Abstract
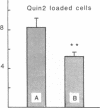
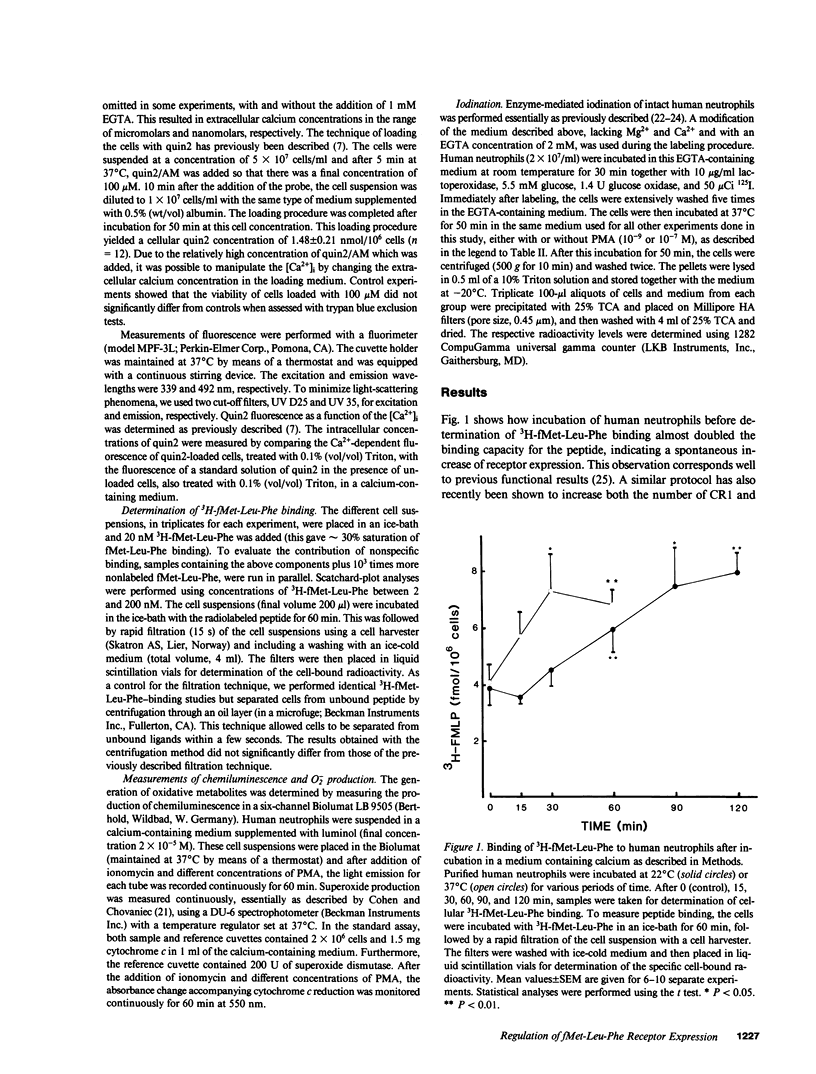
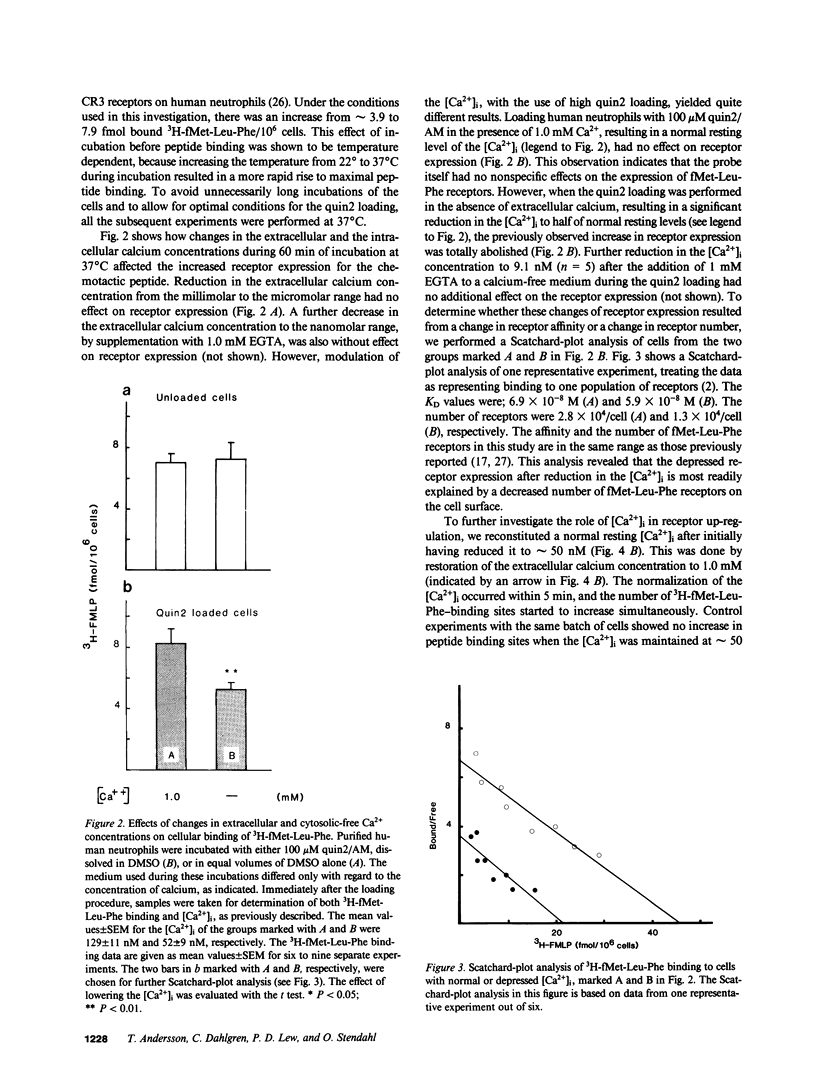
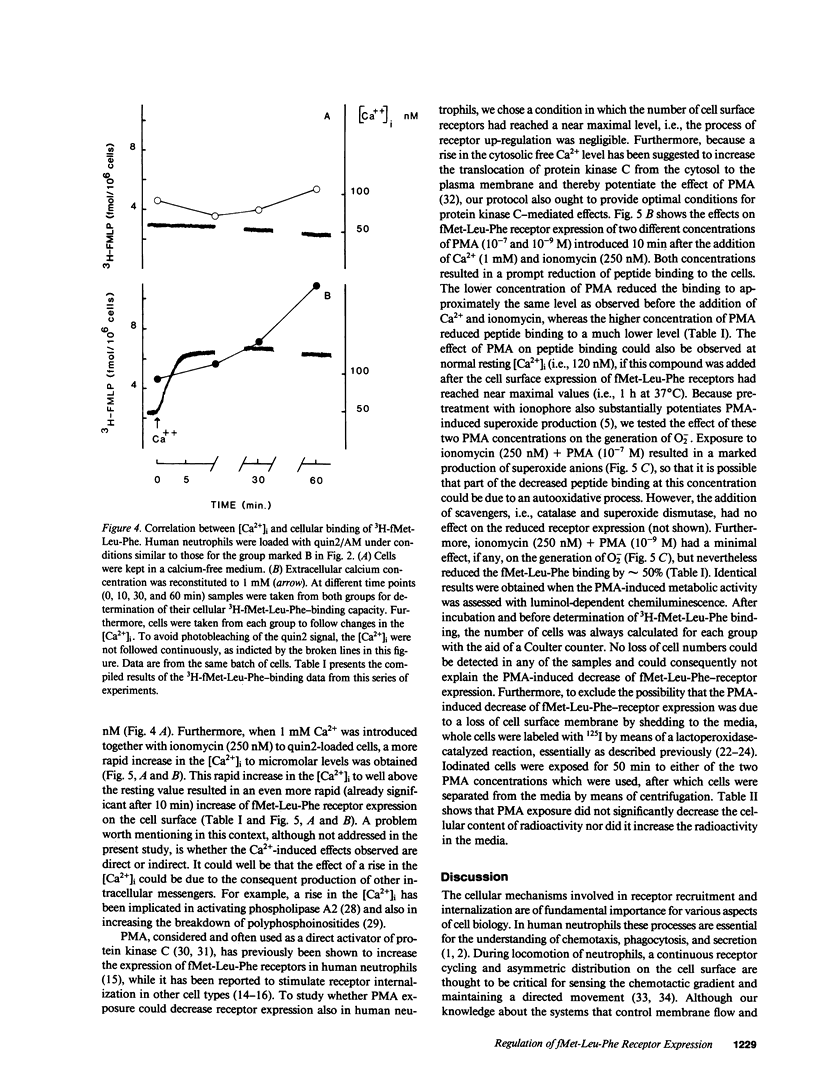
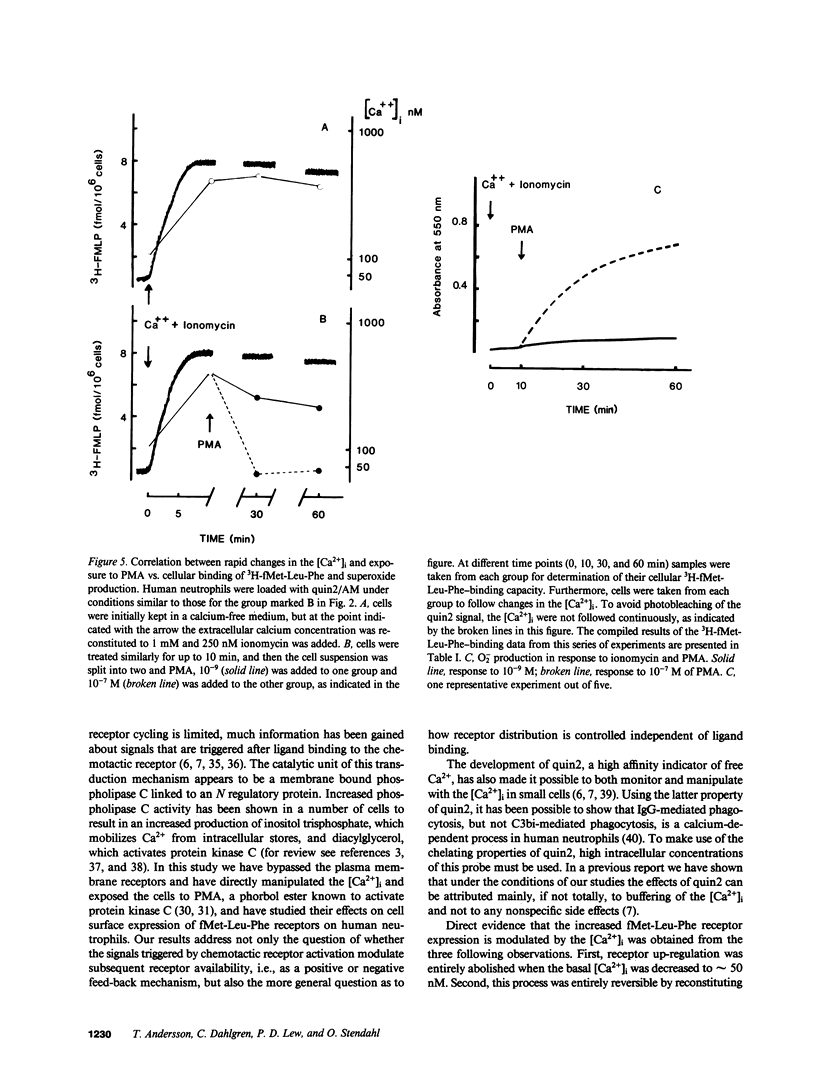
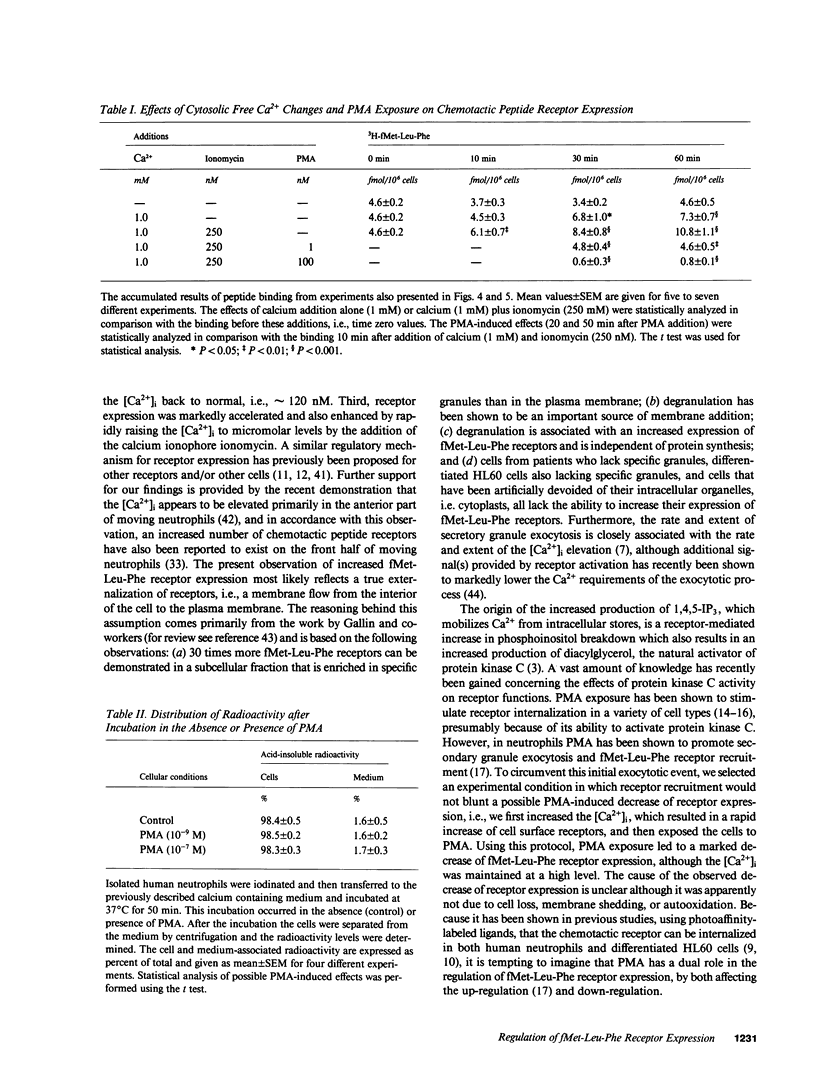
We have studied how cytosolic free Ca2+ ([Ca2+]i) changes and phorbol myristate acetate (PMA) exposure affects ligand-independent cell surface expression of fMet-Leu-Phe receptors on human neutrophils. Mere incubation primed neutrophils to double their binding of fMet-Leu-Phe. This spontaneous increase of peptide binding was unaffected by changes in the extracellular calcium concentration. However, depression of the [Ca2+]i totally abolished the increased binding of fMet-Leu-Phe. Scatchard-Plot analysis revealed that the observed increase of peptide binding was due to an increased number of receptors. Normalization of the [Ca2+]i in cells where it was initially depressed resulted in a slow but progressive increase in fMet-Leu-Phe binding. The rate of receptor recruitment could be enhanced by rapidly increasing the [Ca2+]i by addition of ionomycin. Addition of PMA to cells with near maximal receptor expression led to a marked reduction of fMet-Leu-Phe binding without affecting [Ca2+]i. These observations suggest the existence of a dual regulatory mechanism for up- and down-regulation of fMet-Leu-Phe receptors