Abstract
Pain is unfortunately a quite common symptom for cancer patients. Normally pain starts as an episodic experience at early cancer phases to become chronic in later stages. In order to improve the quality of life of oncological patients, anti-cancer treatments are often accompanied by analgesic therapies. The P2X receptor are adenosine triphosphate (ATP) gated ion channels expressed by several cells including neurons, cancer and immune cells. Purinergic signaling through P2X receptors recently emerged as possible common pathway for cancer onset/growth and pain sensitivity. Indeed, tumor microenvironment is rich in extracellular ATP, which has a role in both tumor development and pain sensation. The study of the different mechanisms by which P2X receptors favor cancer progression and relative pain, represents an interesting challenge to design integrated therapeutic strategies for oncological patients. This review summarizes recent findings linking P2X receptors and ATP to cancer growth, progression and related pain. Special attention has been paid to the role of P2X2, P2X3, P2X4 and P2X7 in the genesis of cancer pain and to the function of P2X7 in tumor growth and metastasis. Therapeutic implications of the administration of different P2X receptor blockers to alleviate cancer-associated pain sensations contemporarily reducing tumor progression are also discussed.
Keywords: Cancer, Pain, Adenosine triphosphate, P2X2, P2X3, P2X2/3, P2X4, P2X7
Core tip: Cancer pain is an increasing emergency as the number of oncological patients and survivors tends to growth. Oncological patients will greatly benefit of new therapies combining anti-tumor effects with a reduction of pain perception. This review gives an overview of latest literature on P2X receptors role in tumor progression and different types of cancer pain. The potential of P2X receptors as therapeutic targets in tumor is also discussed.
INTRODUCTION
Half of all oncological patients present pain symptoms[1]. This percentage arise in individuals undergoing cancer treatment getting worse, as tumor progress through its advanced stages[2-4]. Pain sensitization is also frequent in long-term cancer survivors following curative treatment[2,3]. Moreover, pain experience of oncological patients ranges from moderate to severe[3]. The most common pain locations in all cancer patients are back, abdomen and hips. Although there are many studies reporting an increased pain prevalence in cancer types involving head, bone, gynecological and gastrointestinal sites[2], other meta-analysis found no significant relationship between pain and cancer type[3].
A growing body of literature attributed a crucial role in pain sensitization and transduction to adenosine triphosphate (ATP) and purinergic P2X receptors[5]. Moreover, P2X antagonists were successful in reducing neuropathic pain[6]. Furthermore, recent reports highlighted the role of P2X receptors in cancer development and progression[7,8]. Thus, it is tempting to speculate a possible P2X-dependent mechanism affecting both tumor growth and cancer pain. P2X receptors are ATP gated ion channels that, upon ligand stimulation, cause Na+ and Ca2+ cellular influx and K+ efflux. The P2X family includes seven transmembrane proteins (P2X1-7) ranging in length from the 362 amino acids of P2X6 to the 595 of P2X7[9]. Single P2X subunits assemble as homo or hetero-trimers, with each subunit formed by N and C terminal intracellular tails, two membrane spanning domains and a long extracellular loop including the ATP binding site[9,10]. Several cell types including neuronal, immune and cancer cells express P2X receptors. The role of ATP and its receptors in pain sensation is well accepted and diverse kind of pain have been associated to activation of different P2X receptors, i.e., acute pain with sensory neurons-P2X3, neuropathic pain with glial cells-P2X4, inflammatory pain with immune cells-P2X7[11].
Here we give an overview of different forms of cancer pain, identifying the role of P2X receptors in each of them.
DIFFERENT TYPES OF PAIN IN CANCER
Although the sensation of pain, at different levels, is a common experience in various pathologies including cancer, it is very difficult to catalog it. Responding to this need the International Association for the Study of Pain (IASP) classified Chronic Pain originally in 1986, and later on in 1994. The IASP system of classification considers the main site of pain, the system affected, the pattern of occurrence, the time since pain onset, and etiology (http://www.iasp-pain.org). In 2010, Woolf[12] proposed another way to classify pain in three main classes: nociceptive, inflammatory, and neuropathic.
Nociceptive pain can be associated with peripheral nerve sensitization and defined as a high-threshold pain only activated in the presence of intense stimuli such as thermal, mechanical and chemical. These stimuli are transduced and encoded to the brain by nociceptive neurons, whose cell bodies are located in both the dorsal root ganglia (DRG) or the trigeminal ganglia[12,13]. Inflammatory pain is spontaneous pain arising by activation of the immune system due to tissue injury or infection[12,13]. The inflammatory response is normally protective towards pathogens, however, when deregulated can cause pathological processes, such as autoimmune diseases and pain[14]. Neuropathic pain is defined by IASP (http://www.iasp-pain.org) as a pain “caused by a lesion or disease of the somatosensory nervous system”; thus, a nerve injury could determine a peripheral sensitization, characterized by an altered sensitivity at the peripheral and central nervous system (PNS and CNS) level[15]. Infections and inflammation are other potential causes for neuropathic pain[16].
