Abstract
Sepsis-induced acute kidney injury (SAKI) is a frequent complication of infant sepsis that approximately doubles the mortality rate. The poor prognosis of these patients is a result of care that is mainly supportive, nontargeted, and usually begun only after symptoms of the systemic inflammatory response syndrome are observed. Preclinical studies from relevant rodent models of SAKI suggest that mitochondria-targeted antioxidants may be a new mode of therapy that could promote recovery.
Sepsis is the seventh leading cause of infant mortality in the United States and is one of the most common causes of death in children worldwide. Severe sepsis is defined as sepsis with cardiovascular distress and multiple organ dysfunction syndrome. It is now clear that the pathophysiology of pediatric sepsis is not the same as that in the adult. Moreover, recent findings suggest that SAKI represents a unique type of kidney injury in infants that is not identical to SAKI in adults. Unfortunately, current therapy is largely ineffective, often progressing to renal replacement therapy such as dialysis.1 The prognosis is poor in these patients because therapy for sepsis is usually begun only after symptoms are observed and after renal injury may have already been initiated.
The pathophysiology of organ dysfunction during severe sepsis is multifactorial and poorly understood. Although systemic hemodynamic decline during sepsis can contribute to organ hypoperfusion, there is a growing appreciation of the importance of the microcirculation in the development of organ injury. Microvascular failure, which includes hypoperfusion and/or leakage, is newly recognized as a strong predictor of death in patients with severe sepsis.2 Nevertheless, early goal-directed therapy intended to maintain systemic hemodynamics and thereby preserve organ perfusion has been shown to reduce mortality in septic pediatric patients. This is especially important in the infant patient because, as compared with adults, infants with severe sepsis are more likely to have low cardiac output, microvascular constriction, and prolonged capillary refill time. Nevertheless, even with adequate resuscitation, mortality approaches 25% in severe sepsis and nears 45% in patients with SAKI.1
The mechanism by which microvascular failure contributes to parenchymal cell injury remains a key unanswered question. Animal models of sepsis suggest that oxidative stress, a hallmark of sepsis in humans, may represent a link between microvascular failure and multiple-organ dysfunction.3 Increased oxidant generation and the resulting oxidative injury to the kidney during sepsis may have even more serious consequences in the infant patient because of a still-developing antioxidant defense system and a lower functional renal reserve. This combination may not only worsen injury but also impede recovery. Adult rodent models of sepsis have suggested that impaired renal microcirculation is a very early event in SAKI. Decreased microvascular perfusion leads to a hypoxic, pro-oxidant microenvironment, which promotes tubular epithelial injury and worsens renal function. It is becoming clear that renal microvascular hypoperfusion and oxidative stress are linked in a self-amplifying cycle of microvascular and epithelial cell injury.
The renal epithelium is uniquely rich in mitochondria due to the high-energy demands of ion transport. The inner mitochondrial membrane contains four respiratory complexes (I, II, III, and IV) and adenosine triphosphate synthase, and there is evidence, from human muscle biopsies, of ultrastructural mitochondrial damage and respiratory complex inactivation during sepsis. The level of understanding of the mitochondrial defects in the kidney during sepsis remains poor due to the lack of kidney biopsies from septic patients. Superoxide is the primary oxidant produced by mitochondrial respiratory complexes I and III. However, under normal physiology, the levels of superoxide are kept low by the mitochondrial antioxidant enzyme, manganese superoxide dismutase. Although not yet confirmed in infants, the levels of antioxidant enzymes such as total superoxide dismutase and catalase are lower in the developing rodent kidney as compared with the levels in the adult kidney. During sepsis, changes in oxygen delivery, along with disruption of the respiratory electron transport chain, can result in increased superoxide generation, which could overwhelm antioxidant defenses and damage mitochondrial respiration even further. Compelling evidence from studies on adult animals and from a limited number of studies on adult humans supports the notion that therapies targeting mitochondrial oxidants might be beneficial. For example, in the septic aged mouse, inhibition of the mitochondrial respiratory complex in the kidney is associated with generation of superoxide and peroxynitrite (the reaction product of superoxide and nitric oxide), and targeting mitochondrial oxidants prolongs survival.4
There is a growing realization that the adult animal is not the appropriate model for SAKI in the infant population because the immunological and cardiovascular systems, including the kidney, are still developing in the infant. In the septic infant, preservation of microcirculatory perfusion is the focus of supportive goal-directed therapy, and recent clinical evidence suggests that renal blood flow—rather than mean arterial pressure—is the more important factor in maintaining the renal microcirculation, in contrast to the case in adults. These age-dependent differences could profoundly affect the appropriate selection of therapy for infants with sepsis. Consequently, the selection of the preclinical model becomes critically important. Similar to human infants, rat pups have lower resting mean arterial pressure, glomerular filtration rate, and renal blood flow than adults. From a translational perspective, rat pups are generally considered a more appropriate model for infant AKI than adult rats.