on the cell surface of human neutrophils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Niedel J. Processing of the formyl peptide receptor by HL-60 cells. J Biol Chem. 1984 Nov 10;259(21):13309–13315. [PubMed] [Google Scholar]
- Berger M., Birx D. L., Wetzler E. M., O'Shea J. J., Brown E. J., Cross A. S. Calcium requirements for increased complement receptor expression during neutrophil activation. J Immunol. 1985 Aug;135(2):1342–1348. [PubMed] [Google Scholar]
- Berger M., O'Shea J., Cross A. S., Folks T. M., Chused T. M., Brown E. J., Frank M. M. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys S. S., Keogh E. A., Kaplan J. Fusion of intracellular membrane pools with cell surfaces of macrophages stimulated by phorbol esters and calcium ionophores. Cell. 1984 Sep;38(2):569–576. doi: 10.1016/0092-8674(84)90511-7. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Changelian P. S., Jack R. M., Collins L. A., Fearon D. T. PMA induces the ligand-independent internalization of CR1 on human neutrophils. J Immunol. 1985 Mar;134(3):1851–1858. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Magnusson K. E., Stendahl O., Sundqvist T. Modulation of polymorphonuclear leukocyte chemiluminescent response to the chemoattractant f-Met-Leu-Phe. Int Arch Allergy Appl Immunol. 1982;68(1):79–83. doi: 10.1159/000233070. [DOI] [PubMed] [Google Scholar]