Considering different cancer types, patients’ experiences are ascribable to all the three subclasses of pain. In fact, cancer patients’ pain sensations are characterized by different sensory abnormalities or a combination of them: hypersensitivity or hyposensitivity; paresthesia (abnormal sensation that is not painful or unpleasant), dysaesthesia (unpleasant abnormal sensation); allodynia (pain produced by normally non-painful stimuli); or hyperalgesia (increased response to a stimulus that is normally painful)[4].
Cancer-related pain is present at any stage of cancer progression, with different intensity of frequency, and it is progressively increasing[17]. Initially cancer pain is the direct consequence of primary or metastatic tumor masses themselves, and then arises as side effect of treatments to eradicate cancer[1-3,15]. Pain perception could be considered also a useful alarm: most of nociceptive syndromes are easily discovered, and in some cases, their diagnosis could precede that of the neoplasia. On the other hand cancer associated-neuropathic syndromes are highly variable by individual[18]. Considering the impact of pain in patients’ quality of life, it is not possible to define it as a simple side effect of tumor progression, for this reason a great interest is arising about the specific origin and mechanism of cancer pain (Figure 1).
Figure 1.
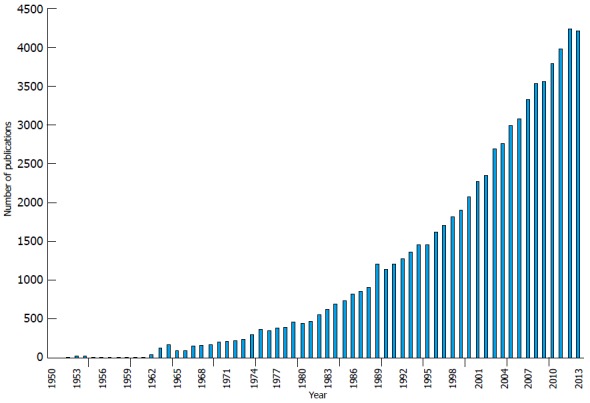
Number of publications per year with keywords “cancer pain”. From MEDLINE®/PubMed®, a database of the United States National Library of Medicine.
Based on cancer stage and treatment, it is possible to define three diverse classes of cancer pain.
Tumor mass-induced pain
Cancer itself is the first source of pain: the extension and invasiveness of primary/metastatic cancer masses generates obstruction, infiltration, or peripheral nerve sensitization inducing nociceptive or neuropathic pain[4,19].
Bone cancer pain is an explicative example: it is caused by tumors arising in bone as osteosarcomas[20], or it is a consequence of skeletal metastases of lung, breast and prostate tumors[21]. When tumor cells infiltrate into bone, different processes begin including bone resorption, compression and damage of the sensory fibers, infiltration of immune cells followed by release of inflammatory cytokines[22]. Thus, cancer bone pain can be defined as nociceptive and inflammatory pain. Furthermore, neuropathic and inflammatory pain pathway activation occurs even at the spinal cord level[23].
Pain induced by anti-cancer treatments
Oncologic patients also suffer pain induced not directly by cancer itself, but by the different treatments that they are undergoing, i.e., chemotherapy, radiation or surgery[18]. These pain experiences belong principally to nociceptive and neuropathic pain syndromes.
Surgery represents one of the most used therapeutic approaches to eradicate cancer. Thus, it is quite common that removal of tumor masses causes nerve sensitization or damage, inducing nociceptive pain. In case of amputation, patients develop multiple form of discomfort in the missing limb. Both painful and non-painful sensation are defined as phantom limb phenomenon: a painful sensation in the residual portion of the amputated limb (stump pain) is associated with a painless sensation originating in the limb that no longer exists (phantom sensation)[24]. In this particular form of pain, the pathophysiology is not clearly defined, as it is not clear whether this is a phenomenon univocally dependent on PNS or CNS[24]. However, it was demonstrated that DRG neurons are a critical source of ectopic impulse discharge[25].
Anti-tumor chemotherapeutic agents are known to cause pain[26]. Chemotherapy-induced neuropathy, that causes pain increase in drug-dose-dependent manner[4], include neuropathic, non-neuropathic[27] and inflammatory pain, inflicting damage both on sensory neurons and satellite glial cells in DRG[28].
