Abstract
Brain-derived neurotrophic factor (BDNF) signaling through its receptor, tropomyosin receptor kinase B (TrkB), plays a critical role in neural plasticity and its dysregulation in striatum and prefrontal cortex (PFC) has been implicated in the etiology of mental health disorders such schizophrenia and drug addiction. In the present study we characterized age-dependent differences in BDNF signaling and TrkB expression within the nucleus accumbens (NAc), caudate putamen (CP) and PFC in rats and determined the effects of administration of the dopamine agonist, SKF 83959, previously which selectively activates the Gq-coupled dopamine receptors, the dopamine D5 receptor and the D1-D2 receptor heteromer. As proBDNF binds with high affinity to the p75 neurotrophin receptor (p75NTR), expression levels of these proteins were also assessed. The present findings showed that juvenile rats (age-26-28 days) exhibited significantly elevated basal BDNF expression and activation of full length TrkB (TrkBfull) in NAc compared to their adult counterparts, as evidence by increased TrkBfull phosphorylation. These changes were concomitant with an increase in the relative expression of TrkBfull compared to the truncated isoform, TrkB.T1, in NAc and CP. Conversely, in PFC the basal expression of BDNF in juvenile rats was significantly lower than in adult rats with an elevated relative expression of TrkBfull. Acute administration of SKF 83959 to juvenile rats abolished the age-dependent differences in BDNF expression in NAc and PFC, and in the relative expression of TrkBfull in NAc and CP. Together these findings indicate that the expression and/or signaling of BDNF and TrkB in striatum and PFC of juvenile rats is fundamentally different from that of adult rats, a finding that may have implications in neuropsychiatric disorders that exhibit age-dependent susceptibility such as schizophrenia and drug addiction.
Keywords: SKF 83959, brain-derived neurotrophic factor, tropomyosin receptor kinase B, juvenile, prefrontal cortex, nucleus accumbens
Introduction
Adolescence is a period of maturation in the corticolimbic regions of the brain and evidence suggests that this stage of development may coincide with the initial manifestation of symptoms associated with neuropsychiatric disease. Symptoms of depression and schizophrenia, for example, show significant increases during adolescence [1,2], a developmental period also associated with increased sensitivity to psychostimulant-induced reward [3-6]. Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, acts within the central nervous system via the tropomyosin receptor kinase B (TrkB) to support the survival of existing neurons [7,8], maintain neuronal synapse integrity [9], and promote neuronal growth and differentiation [8,10-12]. In view of these critical actions on neuronal plasticity, BDNF has been postulated to be associated with a number of mental health disorders that develop during adolescence including depression, schizophrenia and drug addiction (for review, [13])
Given the reported additional roles of mesocorticolimbic BNDF in the regulation of behaviours associated with neuropsychiatric disease such as anhedonia [14,15] and cognitive dysfunction [16], as well as addiction-related behaviours [17-19], it is possible that transient changes in BDNF signaling during development may contribute to the age-dependent susceptibility to neuropsychiatric disease. One mechanism by which BDNF expression is mediated is through the activation of specific dopamine receptors, and indeed increased expression of BDNF in the striatum and prefrontal cortex (PFC) has been linked to the D1 receptor (D1R) [20], the D5 receptor (D5R) [21] and the D1-D2 receptor heteromer [11,22]. In line with this reasoning, it has also been hypothesized that age-dependent differences in dopamine receptor expression may contribute to the increased risk of adolescents to develop drug addiction [23]. For example, it has been reported that delayed drug extinction in adolescence may be mediated by an overexpression of D1R on glutamatergic output neurons in the PFC, whereas in the adult the D1R is preferentially expressed on GABAergic interneurons [23]. Increased expression levels of striatal D1R in striatum and PFC have also been shown to attain peak densities during adolescence with a slow decline in receptor expression into adulthood [24,25]. Although the age-dependent expression of the D5R in striatum and PFC have not been characterized to our knowledge, a transient increase in the mRNA levels of the D5R from birth to adulthood in both regions have been reported in rats [26]. However, as the abundance of the D5R is very low in striatal neurons (~1-2% of neurons), this would suggest that the impact of the D5R on BDNF signaling would be more physiologically relevant in PFC where D5R expression is much higher and consistent with this idea, it has been recently demonstrated that activation of the D5R in adult rats resulted in increased BDNF expression in PFC, an effect that was not observed in striatum [21].
The expression of BDNF in NAc has also been linked to the dopamine D1-D2 receptor heteromer [11,22], a receptor complex with pharmacological and functional properties that are distinct from its constituent receptors [22,27-29], and which is localized to a unique subset of striatal medium spiny neurons that coexpress GABA and glutamate, as well as dynorphin and enkephalin, in addition to the D1R and D2R [22]. Similar to the D1R and D2R, the expression and functional activity of the D1-D2 heteromer in striatum has been shown to exhibit age-dependent changes in rodents [29,30]. However, in contrast to the D1R which show increased densities in the striatum of younger animals, in the shell region of the NAc and caudate putamen (CP) the expression of the D1-D2 heteromer is reduced [30]. It was demonstrated that the lower expression levels were not a consequence of reduced formation of the D1-D2 heteromer, but were the direct result of fewer numbers of D1R and D2R coexpressing neurons in these regions [30]. Consistent with these findings of an age-dependent increase in D1-D2 heteromer expression, the activity of the D1-D2 receptor heteromer has also been shown to be elevated in older animals compared to young adult animals [29].
Although evidence indicates that dopamine receptor expression is dynamically regulated with age, dopamine-mediated differences in BDNF expression between young and adult rats have not been elucidated, information that may be particularly relevant to neuropsychiatric diseases such as addiction, where adolescents have been shown to exhibit enhanced reward perception [31]. Thus in the present study we sought to examine age-dependent differences in basal levels of BDNF expression and signaling in regions of the corticostriatal circuitry, the striatum and PFC. As the precursor to BDNF, proBDNF, has also been implicated in disorders of cognitive dysfunction [32] and binds with high affinity to the p75 neurotrophin receptor (p75NTR) [33], the expression of this proneurotrophin and p75NTR was also assessed. In addition, as both the D5R and the D1-D2 receptor heteromer have been linked to the regulation of BDNF expression, age-dependent effects of D5R and D1-D2 heteromer activation on BDNF and proBDNF expression and signaling was determined. To achieve this the dopamine agonist SKF 83959 was utilized, an agonist that does not activate the Gs-linked D1R [34], but has been linked to phospholipase C (PLC) activation mediated either by the D5R [35], or the dopamine D1-D2 receptor heteromer [27], and regulated by the Gq protein [29,36]. We showed that juvenile rats exhibited brain region-dependent differences in the basal expression of BDNF with increased expression in NAc and reduced expression in PFC compared to adult rats. These changes were associated with increased activation of the BDNF receptor TrkB in NAc. We further showed in CP that the expression of the truncated isoform of the TrkB receptor, TrkB.T1, was significantly lower in juvenile rats. Finally, we showed that following acute administration of SKF 83959 many of these age-dependent differences in protein expression were abolished signifying that Gq-coupled dopamine receptor-dependent effects on BDNF signaling were age-dependent.