- Davies W. A., Stossel T. P. Peripheral hyaline blebs (podosomes) of macrophages. J Cell Biol. 1977 Dec;75(3):941–955. doi: 10.1083/jcb.75.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Degranulating stimuli increase the availability of receptors on human neutrophils for the chemoattractant f-met-leu-phe. J Immunol. 1980 Apr;124(4):1585–1588. [PubMed] [Google Scholar]
- Gallin J. I. Neutrophil specific granules: a fuse that ignites the inflammatory response. Clin Res. 1984 Sep;32(3):320–328. [PubMed] [Google Scholar]
- Gallin J. I., Seligmann B. E. Neutrophil chemoattractant fMet-Leu-Phe receptor expression and ionic events following activation. Contemp Top Immunobiol. 1984;14:83–108. doi: 10.1007/978-1-4757-4862-8_3. [DOI] [PubMed] [Google Scholar]
- Gennaro R., Florio C., Romeo D. Activation of protein kinase C in neutrophil cytoplasts. Localization of protein substrates and possible relationship with stimulus-response coupling. FEBS Lett. 1985 Jan 28;180(2):185–190. doi: 10.1016/0014-5793(85)81068-1. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. II. Metabolic fate of iodinated polypeptides of mouse L cells. J Cell Biol. 1975 Feb;64(2):461–479. doi: 10.1083/jcb.64.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopetta B., Carpentier J. L., Pozzan T., Lew D. P., Gorden P., Orci L. Role of intracellular calcium and protein kinase C in the endocytosis of transferrin and insulin by HL60 cells. J Cell Biol. 1986 Sep;103(3):851–856. doi: 10.1083/jcb.103.