Even radiotherapy causes pain as side effect. For example, in breast cancer, radiotherapy successfully decreases mortality but can determine a long-lasting breast pain[29]. The administration of opioids is a good approach to prevent predictable pain due to radiotherapy in bone cancer patients[30].
Chronic non-cancer pain in cancer patients
Chronic non-cancer pain is a debilitative problem that compromises every aspect of patients’ life. Normally, it could be defined as a nociceptive or neuropathic pain, lasting longer than the expected period of healing. Chronic pain sensation is due by persistent stimulation, changes or damages to the CNS or PNS, or both[31]. There is not a common therapeutic approach for these different types of patients’ pain, because the pain type and severity is a result of genotype, and administered treatment has to be customized[31].
ATP AND P2X RECEPTORS IN TUMOR
A growing body of evidence indicates ATP and purinergic signaling as key players in both cancer and pain[32-34]. ATP is released in physiologic conditions by healthy cells exerting beneficial effects via several mechanisms for example acting as fast excitatory neurotransmitter or playing long-term (trophic) roles in cell proliferation, growth and development[5,35,36]. Nonetheless, high levels of ATP can have detrimental effects as this nucleotide acts as pain stimulus and is involved in different inflammatory and non-inflammatory pathologies, including cancer[9,37]. In vivo measure of extracellular ATP in tumor microenvironment allowed to estimate a cancer surrounding concentration of the nucleotide in the micromolar range[38]. Tumor cells can actively secrete ATP[39] or release it as consequence of spontaneous or chemotherapy-induced cell death[40]. Platelets are an extra source of ATP in tumor context, and thanks to release of the nucleotide they facilitate cancer cell extravasation and spreading[41]. Another, good candidate for ATP secretion is its own receptor P2X7 as its stimulation with the synthetic agonist BZ-ATP caused release of ATP requiring P2X7 large pore opening[42,43]. Once in the cancer milieu, ATP acts as growth promoting and pro-metastatic factor[7,8], neuronal and immune-cells stimulus[5,44] and main source of the immunosuppressant adenosine[45] .
Several studies implicated ATP-receptor P2X7 in cell proliferation under nutrient deprivation conditions, similar to those present in cancer microenvironment, such as growth factors and glucose reduced availability[43,46-48]. Moreover, it was recently demonstrated that P2X7 facilitates tumor engraftment, growth and vascularization[7] and that P2X7 blockade or silencing effectively reduces tumor growth in animal models of colon cancer, melanoma and neuroblastoma[7,49]. P2X and P2Y receptors expression has been reported both in tumor and tumor-associated cells in several cancers (for a recent review see[34]). However, and in depth characterization of the role of ATP in the crosstalk among tumor and immune or neuronal surrounding cells is still missing.
P2X IN CANCER PAIN
Extracellular ATP triggers pain perception through activation of different P2X receptors. Indeed, P2X receptors are essential players in nociceptive, inflammatory and pathological pain transduction[38,50,51]. All the different compartments involved in pain transduction, sensory ganglia, DRG and spinal cord, are characterized by high expression of P2X receptors in excitable and non-excitable cells[51].
Different studies demonstrated the involvement of ATP and P2X receptors in cancer pain[22]. The use of diverse animal pain models allowed identifying P2X receptors as mediators of cancer pain perception trough DRG and spinal cord[28,52,53].
