Abstract
Objective:
To investigate whether planning target volume (PTV) margins may be safely reduced in radiotherapy of localized prostate cancer incorporating daily online tube potential-cone beam CT (CBCT) image guidance and the anticipated benefit in predicted rectal toxicity.
Methods:
The prostate-only clinical target volume (CTV2) and rectum were delineated on 1 pre-treatment CBCT each week in 18 randomly selected patients. By transposing these contours onto the original plan, dose–volume histograms (DVHs) for CTV2 and the rectum were each calculated and combined, for each patient, to produce a single mean DVH representative of the dose delivered over the treatment course. Plans were reoptimized using reduced CTV2 to PTV2 margins and the consequent radiobiological impact modelled by the tumour control probability (TCP) and normal tissue complication probability (NTCP) of the rectum.
Results:
All CBCT images were deemed of sufficient quality to identify the CTV and rectum. No loss of TCP was observed when plans using the standard 5-mm CTV2 to PTV2 margin of the centre were reoptimized with a 4- or 3-mm margin. Margin reduction was associated with a significant decrease in rectal NTCP (5–4 mm; p < 0.05 and 5–3 mm; p < 0.01).
Conclusion:
Using daily online image guidance with CBCT, a reduction in CTV2 to PTV2 margins to 3 mm is achievable without compromising tumour control. The consequent sparing of surrounding normal tissues is associated with reduced anticipated rectal toxicity.
Advances in knowledge:
Margin reduction is feasible and potentially beneficial. Centres with image-guided radiotherapy capability should consider assessing whether margin reduction is possible within their institutes.
Adenocarcinoma of the prostate accounts for >10,000 deaths in the UK each year.1 External beam radiotherapy is a potentially curative treatment option for localized disease but is associated with a risk of acute and late side effects, as a consequence of radiation effects on surrounding normal tissues. The rectum is the critical dose-limiting structure, and there is a close relationship between the radiation dose received and subsequent risk of late toxicity.2 Late effects, including diarrhoea, incontinence, tenesmus and bleeding can have a significant impact on quality of life.3
A course of radical radiotherapy is delivered over more than 7 weeks but is usually planned on a single planning CT scan. This introduces geometric uncertainty such that the initial planning scan is unlikely to be representative of the position of the prostate or normal structures throughout the course of treatment. The anatomical proximity of the prostate and mid-rectum means that rectal distension, secondary to gaseous or faecal filling, is the major contributor to prostate motion. Studies using implanted fiducial markers,4,5 serial CT scans6 and ultrasound7 demonstrate prostatic displacements of up to 20 mm.8 The position of the prostate gland in relation to bony landmarks is also seen to change in an unpredictable way.9 To ensure adequate dose coverage of the tumour, a safety margin is added to the clinical target volume (CTV) to accommodate for positional uncertainties called the planning target volume (PTV). A larger CTV to PTV margin results in the irradiation of a larger volume of normal tissue within the high-dose envelope.
Image-guided radiotherapy (IGRT) uses images taken at the time of treatment to assess the accuracy of patient set-up, allowing real-time correction to be made if required. Its aim is to reduce the geometric uncertainties that occur between planning and delivery of radiotherapy. Strategies in current use include ultrasound,10 megavoltage CT (TomoTherapy®; Accuray, Sunnyvale, CA),11 CBCT12,13 and implanted fiducial markers.14,15
In this study, all patients were treated using intensity-modulated radiotherapy (IMRT) with daily online IGRT using tube potential-cone beam CT (CBCT). Our aim was to generate and assess new margin strategies based on the premise that the reduction in geometric uncertainty afforded by IGRT may permit PTV margin reduction without unacceptably compromising tumour control.
IGRT reduces positional uncertainty but does not eliminate interfractional set-up variation. In order to more accurately assess the actual doses delivered to the pelvic organs, pre-treatment CBCT images were used to delineate the prostate and rectum in the treated position. Dose–volume histograms (DVHs) for the CBCT-outlined structures were estimated by transposing these contours onto the original plan and using them to sample the original dose distribution. These were compared with the DVHs of the original plan and to the DVHs created using reduced margins with corresponding dose distributions generated by reoptimization. Data were evaluated using radiobiological modelling of the tumour control probability (TCP) and normal tissue complication probability (NTCP) of the rectum to assess whether margin reduction could be achieved safely and the likely benefit in terms of spared rectal toxicity anticipated from such a strategy.