Methods
Animals
Experimentally naïve juvenile (aged 26-28 days) or adult male Sprague-Dawley rats (aged ~4 months) (Charles River, Canada), weighing 50-70 g or 400-450 g at the start of the experiment were used (N=6-7/group per experiment). Rats were pair-housed in polyethylene cages in a colony room maintained on a 12-h light–dark cycle with free access to food and water. Following arrival, rats were handled for 2 minutes daily for 3 days before the start of experiments. All treatments were performed during the light phase of the day–night cycle. Animals were housed and tested in compliance with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993).
Drugs
SKF 83959 hydrobromide (Tocris Bioscience) was dissolved in physiological saline containing 5% DMSO and administered subcutaneously at a dose of 1.5 mg/kg. This dose was chosen as we have previously shown increased BDNF expression in adult rat NAc and ventral tegmental area following administration of SKF 83959 at this dose [22]. SKF 83959 administered at this dose is also sufficient to induce significant increases in behavioral responses, such as grooming, in rodents [22,28]. SKF 83959 has been previously shown to activate the Gq-coupled D1-D2 receptor heteromer [29] or the dopamine D5 receptor [21,35]. For non-drug injections, an equivalent volume of saline was administered. All injections were administered at a volume of 1.0 ml/kg.
Immunoblot
Tissue from NAc, CP and PFC was obtained 90 minutes following SKF 83959 administration, and flash-frozen until ready for use. Tissue from NAc, CP and PFC was obtained 90 minutes following SKF 83959 administration, and flash-frozen until ready for use. This time point was chosen based on our previous reports that showed a significant increase in BDNF expression in cultured striatal neurons 1-2 hours following SKF 83959 administration [11] and in adult rat NAc 90 minutes following an acute SKF 83959 injection [11,22]. Tissue was thawed on ice and suspended in cell lysis buffer containing 5 mM Tris, 0.2 mM EDTA, protease and phosphatase inhibitors, and sonicated briefly on ice. Protein concentrations were measured using the Bradford assay. 40 μg of protein were incubated in sample buffer containing Tris-HCl 125 mM pH 6.5, SDS 4%, glycerol 20%, bromophenol blue 0.005% and β-mercaptoethanol 5% for 3 minutes at 95° C. Samples were separated by SDS-PAGE on a 10% or 14% gel and electroblotted on PVDF transfer membrane for 2.5 hours. Membranes were blocked for 1 hour in 3-5% skim milk in TBS with 0.1% Tween 20 and incubated overnight at 4° C with gentle shaking with primary antibody to BDNF 1:10000 (Millipore), proBDNF (Abcam), p75 neurotrophin receptor (p75NTR) 1:2000 (Millipore), TrkB 1:2500 (BD Biosciences), and to phosphorylated TrkB at Y817 (Epitomics). GAPDH 1:10000 (Abcam) was used as a loading control. Membranes were then washed in TBS-Tween and incubated for 2 hours at room temperature with the appropriate species-specific secondary antibody (Bio-Rad Laboratories, Hercules, CA, USA). Antibody labeling of proteins were detected with enhanced chemiluminescence (Amersham Biosciences, UK) and signal intensity was quantified using Zeiss AxioVision4 software.
Data Analysis
All values are reported as mean ± SEM. The statistical significance of each dependent measure was evaluated using ANOVA with Drug and Age as the between subject factors followed by posthoc tests for planned comparisons. The immunoblot data was collected by obtaining both the densitometry and area of the band and the main dependent variable was Grey × area, expressed as a percent of saline-treated adult controls. Computations were performed using the SPSS/PC+ statistical package. Statistical criteria for significant differences were set at P<0.05.
Results
SKF 83959, an agonist for the Gq-coupled D5R and D1-D2 heteromer, but which does not activate the Gs-coupled D1R or the Gi-coupled D2R [29,34], has been shown to increase the expression of BDNF in neonatal striatal culture and adult rat NAc via a D1-D2 receptor heteromer-mediated mechanism [11,22]. As the D1-D2 receptor heteromer has been recently shown to exhibit age-dependent differences in striatal expression levels [29,30], age-related basal and SKF 83959-induced changes in BDNF levels were assessed (fig. 1). Examination of basal BDNF levels revealed significantly higher expression in juvenile rat NAc compared to adults (P=0.040) (fig. 1a). In accordance with our previous findings, activation of the D1-D2 heteromer induced a substantial increase in NAc BDNF expression in adults (P=0.008), but had a lesser effect on BDNF levels in juveniles that was not significant (P=0.071, n.s.). There were no significant effects of SKF 83959 on BDNF expression in CP in either age group (fig. 1b). As SKF 83959 also increases BDNF expression in adult rat PFC via a D5R-mediated mechanism [21], age-dependent differences in the induction of BDNF by the agonist were additionally assessed. In contrast to that observed in NAc, juvenile rats exhibited significantly lower basal levels of BDNF in PFC compared to their adult counterparts (P=0.01, fig. 1c). Administration of SKF 83959 induced an increase in BDNF expression in both age groups, although the effect was more robust in the juvenile rats (adult vs saline: P<0.03; juvenile vs saline: P<0.001).
Fig. 1.