As mentioned before, one of the most studied cancer pain models is bone cancer: many common tumors preferentially metastasize to multiple bones and bone cancer patients suffer of moderate to severe chronic pain[20,54]. Interestingly, up-regulation of P2X3 receptor was founded in DRG of animals experiencing bone cancer pain[52,53,55]. Homomeric P2X3 and heteromeric P2X2/3 receptors are the main responsible for ATP evoked nociceptive pain[56,57], and were accordingly involved in bone cancer pain perception[55]. Recently, Liu et al[52] (2013) showed that , in rat bone cancer, DRG neurons-P2X3 receptor is functionally up-regulated by the neuronal calcium sensor VILIP-1, and contributes to the development of bone cancer pain. Moreover, P2X3 receptor antagonist A-317491, which successfully reduces chronic inflammatory and neuropathic pain behavior in animals[58,59], also relieves cancer bone pain[60]. However, A-317491 effect was limited to attenuation of bone pain related behaviors in the early stage of tumor development, while had no effect as tumor progressed to an advanced point. Similarly, oral administration of AF-353, another potent and selective P2X3 and P2X2/3 receptor antagonist, attenuated bone cancer pain, but had no preventing effect on cancer-induced bone destruction[55]. P2X receptors blockade was found to be an efficacious pain easing strategy also in a model of melanoma developed in mice paw[61]. In this context, tumor cell inoculation caused a pain related behavior that was increased by intra-plantar injection of ATP, and reduced, in a dose-dependent fashion, by P2 receptor broad-spectrum antagonists. Interestingly, the authors reported a contemporary up-regulation of P2X3 receptors in DRG. Discussed evidence suggests that specific blockade of P2X3 or P2X2/3 receptors will not be sufficient to minimize cancer related pain. In fact, the role of P2X receptors in cancer pain is not restricted to P2X3 and neuronal cells. On the contrary, non-neuronal cells play a pivotal role in chronic pain states[51,57,62] and immune-cancer cells expressed P2X4 and P2X7 receptors are involved in inflammatory and neuropathic pain development[29,30,47,53,54,60]. P2X4 or P2X7 null mice show reduced nociceptive sensitivity and development of neuropathic pain states. Moreover, similar results can be obtained by in vivo administration of P2X7 antagonists[6] However, neither P2X7 genetic ablation nor receptors’ antagonist administration was able to alleviate bone cancer pain[63]. The different efficacy of P2X7 antagonist in reducing inflammatory and neuropathic pain versus bone cancer pain can be ascribed to a diverse mechanism of generation of the painful stimulus, which is probably more complex in cancer than in other pathological situations.
P2X RECEPTORS AS PHARMACOLOGICAL TARGETS FOR CANCER PAIN
Pharmacological approaches to different kind of cancer pain are based on patient’s status. There are three main classes of drugs for cancer pain management: opioids, non-opioids and adjuvant analgesics[64]. Normally, pharmacotherapy consist in an appropriate selection of drugs, belonging to the three categories mentioned, and it is structured to optimize positive outcomes and minimize side-effects[4]. Terminal oncological patients generally experience chronic pain that can be eased by palliative care improving their quality of life and helping them to face the prospect of death[1]. Suramin, a broad spectrum P2 receptor antagonist, showed clinically relevant antitumor activity in prostate cancer, as reflected by reduction of prostate specific antigen levels, pain relief, and a relatively long time to disease progression[65,66]. Moreover, suramin underwent phase III clinical trials for refractory prostate cancer, giving encouraging results as palliative therapy and delaying disease progression[67]. However, the mechanism of action of suramin is essentially unknown as it binds to several proteins including growth factors and is not only a P2 receptor antagonist but also acts as agonist of ryanodine receptors. Nevertheless, pain-easing activity of suramin could be attributed, at least partially, to P2X3 or P2X2/3 blockade. Indeed, as mentioned before, different P2X3, P2X2/3 specific antagonists proved effective in reduce bone cancer pain perception in animal models[53,60,68] and refractory prostate cancer generally metastasize to bone. P2X2 and P2X2/3 receptors have also been involved in development of opioid treatment resistance in severe pain states associated to cancer metastasis[68]. Chizhmakov et al[69] demonstrated that opioids are able to inhibit P2X3 and P2X2/3 receptor responses in sensory neurons[69], but also that cancer cells, co-cultured with neurons, release diffusible factors able to alter opioid-dependent P2Xs inhibition, probably causing opioid resistance[69].
Another interesting target for the development of anti-cancer, pain-easing therapies is P2X7. At the moment different P2X7 antagonists are undergoing clinical trials for their efficacy in reducing neuropathic and inflammatory pain[70,71], and its role in pain pathways is confirmed by the involvement of P2X7 receptor, expressed by spinal microglia, in induction of morphine tolerance[72]. Moreover, P2X7 potent and selective antagonists A-740003 and A-438079 both showed anti-nociceptive activity in rodents[69,73]. Accordingly, P2X7 receptor antagonist brilliant blue G reduced pain perception in a rat model of bone cancer[74]. However, in a similar mice model A-438079 failed to alleviate bone cancer pain[63]. These discrepancies could be ascribed to the different animal models, cell players examined, and dissimilar specificity of the drugs administered. A strategy based on P2X7 blockade could not only be efficacious in reducing cancer pain but also tumor growth, vascularization and spreading. In fact, we recently demonstrated that P2X7 receptor affects cancer development, causing increased tumor engraftment, growth rate and angiogenesis[75]. Interestingly, both P2X7 antagonist AZ-10606120 and P2X7 inhibitor oxidized-ATP caused an evident tumor regression accompanied by reduced vascular endothelial growth factor secretion and consequently a decrease in tumor blood vessels[76]. Accordingly, different studies show a P2X7 blockade dependent reduction of melanoma and glioma growth[49]. However, data on glioma are debatable, in fact Fang and colleagues also reported Brillant Blue G dependent glioma growth acceleration[75].