METHODS AND MATERIALS
Local prostate protocol
18 patients treated with primary radical radiotherapy for localized prostate cancer were selected at random from the previous year's caseload. All patients were planned and treated supine using external immobilization, including knee rests and ankle stocks. Patients received specific dietary advice to minimize faecal loading and flatus and were treated with the bladder comfortably full.
Non-contrast-enhanced CT images with a 2-mm slice thickness were obtained and transferred to ProSoma® virtual simulation software (Medcom, Darmstadt, Germany) for target and critical structure delineation. Target and critical structure delineation was undertaken following standard departmental protocols derived from the CHHiP trial protocol.16 Prostate and seminal vesicle CTV (CTV1), covering the prostate and bilateral proximal seminal vesicles (SVs), and prostate-only CTV (CTV2), covering the prostate alone, were contoured on the planning CT images. A 10-mm margin was grown isotropically from CTV1 to form PTV1 and a 5-mm margin from CTV2 to form PTV2. The rectum was delineated from the anus to the rectosigmoid junction. IMRT plans were generated using the Varian Eclipse™ v. 10.0 inverse planning system (Varian Medical Systems, Palo Alto, CA) and delivered using Varian Clinac®-iX with RapidArc® (Varian Medical Systems) to a planned total dose at the isocentre of 74 Gy in 37 fractions (60 Gy in 37 fractions to PTV1 with a synchronous boost of 14 Gy to PTV2).
Prior to daily treatment delivery, a full pelvic tube potential-CBCT was acquired as part of routine practice. Planning CT and CBCT images were automatically matched using soft-tissue registration before visual inspection to ensure that the PTV1 encompassed the prostate and SVs. If the rectum encroached >50% across the diameter of the CTV1 outline (which was superimposed onto the CBCT as part of the matching process), the patient was asked to empty their bowels before treatment, and the CBCT was repeated. If it encroached by <50%, the radiographers attempted to match to the target, only asking the patient to re-empty their bowels if no good match could be achieved. Once a satisfactory match was achieved, isocentre shifts were calculated and applied by the treating radiographer before treatment delivery.
All matching was independently verified offline by a senior radiographer to ensure the accuracy of the matching process. The size of any discrepancy between online and offline matches were recorded and an action required status issued for discrepancies exceeding 2 mm. If an action required status had been issued, it would be reviewed by the treating radiographers before delivery of the next treatment fraction. If a single non-conformance of >5 mm was recorded or, on three occasions during treatment non-conformance of >3 mm, the incident was escalated to a senior team consisting the IGRT lead, treatment floor supervisor and lead dosimetrist to investigate the cause. This may be an issue with patient set-up, in these cases prompt remedial action would be taken, or a training issue, which would be addressed and additional supervision put in place if required.
Image selection
CBCT images were stored in the Eclipse planning software in the treated position with the couch shifts applied. For each patient, CBCT images at Days 1, 6, 11, 16, 21, 26 and 31 were selected for analysis. For the purposes of the study, a single CTV corresponding to the CTV2 of the original plan was contoured on each CBCT. A separate CTV1 (prostate and SVs) was not contoured. The rectum was contoured from the level of the anus to the rectosigmoid flexure. A single oncologist contoured all structures.
The standard of image quality for target localization and contouring were assessed by both visual assessment by the contouring oncologist, with any images deemed of insufficient quality to allow accurate structure delineation excluded from analysis, and by volumetric analysis, whereby the CTV2 contoured on the CBCT images were compared with the CTV2 on the planning CT, and the size of any discrepancy between the volumes taken as an indicator of the accuracy of contouring on CBCT.
Dose calculations and radiobiological analysis
The CBCT contours for each evaluated fraction were used to sample the original dose distribution, allowing calculation of a DVH for the CTV2 and rectum in the treated position. For each patient, the seven CBCT DVHs for both the CTV2 and rectum were each combined to produce a mean, representative, DVH for the variably positioned structures over the course of treatment. These were compared with the original DVHs in order to assess any differences between the planned and delivered distributions to those structures.