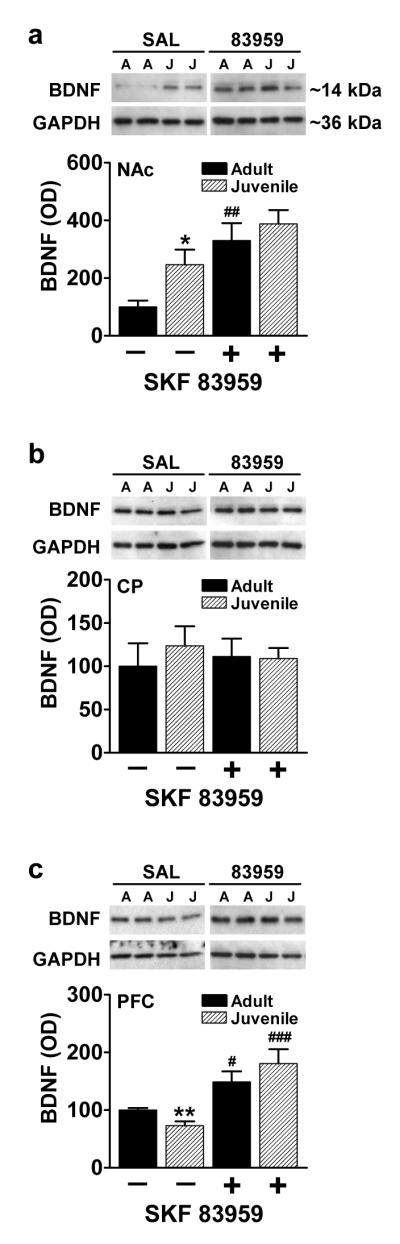
Age-specific and SKF 83959-dependent effects on BDNF expression in NAc, CP and PFC of adult and juvenile rats. (a) Animals exhibited an age-dependent difference in basal BDNF expression in NAc, with juvenile rats exhibiting significantly higher expression than their adult counterparts. SKF 83959 (1.5 mg/kg s.c.) administration resulted in a modest but insignificant increase in BDNF levels in juvenile animals, however a substantial increase in BDNF expression was induced in adult rats (b) Adult and juvenile rats showed similar basal BDNF levels in CP. SKF 83959 treatment had no effect on BDNF expression in this region. (c) Juvenile rats exhibited significantly lower basal BDNF expression in PFC than adult rats. SKF 83959 significantly elevated BDNF expression in both age groups. Representative blots for each region are also shown. GAPDH was used as a loading control. Data are represented as mean ± SEM, and are expressed as a percent of adult/saline controls. {NAc: Drug, F(1,22)=13.0, P=0.002; Age, F(1,22)=3.8, P=0.06, n.s.; Drug × Age, F(1,22)=0.7, P=0.40, n.s.; CP: Drug, F(1,22)=0.01, P=0.94, n.s.; Age, F(1,22)=0.3, P=0.61, n.s.; Drug × Age, F(1,22)=0.4, P=0.54, n.s.; PFC: Drug, F(1,23)=22.3, P<0.001; Age, F(1,23)=0.02, P=0.88, n.s; Drug × Age, F(1,23)=3.1, P=0.09, n.s.}. N=6-7/group per experiment. *P<0.05 compared to basal expression of adult rats; ##P<0.01 compared to saline controls of the same age.
The BDNF receptor, TrkB, can be expressed as a full-length isoform (TrkBfull) in which the signaling capabilities are dependent on ligand-mediated phosphorylation of tyrosine residues. To determine whether the activation state of TrkBfull differed between juvenile and adults animals, the phosphorylation of TrkBfull at tyrosine 817 (Y817) was determined. In NAc, the basal phosphorylation state of TrkBfull was higher in juvenile rats compared to the adult rats (P=0.046, fig. 2a) with no age-dependent differences observed in CP or PFC (fig. 2b,c) although differences in BDNF expression were seen 90 minutes followings SKF 83959 administration, a corresponding increase in TrkBfull phosphorylation at this time point was not evident (fig. 2a-c).
Fig. 2.
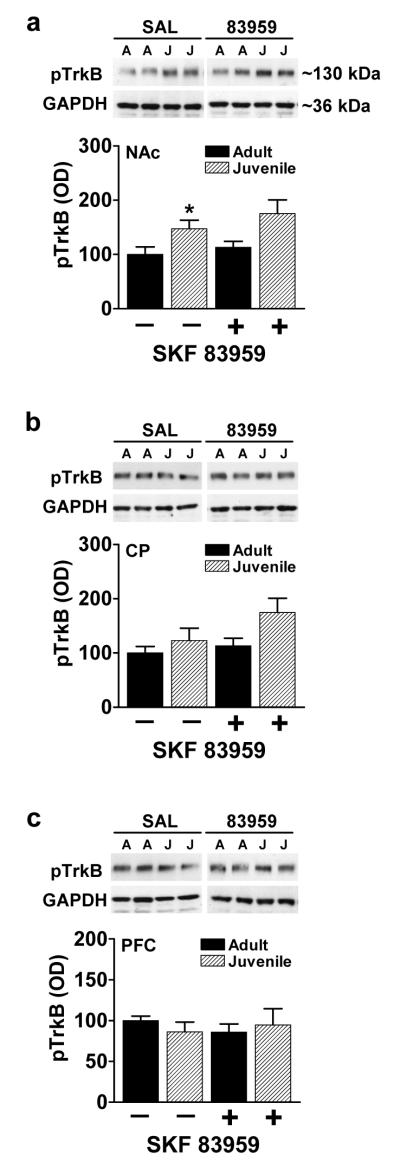
Juvenile rats exhibit enhanced TrkBfull activation in NAc. (a) Juvenile rats exhibited increased basal phosphorylation of TrkBfull compared to their adult counterparts. SKF 83959 (1.5 mg/kg s.c.) had no effect on TrkBfull phosphorylation 90 minutes following administration. (b-c) Adult and juvenile rats showed similar phosphorylation levels of TrkBfull in both CP and PFC. SKF 83959 did not alter the phosphorylation status of TrkB in CP or PFC of either age group. Representative blots for each region are also shown. GAPDH was used as a loading control. Data are represented as mean ± SEM, and are expressed as a percent of adult/saline controls. {NAc: Drug, F(1,21)=1.3, P=0.27, n.s.; Age, F(1,22)=9.0, P=0.007; Drug × Age, F(1,22)=0.16, P=0.69, n.s.; CP: Drug, F(1,22)=3.8, P=0.06, n.s.; Age, F(1,22)=6.5, P=0.02; Drug × Age, F(1,22)=1.4, P=0.25, n.s.; PFC: Drug, F(1,21)=0.04, P=0.85; Age, F(1,21)=0.03, P=0.86, n.s; Drug × Age, F(1,21)=.68, P=0.42, n.s.}. N=6-7/group per experiment. *P<0.05 compared to basal expression of adult rats.