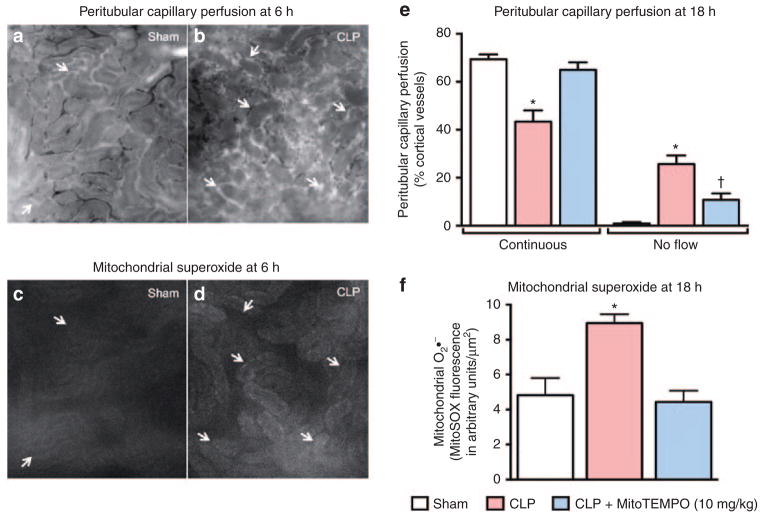
Although no single animal model completely duplicates the complexities of sepsis in humans, the cecal ligation and puncture sepsis model is widely accepted as the most relevant because it exhibits many of the key pathogenic features observed in humans. We have developed a rat pup cecal ligation and puncture model to identify, and then evaluate, new therapeutic targets to prevent or treat SAKI. Using weaning as the scaling factor, rat pups at 17–18 days old are estimated to be at a stage of development comparable to a 5- to 6-month-old human infant. We have used this model to identify changes that occur in the renal macro- and microcirculation and to describe the development of renal epithelial oxidative stress during sepsis.5 We have now begun to evaluate mitochondrial oxidant generation as a possible therapeutic target. MitoTEMPO ((2-(2,2,6,6-tetramethylpiperidin- 1-oxyl-4-ylamino)-2-oxoethyl) triphenyl phosphonium chloride monohydrate) is a nitroxide antioxidant conjugated to a lipophilic triphenyl phosphonium cation, which targets it to the mitochondria (Figure 1). Although its exact antioxidant mechanisms are unclear, the catalytic cycling of MitoTEMPO is thought to scavenge oxygen radicals in the mitochondria. Using intravital video microscopy of the kidney to measure, in real time, peritubular capillary perfusion and mitochondrial superoxide generation in the tubular epithelium of rat pups, we found that sepsis causes a decrease in renal cortical capillary perfusion and an associated increase in epithelial mitochondrial superoxide generation (Figure 2). MitoTEMPO administered at the time of cecal ligation and puncture not only reduced mitochondrial superoxide levels, it also improved renal microcirculatory perfusion. This suggests a cross talk between the renal tubules and the adjacent peritubular capillaries that can regulate the microcirculatory microenvironment. Studies in aged mice have suggested a therapeutic potential of treatments that are designed to break the cycle of injury and restore renal function by targeting oxidants and allowing the renal microcirculation and tubular epithelium time to recover. In fact, even delayed therapy with MitoTEMPO promotes recovery of mitochondrial function, microcirculatory perfusion, and overall renal function, as well as, importantly, prolonging survival in aged septic mice.4 Studies are ongoing to investigate whether delayed therapy with MitoTEMPO will also be protective in the rat pup model.
Figure 1.
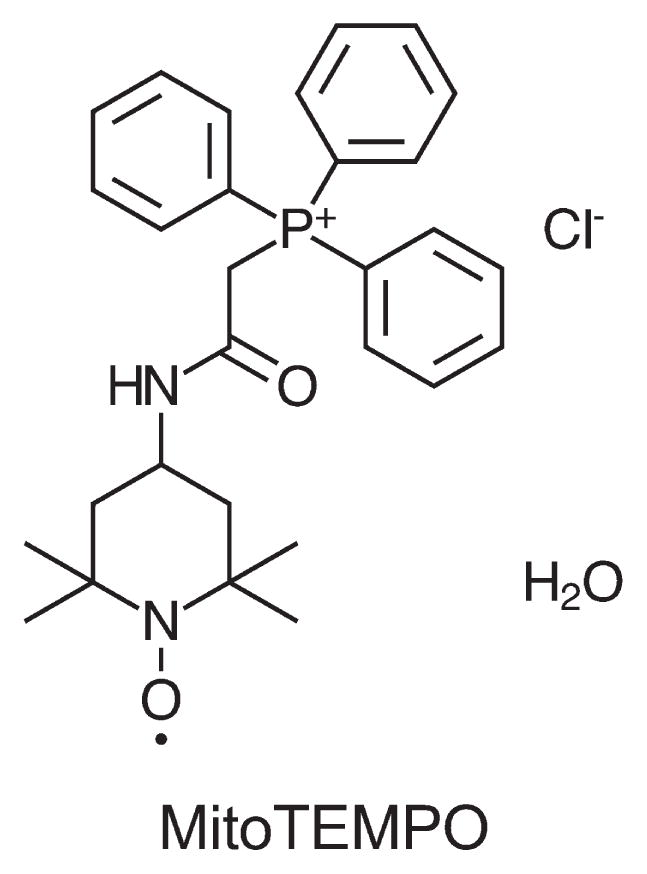
Chemical structure of MitoTEMPO. MitoTEMPO ((2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenyl phosphonium chloride monohydrate) is a nitroxide conjugated to a lipophilic triphenyl phosphonium cation, which targets it to the mitochondria. The negative membrane potential across the mitochondrial inner membrane drives the accumulation of lipophilic cations within the mitochondria.