To assess the feasibility of margin reduction, plans were reoptimized using a 4- and a 3-mm CTV2 to PTV2 margin. For reoptimization calculations, this was used in conjunction with the original CTV1 with a margin of 10 mm. The resulting mean DVHs of the CTV2 and rectum were evaluated against those of the original plan.
Radiobiological evaluation of radiotherapy treatment plans was undertaken using the BIOPLAN software package17 applying default parameter settings for TCP [radiosensitivity (α), 0.292; α/β, 10 Gy; clonogenic cell density (N0), 107 cm3].18
The modified NTCP for the rectum was calculated using the Lyman–Kutcher–Burman (LKB) model.19,20 The values used for the α/β ratio and LKB parameters (Table 1) were based on those proposed by quantitative analyses of normal tissue effects in the clinic (QUANTEC) for grade ≥2 late rectal toxicity and bleeding.21
Table 1.
Parameters used for normal tissue complication probability (NTCP) calculation
| Parameter | Value |
|---|---|
| α/β ratio | 3 Gy |
| TD50 | 78.5 |
| M | 0.13 |
| N | 0.09 |
M, parameter describing the slope of NTCP vs dose; N, parameter describing volume dependence of the NTCP; TD50, dose that uniformly delivered to the whole organ produces a 50% chance of complications.
Rectal volumes were calculated for the initial planning CT and for each CBCT image to allow assessment of interfractional rectal volume variation and to determine whether any systematic changes in rectal volume could be identified over the course of treatment.
RESULTS
Image quality and accuracy of prostate identification
All images (126/126) were deemed of sufficient quality to allow delineation of the prostate and rectum (Figure 1). The main obstacle to contouring was artefact caused by air within the rectum, which required interpolation of contours over short distances to overcome loss of anatomical definition in the images. This was not deemed to reduce the overall accuracy of delineation.
Figure 1.
Comparison of image quality between planning CT (left) and CBCT (right).
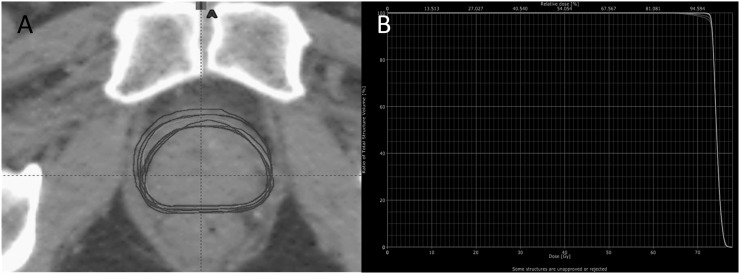
Figure 2a illustrates the superimposed weekly CBCT CTV2 contours of a typical patient on the planning CT; the tightly grouped contours indicate close agreement between the position of the prostate as determined by the radiographers and that delineated as CTV2 by the oncologist. The cumulative DVH plotted using these data (Figure 2b) shows good conformity of target coverage over the course of treatment. There was generally excellent agreement between the planning CT to CBCT matches made online and those independently verified offline by a senior radiographer with a total of 31 action required statuses issued to indicate a discrepancy in excess of 2 mm. The standard deviations of all discrepancies between the online and offline matches were 1.0, 0.8 and 0.6 mm in the vertical, longitudinal and lateral dimensions, respectively.
Figure 2.
(a) Superimposed clinical target volume (CTV2) contours as delineated on planning scan and weekly cone beam CTs (CBCTs) of a typical patient in axial view. (b) Comparison of the cumulative dose–volume histograms for CTV2 derived from the planning scan and weekly CBCTs of a typical patient.
The comparison of the CTV2 contour on planning CT to the CTV2 as contoured on the CBCT showed that the mean percentage difference between the volumes was −9.3%. If the prostate is assumed to be a spherical structure, this is equivalent to a change in radius of <1 mm of the equivalent sphere. The range was −28.5% to +14.9%, corresponding to a change in radii of the equivalent sphere of −2.1 to +1.3 mm.