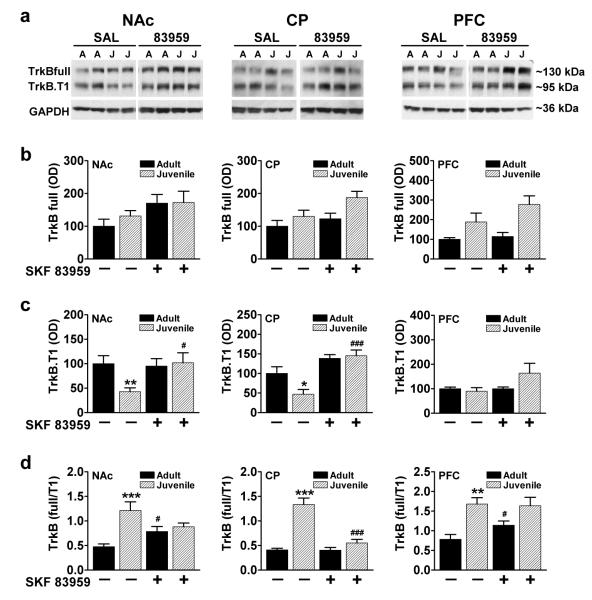
The truncated isoform of TrkB, TrkB.T1, attenuates the physiological responses that would normally be induced by the neurotrophin at TrkBfull by sequestering BDNF [37,38] or via dominant negative inhibition through the formation of inactive heterodimers [39]. TrkB.T1 has also been shown to induce its own signaling cascades independent of TrkBfull [40]. Adult and juvenile rats exhibited similar basal expression levels of the full length isoform, TrkBfull, in all three regions examined (fig. 3a,b). SKF 83959 did not alter TrkBfull expression in NAc or PFC with a trend towards increased expression in the CP of juvenile rats (P=0.064, n.s., fig. 3b, middle panel). Conversely, the basal expression of TrkB.T1 (fig. 3a,c) was substantially different between juvenile and adult rats in both NAc (P=0.009) and CP (P=0.031), with juveniles showing significantly reduced levels of this isoform compared to their adult counterparts (fig. 3c, left and middle panels). SKF 83959 induced a significant increase in TrkB.T1 expression in juvenile rat NAc (P=0.022) and CP (P=0.0001), but did not influence levels in adults. No age- or drug-dependent effects were observed in PFC (fig. 3c, right panel).
Fig. 3.
Effect of age and SKF 83959 on expression of TrkBfull and TrkB.T1 in NAc, CP and PFC of adult and juvenile rats. (a) Representative blots depicting age-related and SKF 83959-induced changes in full length (TrkBfull) and truncated TrkB (TrkB.T1) are shown. GAPDH was used as a loading control. (b) Adult and juvenile rats exhibited similar basal expression levels of the active isoform TrkBfull in all three regions examined. SKF 83959 (1.5 mg/kg s.c.) did not alter TrkBfull expression in NAc, CP or PFC {NAc: Drug, F(1,21)=4.4, P=0.049; Age, F(1,21)=0.4, P=0.54, n.s.; Drug × Age, F(1,21)=0.3, P=0.59, n.s.; CP: Drug, F(1,21)=6.7, P=0.017; Age, F(1,21)=4.7, P=0.042; Drug × Age, F(1,21)=0.9, P=0.35, n.s.; PFC: Drug, F(1,22)=2.2, P=0.15, n.s.; Age, F(1,22)=13.2, P=0.001; Drug × Age, F(1,22)=1.2, P=0.29, n.s.}. (c) Juvenile animals showed lower basal levels of TrkB.T1 expression compared to adults in NAc and CP, with no differences in PFC. SKF 83959 did not alter TrkB.T1 expression in any region in adult rats, but elevated the expression of this TrkB isoform in NAc and CP, but not PFC, of juvenile rats {NAc: Drug, F(1,21)=3.0, P=0.10; Age, F(1,21)=2.6, P=0.12; Drug × Age, F(1,21)=4.2, P=0.048; CP: Drug, F(1,21)=24.1, P=0.0001; Age, F(1,21)=2.8, P=0.11, n.s.; Drug × Age, F(1,21)=4.6, P=0.044; PFC: Drug, F(1,21)=2.7, P=0.11, n.s.; Age, F(1,21)=1.4, P=0.25, n.s.; Drug × Age, F(1,21)=2.7, P=0.11, n.s.}. (d) The ratio of expression of full to truncated TrkB isoforms. Juvenile rats showed a significantly higher basal TrkB ratio (TrkBfull:TrkB.T1) compared to adults in NAc, CP and PFC. SKF 83959 increased the TrkB ratio in adult rats in NAc and PFC, with no effects in these regions in juvenile rats. In CP, SKF 83959 significantly reduced the TrkB ratio in juvenile rats with no effect in adults. Data are represented as mean ± SEM, and are expressed as a percent of adult/saline controls. {NAc: Drug, F(1,20)=0.01, P=0.92, n.s.; Age, F(1,20)=16.1, P=0.001; Drug × Age, F(1,20)=9.5, P=0.006; CP: Drug, F(1,21)=27.8, P=0.0001; Age, F(1,21)=51.2, P=0.0001; Drug × Age, F(1,21)=26.9, P=0.0001; PFC: Drug, F(1,22)=0.1, P=0.71, n.s.; Age, F(1,22)=22.5, P=0.0001; Drug × Age, F(1,22)=0.002, P=0.97, n.s.}. N=6-7/group per experiment. **P<0.01, ***P<0.001 compared to basal expression of adults rats; #P<0.05, ###P<0.001 compared to saline controls of the same age.
Changes in the relative proportion of TrkBfull and TrkB.T1 could have significant implications in the regulation of dendritic growth [41] and potentially impact on overall BDNF-induced signaling as a result of the dominant-negative effect of TrkB.T1 on TrkBfull-mediated signaling. Juvenile rats innately showed a higher proportion of TrkBfull compared to TrkB.T1 in the NAc (P=0.001), CP (P=0.0001) and PFC (P=0.004) compared to adults (fig. 3d). Interestingly, SKF 83959 treatment resulted in discrepant effects on the ratio of TrkBfull:TrkB.T1 in juvenile and adult animals. Specifically, although SKF 83959 increased the ratio in adult NAc (P=0.024) and PFC (P=0.050) (fig. 3d, left and right panels), no effects were observed in these regions in the juvenile animals. Conversely, whereas SKF 83959 had no effect on the TrkB ratio in adult CP, a robust drug-induced decline in the ratio was evident in juvenile CP (P=0.0001) (fig. 3d, middle panel).