Figure 2.
Changes in the renal microcirculation and oxidant generation during sepsis in rat pups. Rat pups at 17–18 days of age were subjected to cecal ligation and puncture (CLP) surgery to induce sepsis or to a sham surgical procedure (no cecal ligation or puncture).5 Representative intravital video microscopy (IVVM) images of the kidney in a live rat pup 6 h after CLP or the sham procedure are shown in a–d (original magnification ×200). Arrows indicate capillaries with no flow. (a, b) Peritubular capillary perfusion and (c, d) the corresponding mitochondrial superoxide (O2•−) measured by MitoSOX fluorescence. Detailed methods are described in the study by Patil et al.4 White areas in c and d indicate mitochondrial O2•− generation in tubular epithelial cells. (e, f) The effects of MitoTEMPO (10 mg/kg, i.p.) at 18 h after CLP. MitoTEMPO was administered at the time of CLP. Data are mean ± SEM from n = 4–6 pups per group. *P < 0.05 as compared with Sham; †P < 0.05 as compared with Sham and CLP.
Although not yet demonstrated in septic patients, animal studies indicate that microvascular leakage and redistribution of flow through the renal microcirculation occur within the first few hours during sepsis and result in areas of localized hypoxia, which are spatially associated with oxidant generation by the tubular epithelial cells. Consequently, attempts to target the initial renal capillary injury alone may have limited therapeutic value because microcirculatory failure might occur too rapidly. Moreover, the fragility of the immature renal vasculature and the changing systemic hemodynamics during septic shock not only would make targeting renal vascular resistance challenging but could reduce perfusion even further.
The use of general antioxidant therapies, which are not targeted to the mitochondria, has been largely ineffective in septic patients or still requires additional clinical trials to fully demonstrate efficacy. Initial preclinical studies with MitoTEMPO suggest that it may be possible to not only target the source of oxidants directly but also improve mitochondrial function, which could more effectively promote recovery. Although the development of SAKI in infants and adults may be different, mitochondrial dysfunction could be a common mechanism of renal epithelial injury and hence may be amenable to therapy with mitochondria-targeted antioxidants such as MitoTEMPO and other compounds such as mitoquinone. Another very intense area of research aims to identify agents that can promote organ recovery through mitochondrial biogenesis. Because mitochondrial injury probably occurs in other organs as well, this targeted approach should be investigated further in preclinical studies. However, a number of important considerations regarding mitochondria-targeted therapy in the septic infant should be incorporated into these studies. The optimal dosage and administration routes may need to be tailored due to changing pharmacokinetic/pharmacodynamic parameters, metabolism, and blood flow distribution in the developing infant. Another important consideration is that mitochondria-targeted agents could themselves alter mitochondrial membrane potential as they accumulate and thereby actually worsen mitochondrial function.
Considering that therapy for the septic infant is probably begun only after the initiation of organ injury, new therapies should be evaluated using a clinically relevant delayed-dosing paradigm. Moreover, the complexities of sepsis suggest that targeting multiple pathways may be synergistic and might have the greatest impact on outcomes. Mitochondria-targeted therapies could be a new strategy in this approach to break the cycle of injury and promote recovery of organ function.
Acknowledgments
This work was supported by grants R01 GM106419 (L.A.M.-C. and P.R.M.) and T32 GM106999 (P.R.M.) from the National Institutes of General Medical Sciences and by grant 14PRE20450050 (C.R.S.) from the American Heart Association.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Romanovsky A, Morgan C, Bagshaw SM. Pathophysiology and management of septic acute kidney injury. Pediatr Nephrol. 2014;29:1–12. doi: 10.1007/s00467-013-2427-6. [DOI] [PubMed] [Google Scholar]
- 2.De Backer D, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41:791–799. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 3.Gomez H, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306:F734–F743. doi: 10.1152/ajprenal.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seely KA, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;301:F209–F217. doi: 10.1152/ajprenal.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]