The impact of margin reduction on tumour control probability
Across all patients, the mean TCP of the original plan was 81.8% (Table 2). No reduction in TCP was found when using the mean DVHs of the CBCT contours when compared with the DVHs of the planning CTs, either at the original 5-mm margin or for the plans re-optimized with reduced 4- and 3-mm margins, indicating that margin reduction may be achieved without compromising tumour control. For the 3-mm margin, no significant difference was found between the TCP values calculated using the planning CT or CBCT images incorporating organ motion. For the 4- and 5-mm margins, a small but statistically significant increase in TCP was indicated using the CBCT compared with planning CT data (Table 2).
Table 2.
Comparison of tumour control probability (TCP) values for planning and mean cone beam CT (CBCT) clinical target volume 2 (CTV2) dose–volume histograms (DVHs) for all patients using decreasing CTV2 to planning target volume 2 (PTV2) margins
| CTV2 to PTV2 margin (mm) | TCP (planning CTV2 DVH) | TCP (mean CBCT CTV2 DVH) | p-value (two-tailed)a | Mean CBCT TCP relative to original plan TCP (%) |
|---|---|---|---|---|
| 5 | 81.8% (78.9–83.8) | 82.0% (79.5–84.0) | 0.009 | 0.3 (−1.1 to 1.0) |
| 4 | 81.7% (78.7–83.8) | 81.8% (79.2–83.9) | 0.034 | 0.1 (−1.5 to 0.7) |
| 3 | 81.9% (78.8–83.8) | 81.9% (79.2–83.6) | 0.272 | 0.2 (−2.0 to 0.7) |
Data are represented as mean (range).
Wilcoxon signed rank test.
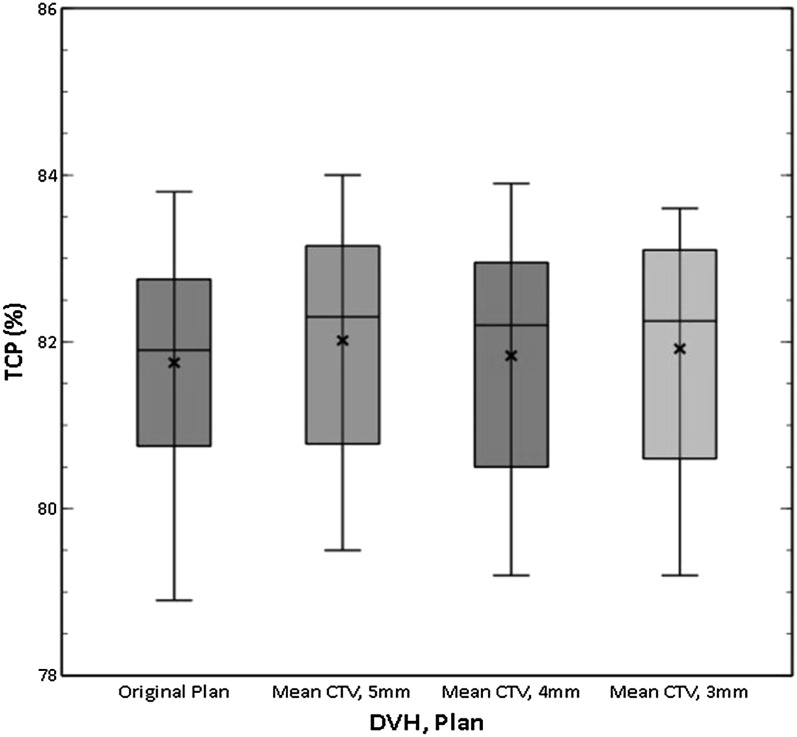
Overall, there was a good level of consistency with low-level variation in TCP across all patients and plans (Figure 3). Relative to the TCP calculated using the original plan, the mean change in TCP using the mean DVH of the CBCTs was 0.3% using the standard 5-mm margin and 0.1% and 0.2% for the reoptimized plans using 4- and 3-mm margins, respectively. For individual patients, the total variation was limited to between −2.0% and +1.0%.
Figure 3.
Box plot comparing tumour control probability (TCP) values for all patients using the original planning data and the mean dose–volume histograms (DVHs) of cone beam CT plans reoptimized at decreased margin sizes. CTV, clinical target volume.