Whereas BDNF binds to p75NTR with low affinity, the precursor to BDNF, pro-BDNF, binds to p75NTR with high affinity [33]. This pan neurotrophin receptor has been linked to apoptosis [42] and has been shown to act as a coreceptor to enhance the affinity and activity of Trk receptors [43]. We therefore examined age- and SKF 83959-dependent changes in proBDNF and p75NTR expression levels in NAc, CP and PFC (figs. 4,5). No age-related difference in basal protein expression of proBDNF was observed in any region, nor did SKF 83959 have any effects (fig. 4a-c). Similarly, no age-dependent differences were evident upon examination of p75NTR expression (Fig. 5a-c). SKF 83959 treatment had no effects on p75NTR expression in NAc or CP (fig. 5a,b), but significantly increased p75NTR levels to a similar degree in both juvenile (P=0.018) and adult PFC (P=0.042) (fig. 5c).
Fig. 4.
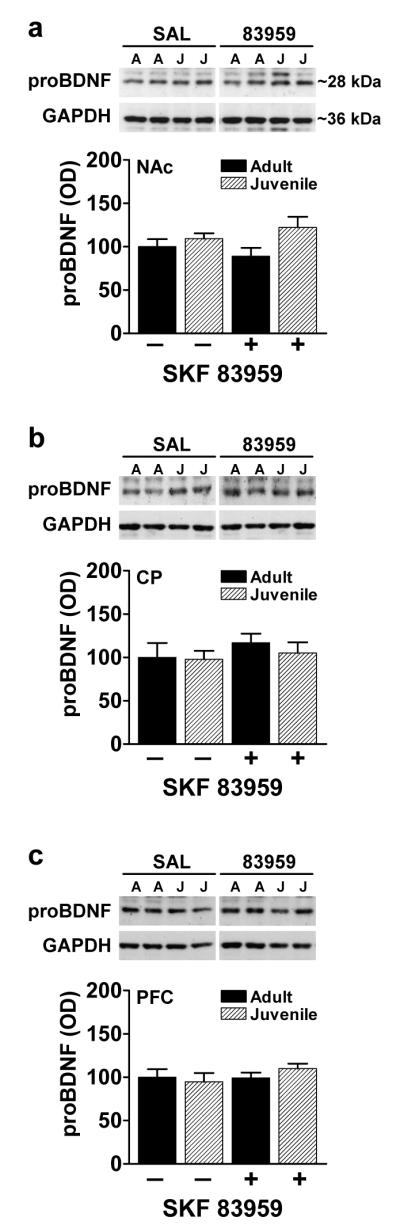
Effect of age and SKF 83959 on proBDNF expression in NAc, CP and PFC. (a-c) No significant differences in the expression of proBDNF were observed between juvenile and adults rats in any of the regions examined. SKF 83959 had no effects on proBDNF expression in either age group.. Representative blots for each region are also shown. GAPDH was used as a loading control. Data are represented as mean ± SEM, and are expressed as a percent of adult/saline controls. {NAc: Drug, F(1,22)=0.01, P=0.91, n.s.; Age, F(1,22)=4.7, P=0.04; Drug × Age, F(1,22)=1.5, P=0.23, n.s.; CP: Drug, F(1,21)=0.9, P=0.34, n.s.; Age, F(1,21)=0.3, P=0.58; Drug × Age, F(1,21)=2, P=0.7, n.s.; PFC: Drug, F(1,22)=0.9, P=0.36; Age, F(1,22)=0.1, P=0.72, n.s; Drug × Age, F(1,22)=1.1, P=0.32, n.s.}. N=6-7/group per experiment. *P<0.05 compared to basal expression of adult rats.
Fig. 5.
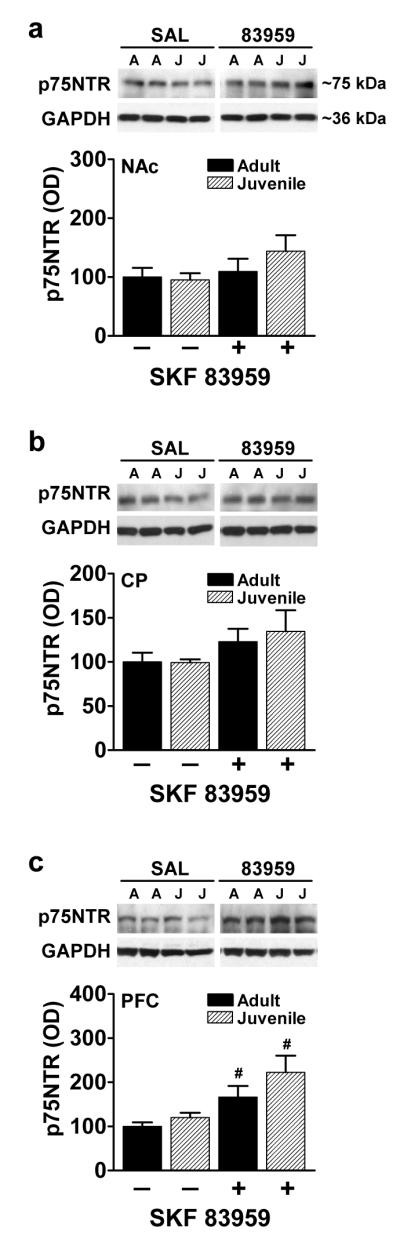
Effect of age and SKF 83959 on p75NTR expression in NAc, CP and PFC of adult and juvenile rats. (a, b) There were no age-related or SKF 83959-induced alterations in p75NTR expression in NAc or CP. (c) In PFC, basal expression of p75NTR did not differ between age groups. However, SKF 83959 (1.5 mg/kg) induced a significant increase in p75NTR levels in both adult and juvenile rats. Representative blots for each region are also shown. GAPDH was used as a loading control. Data are represented as mean ± SEM, and are expressed as a percent of adult/saline controls. {NAc: Drug, F(1,22)=2.0, P=0.18, n.s.; Age, F(1,22)=0.5, P=0.48, n.s.; Drug × Age, F(1,22)=0.9, P=0.36, n.s.; CP: Drug, F(1,21)=2.6, P=0.11, n.s.; Age, F(1,21)=0.30, P=0.59, n.s.; Drug × Age, F(1,21)=0.9, P=0.35, n.s.; PFC: Drug, F(1,22)=13.0, P=0.002; Age, F(1,22)=2.7, P=0.11, n.s.; Drug × Age, F(1,22)=0.59, P=0.45, n.s.}. N=6-7/group per experiment. #P<0.05 compared to saline controls of the same age.