A growing body of literature has also attributed a role to P2X7 in tumor dissemination[65,77-80] and drugs acting at the receptor have been proposed as anti-metastatic agents[8,80]. Interestingly, drugs as different as the traditional Chinese medicine compound Emodin, and the statin atrovastin both act at tumor cells inhibiting their migratory ability via P2X7 blockade[79,80].
Treatment of oncological patients with intravenous infusions of ATP at high doses was also proposed as strategy to fight tumor growth taking advantage of ATP cytotoxic properties. ATP administration intravenously proved to be effective in arresting tumor progression in rodent models of hormone-refractory prostate[81] and colon cancer[82]. However, the possible side effects due to pain development after intravenous administration of millimolar doses of ATP to patients have to be taken into account. In fact, it is known that application/injection of ATP causes pain perception associated with vascular changes[61,62] and this effect was also observed in clinical trials administering high doses of ATP to patients[83]. On the other hand, short infusion of low ATP concentrations did not caused pain when administered to terminal oncological patients, but improved their quality of life[84] and reduced cachexia[85]. However, administration of ATP to patients with less severe forms of cancer did not produce any positive effect on their quality of life or in tumor regression[84].
Interestingly, ATP has been suggested also as adjuvant chemotherapy facilitating the passage of anticancer drugs such as doxorubicin and mytomicin C[81,86,87]. However, chemotherapy itself causes pain, which could be enhanced by ATP administration. Summarizing, even if ATP administration to cancer patients is representing an interesting strategy to eradicate tumor cells, probably will be causing pain as major side effect and will be, then, inadequate for the treatment of tumors associated with severe pain sensation.
CONCLUSION
Cancer pain is one of the most serious problems in oncology, depending on the growing number of cancer patients and, luckily, of cancer survivors. For these reasons identification of adequate anti-pain therapies, improving the quality of life of oncological patients is attracting widespread interest. Due to the different cellular components involved in tumor, classically administered anti-pain pharmacological approaches not always satisfy cancer patient’s needs. In this review, we underline a possible connection between pain perception and tumor growth in cancer mediated by P2X receptors (Figure 2). Described evidence suggests that P2X receptors are good pharmacological targets for the treatment of cancer and associated pain. Reported literature supports the hypothesis that the ideal anti-cancer strategy will be obtained with a calibrated mixture or combination of P2X2, P2X3, P2X4 and P2X7 receptor antagonists, which will reduce/resolve at the same time tumor growth and relative pain.
Figure 2.

Schematic representation of solid tumor generated by cancer cells (red) surrounded by the normal tissue cells (light and dark blue) releasing extracellular adenosine triphosphate in surrounding environment (in yellow). Adenosine triphosphate (ATP) acts through three different ways: (1) binding P2X7 receptors, ATP facilitates tumor growth; (2) binding the P2X2, P2X3, P2X2/3 receptors expressed by neuronal cells (in green), ATP triggers pain perception; and (3) binding P2X4 and P2X7 receptors expressed by immune cells (in yellow), ATP causes inflammation and pain.
Footnotes
Supported by Grants to Elena Adinolfi from the Italian association for Cancer research (MFAG11630), and from the Region Emilia Romagna (Young researchers funds, Bando Alessandro Liberati)
P- Reviewer: Burnstock G, Bruzzone S, Hassan M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 2.Marcus DA. Epidemiology of cancer pain. Curr Pain Headache Rep. 2011;15:231–234. doi: 10.1007/s11916-011-0208-0. [DOI] [PubMed] [Google Scholar]
- 3.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 4.Fallon MT. Neuropathic pain in cancer. Br J Anaesth. 2013;111:105–111. doi: 10.1093/bja/aet208. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic mechanisms and pain--an update. Eur J Pharmacol. 2013;716:24–40. doi: 10.1016/j.ejphar.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Volonté C, Apolloni S, Skaper SD, Burnstock G. P2X7 receptors: channels, pores and more. CNS Neurol Disord Drug Targets. 2012;11:705–721. doi: 10.2174/187152712803581137. [DOI] [PubMed] [Google Scholar]