Interfractional rectal volume changes and anticipated toxicity
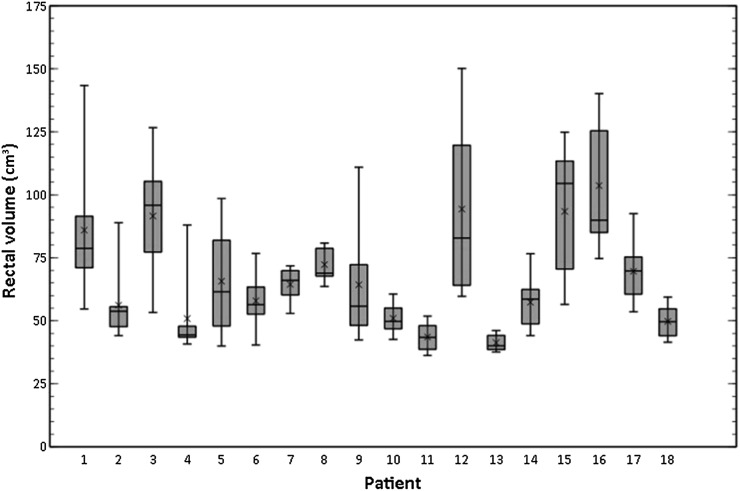
The rectum was easily identifiable on all CBCT images (Figure 4). Considerable variation in the size of the rectum both at planning and during treatment was observed. This translated into large deviations in the cumulative DVHs of some patients (Figure 5). The mean rectal volume contoured on the planning CT was 73.3 cm3 (range, 47.5–104.7 cm3), and on the weekly CBCT, it was 67.4 cm3 (range, 36.2–150.1 cm3). The amount of interfractional rectal volume change over the course of treatment varied significantly between patients (Figure 6). Patient 12 showed the largest variation (between 59.7 and 150.1 cm3), and Patient 13 the smallest (between 37.6 and 46.1 cm3).
Figure 4.
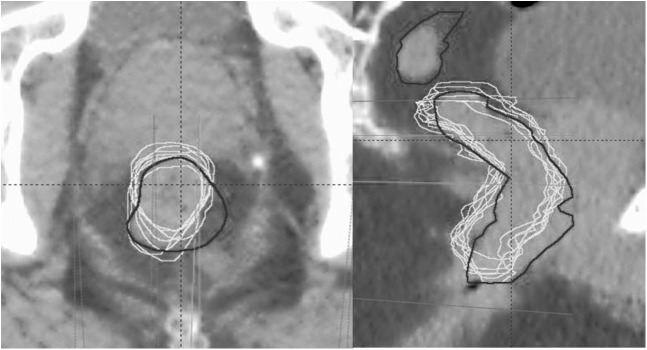
Superimposed rectal contours from the planning CT (black contour) and weekly cone beam CT (white contour) of a typical patient on axial (left) and sagittal (right) views.
Figure 5.
Comparison of the cumulative dose–volume histograms (DVHs) for rectum derived from the planning scan (grey) and weekly cone beam CTs (white) of a typical patient.
Figure 6.
Box plots demonstrating the variation in rectal volume on cone beam CT during treatment for each of patient.
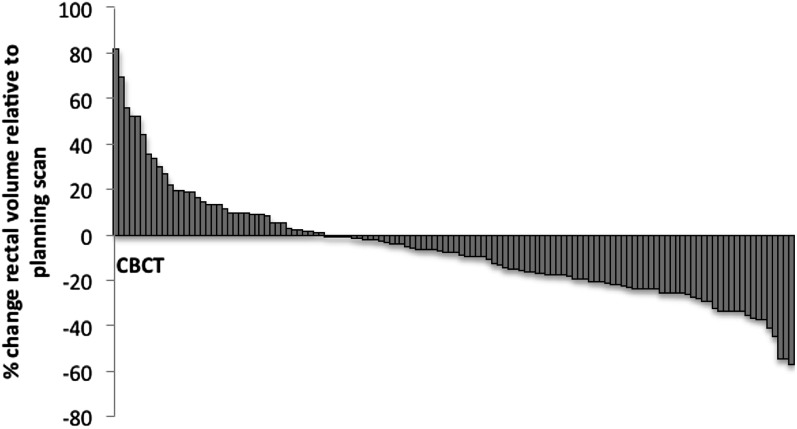
Rectal volumes were decreased compared with the planning volume in 85/126 (67%) CBCTs acquired (Figure 7) by an average of 18.6% (range, −63.6 to 18.3). However, a linear relationship between time through treatment (fraction number) and the rectal volume was not demonstrated (r = −0.02; p = −0.85).