Discussion
The present study showed that BDNF-TrkB signaling differed between juvenile and adult rats with juvenile animals exhibiting heightened BDNF expression and TrkBfull activation in the NAc, and a significant increase in the relative expression of TrkBfull compared to TrkB.T1 in NAc and CP. Conversely in juvenile rat PFC, the basal expression of BDNF was significantly reduced compared to adult rat PFC although the proportion of TrkBfull was elevated. It was further demonstrated that administration of SKF 83959, an agonist for the Gq-coupled D5R and D1-D2 receptor heteromer, had differential effects in juvenile and adults rats and could effectively normalize the differences in BDNF-TrkB signaling observed in the juvenile animals to adult levels. Although the present discussion will focus on BDNF regulation by dopamine, it is important to note that a number of neurotransmitter systems may have contributed to the present findings of differential basal BDNF expression in juvenile rats, as serotonin [44], norepinephrine [45], glutamate [46], and GABA [47] have also been shown to be involved in the neuronal regulation of BDNF. Furthermore, while the regional differences in protein expression will be discussed, it is important to note that the effects reported in the present study may not be mutually exclusive as interactions between the striatum and PFC, both of which are components of the mesocorticolimbic circuitry, are well characterized.
BDNF expression in juvenile and adults rats
It has been suggested that the adolescent striatum exists in a hyperdopaminergic state, with the dopamine system exhibiting a much higher tone relative to that in adults, and at the same time exhibiting blunted pharmacological responsiveness to dopamine receptor stimulation [48]. This hypothesis is supported by our previous behavioural findings, which showed a subsensitivity to grooming responses induced by SKF 83959 in juvenile rats [30], as well as the present findings that showed not only an increase in the basal expression of BDNF in NAc, but blunted SKF 83959-induced BDNF expression in NAc of juvenile rats. Increased striatal BDNF expression has been linked to both D1R and [20] D1-D2 heteromer activation [11,22]. However evidence suggests that BDNF expression reported to be induced by the D1R may in fact be the result of dopamine agonist cross-reactivity with the D1-D2 heteromer. Specifically, in a study by Williams and Undieh [20] the “selective” D1R agonist SKF 38393 was shown to increase striatal BDNF protein in brain slices. However SKF 38393 induces behavioural and neurochemical effects characteristic of dopamine D1-D2 heteromer activation, such as grooming and activation of phospholipase C leading to intracellular calcium release [29,49-51]. In addition, we have previously shown that the highly selective D1R agonist SKF 83822, which activates the Gs-linked D1R but not the D1-D2 heteromer [29], had no effect on BDNF expression in striatum following its administration to rats [11]. Thus although the basal activation state of the D1R may be elevated in young animals as hypothesized [48], it is unlikely that the enhanced expression of BDNF in juvenile rat NAc was mediated by this receptor, but rather through the D1-D2 heteromer. Indeed, an increase in the basal functional activity of the D1-D2 heteromer in striatum under conditions of hyperdopaminergia has been previously reported, whereby there was an increased percentage of D1-D2 heteromers in the high affinity active state in adult rats following repeated amphetamine and, additionally, in the postmortem globus pallidus of schizophrenia patients, who have a neuropsychiatric disorder characterized by elevated striatal dopamine transmission [28]. Therefore, although the expression of the D1-D2 heteromer has been shown to be reduced in NAc in juvenile rats [30], evidence indicates there may be an overall increase in the activity of the receptor complex in this region, potentially contributing to the enhanced basal BDNF expression in juvenile rats.
In contrast to the findings in striatum, the expression of BDNF in juvenile rat PFC was lower than in their adult counterparts. A dopaminergic mechanism underlying these age-dependent differences is difficult to elucidate given that extracellular dopamine levels within the PFC appear to remain relatively consistent across ages [52,53]. In addition, we have demonstrated no role for the D1-D2 receptor heteromer in the regulation of BDNF expression in this region [21]. Activation of the Gq-coupled D5R has been recently shown to regulate BDNF expression in PFC [21], and is thus a likely candidate mediating the observed suppression of BDNF expression in juvenile PFC. However aside from one study which showed a transient increase in the mRNA levels of the D5R from birth to adulthood in striatum and PFC [26], age-dependent differences in D5R expression or functional activity have not been characterized, likely as a result of a complete lack of pharmacological agents selective for this receptor which structurally and pharmacologically resembles the D1R. Finally, one other possibility to explain the reduced BDNF levels in juvenile rat PFC lies with the idea that during development there is a restructuring of striatocortical networks or synaptic remodeling [54,55]. It has been shown, for example, that in juvenile rats neuronal connectivity involving the PFC is immature [54], which may potentially impact on dopamine and BDNF signaling within this region.
TrkB expression in juvenile and adults rats
The levels of the truncated isoform of TrkB, TrkB.T1, were significantly reduced in both the NAc and CP of juvenile rats, with no change in the expression levels of TrkBfull, and resulting in an increased proportion of TrkBfull in young animals compared to adult rats. Similarly, the proportion of TrkBfull was elevated in PFC. Although classically believed to simply be a non-signaling isoform of TrkB, yet capable of binding BDNF and thus BDNF scavenger per se, evidence now indicates that TrkB.T1 may be of further physiological significance (for review [40]). In addition to the role of TrkB.T1 as a dominant negative regulator of TrkBfull signaling, accomplished by competitive binding of BDNF as well as through dimerization of the two TrkB isoforms [39], TrkB.T1 in non-neuronal cells has been shown to be involved in the regulation of extracellular BDNF availability via sequestration of the neurotrophin followed by its degradation or the subsequent presentation of BDNF to neurons by exocytosis [37,38,40]. Furthermore, while not yet established in neurons, TrkB.T1 has also been implicated in the regulation of kinase activity in non-neuronal cells that requires an intact intracellular domain [56]. BDNF-dependent TrkB.T1 stimulation has also been shown to regulate calcium signaling in glial cells [57] as well as to promote the differentiation of neural stem cells to astrocytes by a mechanism involving G protein and PKC [58].