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Naemura J. R., Painter R. G., Schmitt M., Sklar L. A., Cochrane C. G. The fate of the N-formyl-chemotactic peptide receptor in stimulated human granulocytes: subcellular fractionation studies. J Cell Biochem. 1982;20(2):177–191. doi: 10.1002/jcb.240200209. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M., Matrisian L. M., Magun B. E. Cytosolic calcium regulates epidermal growth factor endocytosis in rat pancreas and cultured fibroblasts. Proc Natl Acad Sci U S A. 1984 Jan;81(2):461–465. doi: 10.1073/pnas.81.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagast H., Pozzan T., Waldvogel F. A., Lew P. D. Phorbol myristate acetate stimulates ATP-dependent calcium transport by the plasma membrane of neutrophils. J Clin Invest. 1984 Mar;73(3):878–883. doi: 10.1172/JCI111284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. P., Andersson T., Hed J., Di Virgilio F., Pozzan T., Stendahl O. Ca2+-dependent and Ca2+-independent phagocytosis in human neutrophils. Nature. 1985 Jun 6;315(6019):509–511. doi: 10.1038/315509a0. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Gaither T. A., Takahashi T., Frank M. M. Tumor-promoting phorbol esters induce rapid internalization of the C3b receptor via a cytoskeleton-dependent mechanism. J Immunol. 1985 Aug;135(2):1325–1330. [PubMed] [Google Scholar]
- Penfield A., Dale M. M. Synergism between A23187 and 1-oleoyl-2-acetyl-glycerol in superoxide production by human neutrophils. Biochem Biophys Res Commun. 1984 Nov 30;125(1):332–336. doi: 10.1016/s0006-291x(84)80372-1. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Sawyer D. W., Sullivan J. A., Mandell G. L. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985 Nov 8;230(4726):663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Transductional mechanisms of chemoattractant receptors on leukocytes. Contemp Top Immunobiol. 1984;14:1–28. doi: 10.1007/978-1-4757-4862-8_1. [DOI] [PubMed] [Google Scholar]
- Sullivan S. J., Daukas G., Zigmond S. H. Asymmetric distribution of the chemotactic peptide receptor on polymorphonuclear leukocytes. J Cell Biol. 1984 Oct;99(4 Pt 1):1461–1467. doi: 10.1083/jcb.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark G. R., Kuhn L. J., Niedel J. E. Possible mechanism of phorbol diester-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1984 Feb;73(2):448–457. doi: 10.1172/JCI111231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J., Lauffenburger D. A. Kinetic analysis of chemotactic peptide receptor modulation. J Cell Biol. 1982 Jan;92(1):34–43. doi: 10.1083/jcb.92.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]