Figure 7.
Change in rectal volume on cone beam CT (CBCT) relative to original planning scan volume.
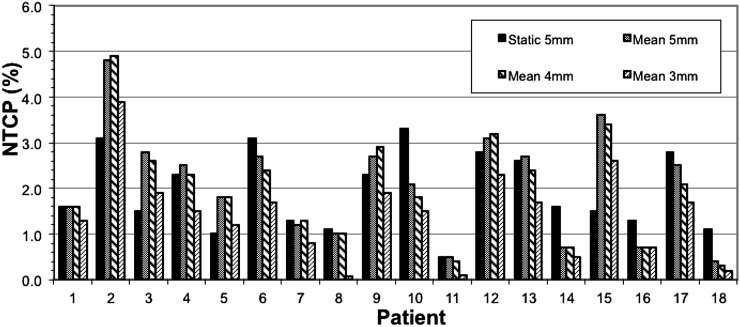
Across all patients, no statistically significant difference was observed between the NTCP calculated using the original “static” rectal DVH of the planning scan and the mean rectal DVH derived from the weekly contoured CBCT images (Table 3), thus indicating, on average, that the planned rectal doses were a fair representation of those relevant over the course of treatment. However, for some patients, the variation was marked (Figure 8). Patient 15 showed the greatest discrepancy between the NTCP anticipated from the planning scan (1.5%) and the value calculated from the mean rectal DVHs (3.6%).
Table 3.
Comparison of normal tissue complication probability (NTCP) values calculated from the rectal dose–volume histogram (DVH) of the “static” planning CT to the mean cone beam CT (CBCT) rectal DVH at the standard 5-mm margin and at reduced clinical target volume 2 (CTV2) to planning target volume 2 (PTV2) margins
| CTV2 to PTV2 margin (mm) | NTCP (planning rectal DVH) | NTCP (mean CBCT rectal DVH) | p-value (two-tailed)a |
|---|---|---|---|
| 5 | 1.9% (0.5–3.3) | 2.1% (0.4–4.8) | 0.72 |
| 4 | 1.9% (0.5–3.2) | 2.0% (0.3–4.9) | 0.78 |
| 3 | 1.3% (0.2–2.5) | 1.4% (0.1–3.9) | 0.78 |
Data are represented as mean (range).
Wilcoxon signed ranks test.
Figure 8.
Bar chart comparing the normal tissue complication probability (NTCP) values of the original “static” plan to the mean rectal dose–volume histogram of cone beam CTs at standard 5-mm clinical target volume 2 (CTV2) to planning target volume 2 (PTV2) margins and for plans re-optimized using decreased margins for each patient.
Margin reduction resulted in a statistically significant improvement in calculated NTCP based on the mean rectal DVH (Table 4). The figures suggest that the gains in terms of reduced NTCP are much greater if margins can be reduced by 2 mm rather than a single 1 mm, with a 2-mm margin reduction achieving a relative decrease in NTCP of 36% compared with just 5% for a 1-mm margin reduction. The absolute reduction in NTCP of a 2-mm margin reduction of 0.7% translates to a modest reduction in ≥G2 rectal toxicity from 150/1000 to 143/1000 patients (based on QUANTEC risk of <15% risk of ≥G2 rectal toxicity using typical dose constraints up to 78 Gy). It was, however, noted that variation between individual patients was large (Figure 8). For some patients, such as Patient 16, reducing the CTV2 to PTV2 margin had no impact on NTCP, whereas for others, large improvements in NTCP were achieved, for example, Patient 8, where a 2-mm margin reduction resulted in a 93% decrease in NTCP.
Table 4.