That the proportion of TrkBfull to TrkB.T1 differed in striatum and PFC between juvenile and adult rats is an interesting finding given reports demonstrating that the ratio of the two isoforms can regulate discrete modes of dendritic neuronal growth [41]. Specifically, while BDNF-induced activation of TrkBfull induced dendritic growth proximal to the cell soma, activation of TrkB.T1 promoted dendritic elongation distal to the cell soma. Moreover, the effects of each of the TrkB isoforms on dendritic growth were mutually inhibitory indicating that the ratio of the two isoforms may serve as a regulatory switch between the two modes of growth [41]. As the neural connectivity of juvenile brain is still developing the present findings may simply reflect possible age-dependent differences in dendritic growth patterns, and future studies examining the neuronal localization of the TrkB isoforms, and their association to dendritic growth patterns in young and adult animals would be insightful. However, that SKF 83959 robustly increased the expression of TrkB.T1 in juvenile animals suggests that this agonist may influence the mode of dendritic growth by promoting distal neurite outgrowth, and indeed this idea is consistent with our previous report that showed increased BDNF release and dendritic elongation in cultured striatal neurons following exposure to SKF 83959 [11]. Although Hasbi and colleagues [11] did not examine a direct role for TrkB.T1 in mediating these SKF 83959-induced effects on dendritic growth, TrkB.T1 expression has been reported in cultured striatal neurons [59] and thus a role for this receptor in mediating the observed effects of SKF 83959 on neurite outgrowth seems plausible.
It is noteworthy that while increased basal activation of TrkBfull was evident in the NAc of juvenile rats compared to their adult counterparts in response to elevated BDNF levels, no effect of SKF 83959 on BDNF-induced TrkB activation was observed. The phosphorylation of TrkBfull has been shown to occur quite rapidly following exposure to BDNF [60]. Although the 90 minute time point following drug administration was chosen to reflect known maximal changes in BDNF expression [11,22], acute SKF 83959-induced increases in BDNF expression is time dependent and becomes evident as early as 60 minutes [11]. Thus while a persistent elevation in basal BDNF expression may result in sustained increased TrkBfull phosphorylation, the acute effects of BDNF on TrkBfull phosphorylation may be more rapid.
proBDNF and p75NTR
Proneurotrophins, such a proBDNF, bind the p75NTR with high affinity [33]. Although p75NTR has been shown to regulate apoptosis [42], this receptor has also been more recently linked to other physiological processes such as functioning as a coreceptor to enhance the affinity and activity of Trk receptors [43]. In line with this, p75NTR has also been implicated as having a role in TrkB.T1-induced dendritic outgrowth [61]. Furthermore, p75NTR signaling has been associated with the facilitation of long term depression (LTD) [62], a synaptic modification thought to play a role in clinical depression [63]. Although there were no differences in the basal expression of proBDNF or p75NTR in the present study, an SKF 83959-induced increase in p75NTR levels in PFC was evident in both age groups. While the physiological importance of this increase could involve a number of processes, as described above, dopamine D1-D2 heteromer activity in PFC has been previously linked to depression [64], an effect that may be mediated in part by increased expression of p75NTR.
Implications and Conclusions
In the present study we showed that young rats exhibited region-specific differences in BDNF expression and signaling compared to that of adult rats and, additionally, exhibited differential responses to SKF 83959-induced changes in BDNF and TrkB expression. BDNF signaling has diverse roles in the regulation of synaptic plasticity and thus the developmental purpose of the observed differences in BDNF and TrkB expression is likely complex, involving a plethora of cellular processes that may or may not be interdependent. While these are most likely processes that contribute to the normal development of neuronal connectivity, the understanding of these processes can provide a critical tool for understanding age-dependent differences in the susceptibility to neuropsychiatric disease. More specifically, adolescence has been shown to be a critical period for susceptibility to specific mental health disorders such as schizophrenia and drug addiction [65,66] both of which has been linked to BDNF signaling in striatum and PFC [17-19,67,68]. For example, schizophrenia patients have been reported to exhibit low levels of cortical BDNF and TrkB [67], and a BDNF polymorphism Val66Met has been directly linked to the age of onset of the disorder [69]. Similarly, the involvement of BDNF signaling in drug addiction is widely accepted and an opposing effect of NAc and PFC BDNF signaling on addiction-like behaviours has been repeatedly documented [17,18,70-76]. While the role of dopamine-mediated BDNF signaling in mental health disorders such as schizophrenia and drug addiction is becoming well characterized, the involvement of BDNF as a contributing factor in the age-dependent susceptibility to these disorders requires further investigation. Clearly BDNF plays an integral role in processes that contribute to the pathophysiology of these disorders and thus future studies are required not only to understand the physiological importance of these age-dependent differences in BDNF and TrkB expression, but additionally how they are regulated by dopamine receptors and their role in developmental processes. Such information would not only provide essential knowledge about normal physiological developmental changes, but would also provide insights into how these changes may coincide with vulnerabilities to neuropsyciatric disease.
Acknowledgements
This work was supported by a grant from the National Institute on Drug Abuse (to S.R.G. and B.F.O.) and a CIHR Postdoctoral Fellowship (to M.L.P.). S.R.G. holds a Canada Research Chair in Molecular Neuroscience.
