA is composed of two box H/ACA snoRNA-like domains. Nucleic Acids Res. 2002;30:4643–4649. doi: 10.1093/nar/gkf592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deryusheva S, Choleza M, Barbarossa A, Gall JG, Bordonné R. Post-transcriptional modification of spliceosomal RNAs is normal in SMN-deficient cells. RNA. 2012;18:31–36. doi: 10.1261/rna.030106.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krol A, Gallinaro H, Lazar E, Jacob M, Branlant C. The nuclear 5S RNAs from chicken, rat and man. U5 RNAs are encoded by multiple genes. Nucleic Acids Res. 1981;9:769–787. doi: 10.1093/nar/9.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Epstein P, Reddy R, Henning D, Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980;255:8901–8906. [PubMed] [Google Scholar]
- 96.Massenet S, Branlant C. A limited number of pseudouridine residues in the human atac spliceosomal UsnRNAs as compared to human major spliceosomal UsnRNAs. RNA. 1999;5:1495–1503. doi: 10.1017/s1355838299991537. [DOI] [PMC free article] [PubMed] [Google Scholar]