- 7.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 8.Jelassi B, Chantôme A, Alcaraz-Pérez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–2122. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 9.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toulme E, Tsuda M, Khakh BS, Inoue K. On the Role of ATP-Gated P2X Receptors in Acute, Inflammatory and Neuropathic Pain. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton, FL: CRC Press; 2010. [PubMed] [Google Scholar]
- 12.Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87:3–11. doi: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Moilanen E. Two faces of inflammation: an immunopharmacological view. Basic Clin Pharmacol Toxicol. 2014;114:2–6. doi: 10.1111/bcpt.12180. [DOI] [PubMed] [Google Scholar]
- 15.Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 2012;12:304–314. doi: 10.2174/187152412803760645. [DOI] [PubMed] [Google Scholar]
- 16.Jay GW, Barkin RL. Neuropathic pain: etiology, pathophysiology, mechanisms, and evaluations. Dis Mon. 2014;60:6–47. doi: 10.1016/j.disamonth.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez Andrade JM, Mantyh P. Cancer Pain: From the Development of Mouse Models to Human Clinical Trials. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton, FL: CRC Press; 2010. [PubMed] [Google Scholar]
- 18.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 19.Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci. 2001;21:9367–9376. doi: 10.1523/JNEUROSCI.21-23-09367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97:866–873. doi: 10.1002/cncr.11144. [DOI] [PubMed] [Google Scholar]
- 21.Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9 Suppl 1:S5. doi: 10.1186/ar2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk S, Uldall M, Heegaard AM. The role of purinergic receptors in cancer-induced bone pain. J Osteoporos. 2012;2012:758181. doi: 10.1155/2012/758181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley KM. Advances in cancer pain. Arch Neurol. 1999;56:413–417. doi: 10.1001/archneur.56.4.413. [DOI] [PubMed] [Google Scholar]
- 24.Probstner D, Thuler LC, Ishikawa NM, Alvarenga RM. Phantom limb phenomena in cancer amputees. Pain Pract. 2010;10:249–256. doi: 10.1111/j.1533-2500.2009.00340.x. [DOI] [PubMed] [Google Scholar]
- 25.Vaso A, Adahan HM, Gjika A, Zahaj S, Zhurda T, Vyshka G, Devor M. Peripheral nervous system origin of phantom limb pain. Pain. 2014;155:1384–1391. doi: 10.1016/j.pain.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Christo PJ, Mazloomdoost D. Cancer pain and analgesia. Ann N Y Acad Sci. 2008;1138:278–298. doi: 10.1196/annals.1414.033. [DOI] [PubMed] [Google Scholar]
- 27.Geber C, Breimhorst M, Burbach B, Egenolf C, Baier B, Fechir M, Koerber J, Treede RD, Vogt T, Birklein F. Pain in chemotherapy-induced neuropathy--more than neuropathic? Pain. 2013;154:2877–2887. doi: 10.1016/j.pain.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Warwick RA, Hanani M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain. 2013;17:571–580. doi: 10.1002/j.1532-2149.2012.00219.x. [DOI] [PubMed] [Google Scholar]
- 29.Lundstedt D, Gustafsson M, Steineck G, Malmström P, Alsadius D, Sundberg A, Wilderäng U, Holmberg E, Johansson KA, Karlsson P. Risk factors of developing long-lasting breast pain after breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:71–78. doi: 10.1016/j.ijrobp.2011.05.065. [DOI] [PubMed] [Google Scholar]
- 30.Di Franco R, Falivene S, Ravo V, Mammucari M, Sarli E, Baffini S, De Palma G, Pepe A, Traettino M, Muto M, et al. Management of painful bone metastases: our experience according to scientific evidence on palliative radiotherapy. Anticancer Res. 2014;34:1011–1014. [PubMed] [Google Scholar]
- 31.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377:2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 32.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. P2X4 receptors and neuropathic pain. Front Cell Neurosci. 2013;7:191. doi: 10.3389/fncel.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol. 2007;36:165–183. doi: 10.1007/s12035-007-0033-y. [DOI] [PubMed] [Google Scholar]
- 34.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9:491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev. 2010;63:222–232. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton SG, McMahon SB. ATP as a peripheral mediator of pain. J Auton Nerv Syst. 2000;81:187–194. doi: 10.1016/s0165-1838(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 38.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berglund E, Berglund D, Akcakaya P, Ghaderi M, Daré E, Berggren PO, Köhler M, Aspinwall CA, Lui WO, Zedenius J, et al. Evidence for Ca(2+)-regulated ATP release in gastrointestinal stromal tumors. Exp Cell Res. 2013;319:1229–1238. doi: 10.1016/j.yexcr.