References
- 1.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 2.Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- 3.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- 6.Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 8.Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventimiglia R, Mather PE, Jones BE, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci. 1995;7:213–222. doi: 10.1111/j.1460-9568.1995.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 11.Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, George SR. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- 13.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, Cirulli F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–772. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Reus GZ, Abelaira HM, Stringari RB, Fries GR, Kapczinski F, Quevedo J. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis. 2012;27:175–182. doi: 10.1007/s11011-012-9281-2. [DOI] [PubMed] [Google Scholar]
- 16.Snigdha S, Neill JC, McLean SL, Shemar GK, Cruise L, Shahid M, Henry B. Phencyclidine (PCP)-induced disruption in cognitive performance is gender-specific and associated with a reduction in brain-derived neurotrophic factor (BDNF) in specific regions of the female rat brain. J Mol Neurosci. 2011;43:337–345. doi: 10.1007/s12031-010-9447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 18.Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, DiLeone RJ, Parada LF, Nestler EJ, Self DW. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGinty JF, Whitfield TW, Jr., Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams SN, Undieh AS. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. Neuroreport. 2009;20:606–610. doi: 10.1097/WNR.0b013e32832a0a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perreault ML, Jones-Tabah J, O’Dowd BF, George SR. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int J Neuropsychopharmacol. 2012;25:1–7. doi: 10.1017/S1461145712000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perreault ML, Fan T, Alijaniaram M, O’Dowd BF, George SR. Dopamine D1-D2 Receptor Heteromer in Dual Phenotype GABA/Glutamate-Coexpressing Striatal Medium Spiny Neurons: Regulation of BDNF, GAD67 and VGLUT1/2. PLoS One. 2012;7:e33348. doi: 10.1371/journal.pone.0033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Teicher MH, Andersen SL, Hostetter JC., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 26.Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 2007;1156:31–45. doi: 10.1016/j.brainres.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O’Dowd BF, George SR. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 28.Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O’Dowd BF, George SR. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perreault ML, Hasbi A, Alijaniaram M, O’Dowd BF, George SR. Reduced striatal dopamine D1-D2 receptor heteromer expression and behavioural subsensitivity in juvenile rats. Neuroscience. 2012;225:130–139. doi: 10.1016/j.neuroscience.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 32.Carlino D, De Vanna M, Tongiorgi E. Is Altered BDNF Biosynthesis a General Feature in Patients with Cognitive Dysfunctions? Neuroscientist. 2012 doi: 10.1177/1073858412469444. [DOI] [PubMed] [Google Scholar]
- 33.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 34.Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- 35.Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75:447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O’Dowd BF, George SR. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor hetero-oligomers. Mol Pharmacol. 2009;75:843–854. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alderson RF, Curtis R, Alterman AL, Lindsay RM, DiStefano PS. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and schwann cells in vitro. Brain Res. 2000;871:210–222. doi: 10.1016/s0006-8993(00)02428-8. [DOI] [PubMed] [Google Scholar]
- 38.Biffo S, Offenhauser N, Carter BD, Barde YA. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121:2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- 39.Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenner BM. Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012;23:15–24. doi: 10.1016/j.cytogfr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- 42.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujii T, Kunugi H. p75NTR as a therapeutic target for neuropsychiatric diseases. Curr Mol Pharmacol. 2009;2:70–76. doi: 10.2174/1874467210902010070. [DOI] [PubMed] [Google Scholar]
- 44.Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- 45.Patel NJ, Chen MJ, Russo-Neustadt AA. Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. Eur J Pharmacol. 2010;633:1–9. doi: 10.1016/j.ejphar.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Liu CY, Jiang XX, Zhu YH, Wei DN. Metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine produces antidepressant effects in rats: Role of brain-derived neurotrophic factor. Neuroscience. 2012;223:219–224. doi: 10.1016/j.neuroscience.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Khundakar AA, Zetterstrom TS. Effects of GABAB ligands alone and in combination with paroxetine on hippocampal BDNF gene expression. Eur J Pharmacol. 2011;671:33–38. doi: 10.1016/j.ejphar.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Andersen SL. Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2002;130:197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- 49.Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem. 1994;62:2045–2048. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- 50.Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- 51.Molloy AG, Waddington JL. Assessment of grooming and other behavioural responses to the D-1 dopamine receptor agonist SK & F 38393 and its R- and S-enantiomers in the intact adult rat. Psychopharmacology (Berl) 1987;92:164–168. doi: 10.1007/BF00177909. [DOI] [PubMed] [Google Scholar]
- 52.Boyce PJ, Finlay JM. Extracellular dopamine and norepinephrine in the developing rat prefrontal cortex: transient effects of early partial loss of dopamine. Brain Res Bull. 2009;79:104–110. doi: 10.1016/j.brainresbull.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ. A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology. 2011;61:544–549. doi: 10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Counotte DS, Li KW, Wortel J, Gouwenberg Y, Van Der Schors RC, Smit AB, Spijker S. Changes in molecular composition of rat medial prefrontal cortex synapses during adolescent development. Eur J Neurosci. 2010;32:1452–1460. doi: 10.1111/j.1460-9568.2010.07404.x. [DOI] [PubMed] [Google Scholar]
- 56.Baxter GT, Radeke MJ, Kuo RC, Makrides V, Hinkle B, Hoang R, Medina-Selby A, Coit D, Valenzuela P, Feinstein SC. Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2. J Neurosci. 1997;17:2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 58.Cheng A, Coksaygan T, Tang H, Khatri R, Balice-Gordon RJ, Rao MS, Mattson MP. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J Neurochem. 2007;100:1515–1530. doi: 10.1111/j.1471-4159.2006.04337.x. [DOI] [PubMed] [Google Scholar]
- 59.Gomes JR, Costa JT, Melo CV, Felizzi F, Monteiro P, Pinto MJ, Inacio AR, Wieloch T, Almeida RD, Graos M, Duarte CB. Excitotoxicity downregulates TrkB.FL signaling and upregulates the neuroprotective truncated TrkB receptors in cultured hippocampal and striatal neurons. J Neurosci. 2012;32:4610–4622. doi: 10.1523/JNEUROSCI.0374-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 61.Hartmann M, Brigadski T, Erdmann KS, Holtmann B, Sendtner M, Narz F, Lessmann V. Truncated TrkB receptor-induced outgrowth of dendritic filopodia involves the p75 neurotrophin receptor. J Cell Sci. 2004;117:5803–5814. doi: 10.1242/jcs.01511. [DOI] [PubMed] [Google Scholar]
- 62.Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 63.Marsden WN. Stressor-induced NMDAR dysfunction as a unifying hypothesis for the aetiology, pathogenesis and comorbidity of clinical depression. Med Hypotheses. 2011;77:508–528. doi: 10.1016/j.mehy.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 64.Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med. 2010;16:1393–1395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- 65.Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012;2012:916198. doi: 10.1155/2012/916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Favalli G, Li J, Belmonte-de-Abreu P, Wong AH, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46:1–11. doi: 10.1016/j.jpsychires.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 69.Chao HM, Kao HT, Porton B. BDNF Val66Met variant and age of onset in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:505–506. doi: 10.1002/ajmg.b.30619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. 2003;119:767–775. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 71.Corominas M, Roncero C, Ribases M, Castells X, Casas M. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology. 2007;55:2–13. doi: 10.1159/000103570. [DOI] [PubMed] [Google Scholar]
- 72.Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- 73.Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr., Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- 75.Whitfield TW, Jr., Shi X, Sun WL, McGinty JF. The suppressive effect of an intraprefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berglind WJ, Whitfield TW, Jr., LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]