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, Séror C, Métivier D, Perfettini JL, Zitvogel L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–3728. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 41.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16:3659–3665. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandonà D, Markwardt F, Schmalzing G, et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010;24:3393–3404. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 44.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 45.Stagg J, Beavis PA, Divisekera U, Liu MC, Möller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 46.Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, Di Virgilio F. Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem. 1999;274:33206–33208. doi: 10.1074/jbc.274.47.33206. [DOI] [PubMed] [Google Scholar]
- 47.Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, Ferrari D. P2X(7) receptor: Death or life? Purinergic Signal. 2005;1:219–227. doi: 10.1007/s11302-005-6322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 2012;3:e370. doi: 10.1038/cddis.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hattori F, Ohshima Y, Seki S, Tsukimoto M, Sato M, Takenouchi T, Suzuki A, Takai E, Kitani H, Harada H, et al. Feasibility study of B16 melanoma therapy using oxidized ATP to target purinergic receptor P2X7. Eur J Pharmacol. 2012;695:20–26. doi: 10.1016/j.ejphar.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 51.Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- 52.Liu M, Yang H, Fang D, Yang JJ, Cai J, Wan Y, Chui DH, Han JS, Xing GG. Upregulation of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in dorsal root ganglions contributes to the bone cancer pain in rats. Pain. 2013;154:1551–1568. doi: 10.1016/j.pain.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 53.Wu JX, Xu MY, Miao XR, Lu ZJ, Yuan XM, Li XQ, Yu WF. Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain. 2012;16:1378–1388. doi: 10.1002/j.1532-2149.2012.00149.x. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181. doi: 10.1111/j.1749-6632.2009.05429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, et al. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain. 2010;133:2549–2564. doi: 10.1093/brain/awq194. [DOI] [PubMed] [Google Scholar]
- 56.Jarvis MF. Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain. Expert Opin Ther Targets. 2003;7:513–522. doi: 10.1517/14728222.7.4.513. [DOI] [PubMed] [Google Scholar]
- 57.Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140:1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen RR, Nasser A, Falk S, Baldvinsson SB, Ohlsson PH, Bahl JM, Jarvis MF, Ding M, Heegaard AM. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur J Pharmacol. 2012;688:27–34. doi: 10.1016/j.ejphar.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Fujita M, Andoh T, Sasaki A, Saiki I, Kuraishi Y. Involvement of peripheral adenosine 5’-triphosphate and P2X purinoceptor in pain-related behavior produced by orthotopic melanoma inoculation in mice. Eur J Neurosci. 2010;31:1629–1636. doi: 10.1111/j.1460-9568.2010.07185.x. [DOI] [PubMed] [Google Scholar]
- 62.Khakpay R, Polster D, Köles L, Skorinkin A, Szabo B, Wirkner K, Illes P. Potentiation of the glutamatergic synaptic input to rat locus coeruleus neurons by P2X7 receptors. Purinergic Signal. 2010;6:349–359. doi: 10.1007/s11302-010-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen RR, Nielsen CK, Nasser A, Thomsen SI, Eghorn LF, Pham Y, Schulenburg C, Syberg S, Ding M, Stojilkovic SS, et al. P2X7 receptor-deficient mice are susceptible to bone cancer pain. Pain. 2011;152:1766–1776. doi: 10.1016/j.pain.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paice JA, Ferrell B. The management of cancer pain. CA Cancer J Clin. 2011;61:157–182. doi: 10.3322/caac.20112. [DOI] [PubMed] [Google Scholar]
- 65.Reyno LM, Egorin MJ, Eisenberger MA, Sinibaldi VJ, Zuhowski EG, Sridhara R. Development and validation of a pharmacokinetically based fixed dosing scheme for suramin. J Clin Oncol. 1995;13:2187–2195. doi: 10.1200/JCO.1995.13.9.2187. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi K, Vokes EE, Vogelzang NJ, Janish L, Soliven B, Ratain MJ. Phase I study of suramin given by intermittent infusion without adaptive control in patients with advanced cancer. J Clin Oncol. 1995;13:2196–2207. doi: 10.1200/JCO.1995.13.9.2196. [DOI] [PubMed] [Google Scholar]
- 67.Small EJ, Meyer M, Marshall ME, Reyno LM, Meyers FJ, Natale RB, Lenehan PF, Chen L, Slichenmyer WJ, Eisenberger M. Suramin therapy for patients with symptomatic hormone-refractory prostate cancer: results of a randomized phase III trial comparing suramin plus hydrocortisone to placebo plus hydrocortisone. J Clin Oncol. 2000;18:1440–1450. doi: 10.1200/JCO.2000.18.7.1440. [DOI] [PubMed] [Google Scholar]
- 68.Chizhmakov I, Yudin Y, Mamenko N, Prudnikov I, Tamarova Z, Krishtal O. Opioids inhibit purinergic nociceptors in the sensory neurons and fibres of rat via a G protein-dependent mechanism. Neuropharmacology. 2005;48:639–647. doi: 10.1016/j.neuropharm.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Chizhmakov I, Mamenko N, Volkova T, Khasabova I, Simone DA, Krishtal O. P2X receptors in sensory neurons co-cultured with cancer cells exhibit a decrease in opioid sensitivity. Eur J Neurosci. 2009;29:76–86. doi: 10.1111/j.1460-9568.2008.06556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alves LA, Bezerra RJ, Faria RX, Ferreira LG, da Silva Frutuoso V. Physiological roles and potential therapeutic applications of the P2X7 receptor in inflammation and pain. Molecules. 2013;18:10953–10972. doi: 10.3390/molecules180910953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King BF. Novel P2X7 receptor antagonists ease the pain. Br J Pharmacol. 2007;151:565–567. doi: 10.1038/sj.bjp.0707266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen ML, Cao H, Chu YX, Cheng LZ, Liang LL, Zhang YQ, Zhao ZQ. Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: multiple glial-neuronal dialogues in the rat spinal cord. J Pain. 2012;13:945–958. doi: 10.1016/j.jpain.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 73.Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 74.Huang ZX, Lu ZJ, Ma WQ, Wu FX, Zhang YQ, Yu WF, Zhao ZQ. Involvement of RVM-expressed P2X7 receptor in bone cancer pain: mechanism of descending facilitation. Pain. 2014;155:783–791. doi: 10.1016/j.pain.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Fang J, Chen X, Zhang L, Chen J, Liang Y, Li X, Xiang J, Wang L, Guo G, Zhang B, et al. P2X7R suppression promotes glioma growth through epidermal growth factor receptor signal pathway. Int J Biochem Cell Biol. 2013;45:1109–1120. doi: 10.1016/j.biocel.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-β1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci. 2012;125:5051–5060. doi: 10.1242/jcs.104976. [DOI] [PubMed] [Google Scholar]
- 77.Takai E, Tsukimoto M, Harada H, Kojima S. Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal. 2014;10:487–497. doi: 10.1007/s11302-014-9411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren S, Zhang Y, Wang Y, Lui Y, Wei W, Huang X, Mao W, Zuo Y. Targeting P2X₇ receptor inhibits the metastasis of murine P388D1 lymphoid neoplasm cells to lymph nodes. Cell Biol Int. 2010;34:1205–1211. doi: 10.1042/CBI20090428. [DOI] [PubMed] [Google Scholar]
- 79.Jelassi B, Anchelin M, Chamouton J, Cayuela ML, Clarysse L, Li J, Goré J, Jiang LH, Roger S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis. 2013;34:1487–1496. doi: 10.1093/carcin/bgt099. [DOI] [PubMed] [Google Scholar]
- 80.Ghalali A, Wiklund F, Zheng H, Stenius U, Högberg J. Atorvastatin prevents ATP-driven invasiveness via P2X7 and EHBP1 signaling in PTEN-expressing prostate cancer cells. Carcinogenesis. 2014;35:1547–1555. doi: 10.1093/carcin/bgu019. [DOI] [PubMed] [Google Scholar]
- 81.Shabbir M, Thompson C, Jarmulowiczc M, Mikhailidis D, Burnstock G. Effect of extracellular ATP on the growth of hormone-refractory prostate cancer in vivo. BJU Int. 2008;102:108–112. doi: 10.1111/j.1464-410X.2008.07578.x. [DOI] [PubMed] [Google Scholar]
- 82.Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, Junger WG, Robson SC, Wu Y. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS One. 2013;8:e60184. doi: 10.1371/journal.pone.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Beijer S, Hupperets PS, van den Borne BE, Wijckmans NE, Spreeuwenberg C, van den Brandt PA, Dagnelie PC. Randomized clinical trial on the effects of adenosine 5’-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage. 2010;40:520–530. doi: 10.1016/j.jpainsymman.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 85.Agteresch HJ, Dagnelie PC, van der Gaast A, Stijnen T, Wilson JH. Randomized clinical trial of adenosine 5’-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2000;92:321–328. doi: 10.1093/jnci/92.4.321. [DOI] [PubMed] [Google Scholar]
- 86.Munerati M, Cortesi R, Ferrari D, Di Virgilio F, Nastruzzi C. Macrophages loaded with doxorubicin by ATP-mediated permeabilization: potential carriers for antitumor therapy. Biochim Biophys Acta. 1994;1224:269–276. doi: 10.1016/0167-4889(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 87.Mo R, Jiang T, DiSanto R, Tai W, Gu Z. ATP-triggered anticancer drug delivery. Nat Commun. 2014;5:3364. doi: 10.1038/ncomms4364. [DOI] [PubMed] [Google Scholar]