Absolute and relative changes in normal tissue complication probability (NTCP) compared with a standard 5-mm margin [calculated using the mean rectal dose–volume histogram (DVH) of the cone beam CT] for plans re-optimized using decreasing clinical target volume 2 (CTV2) to planning target volume 2 (PTV2) margins
| CTV2 to PTV2 margin reduction (mm) | Absolute change in rectal NTCP | p-value (two-tailed)a | Relative change rectal NTCP |
|---|---|---|---|
| 5–4 | −0.10% (−0.4 to +0.2) | p < 0.05 | −5% (−25 to +8) |
| 5–3 | −0.70% (−1.0 to 0.0) | p < 0.01 | −36% (−93 to 0) |
Data are represented as mean (range).
Wilcoxon Signed Ranks Test.
DISCUSSION
The use of CBCT to visualize the prostate prior to treatment delivery improves our confidence that the dose distributions of the planning scan are a fair representation of actual CTV coverage. The results of this study confirm that no reduction in TCP was observed when DVH data, sampled from CBCT-outlined CTV2 contours, were compared with those sampled using contours from the planning scan, using a common dose distribution.
In agreement with previous work,22–24 greater variation in rectal dose distributions was seen, which for individual patients lead to a difference between planned and actual NTCP of sufficient magnitude to be of concern. Random variation in bowel distension means that delivered rectal doses can vary unpredictably, and hence the planned dose may be a poor predictor of actual rectal doses.
As anticipated from earlier studies,24–27 substantial intra- and interpatient variations were observed in measured rectal volumes both at planning and during treatment. Although rectal volumes tended to be smaller at planning than in treatment, no systemic change in rectal volume was identified over the course of treatment. This contradicts other studies25,28 that demonstrate a systematic decrease in rectal volume during treatment.
Despite use of image guidance, rectal distension can significantly influence rectal doses and consequent toxicities8,25,29 and potentially impact on intrafractional prostate motion, especially for hypofractionated protocols with prolonged treatment times. Rectal distension may also result in additional radiation exposure from extra CBCTs performed if the patient is required to empty their bowels after having an overfilled rectum at initial set-up. Rectal emptying protocols, including the use of pre-treatment enemas, may therefore be beneficial in order to obtain a more consistent volume during treatment.
No loss of TCP occurred when plans reoptimized using reduced margins were compared with the standard 5-mm margin plans, indicating that margin reduction with daily online CBCT-based IGRT is feasible at our centre. The finding that at a 5- and 4-mm CTV2 to PTV2 margin, TCP was statistically superior using the CBCT data compared with the planning CT was not anticipated. Analysis of the dose profile demonstrated that the optimizer generates small hot areas, in the order of +2%, just inside the edge of the PTV in order to generate the rapid fall off to 95% whilst maintaining the mean dose to the PTV. Although tightly grouped, the CTV2 as contoured on the CBCT images shows some positional variation when superimposed onto the planning CT (Figure 2a). This movement of the CTV2 within the PTV2 will result in increased sampling of the peripheral high dose region and hence a higher calculated TCP using the weekly CBCT contours. Although this effect is specific to the treatment planning system, it is a feature that is likely shared with other optimizers. It is therefore recommended that other centres perform their own margin reduction studies with their specific systems before adopting reduced margins in clinical practice.
Margin reduction resulted in a statistically significant improvement in anticipated rectal toxicity as modelled by the NTCP, although the predicted clinical benefit is modest. The average relative decrease in NTCP of 36% and 5% observed for a 2- and 1-mm reduction in the CTV2 to PTV2 margin, respectively. The figures suggest that the gains in terms of NTCP are much greater if margins can be reduced to 3 mm than to 4 mm. This result should, however, be interpreted with caution, as since the planning CT is performed using 2-mm slices, when margins are applied, a degree of quantization is applied to the superior–inferior margin such that a 4-mm margin may not truly represent the halfway point between 5 and 3 mm, and hence it is likely that the 4- and 3-mm plans are closer together in terms of NTCP than they would be without this effect.
Hammoud et al30 explored the dosimetric impact of margin reduction by overlaying different IMRT plans (a standard plan with a 10-mm margin except 6-mm posteriorly and a reduced margin plan with 5-mm margin except 3-mm posteriorly) onto daily CBCT images to allow recalculation of prostate and normal tissue DVHs. Patient set-up was performed online using bony matching to orthogonal radiographs, with the CBCT reserved for rectal filling examination and offline analysis. Whilst differences in the dose delivered to the prostate as a percentage of dose planned were minimal between the plans, the reduced PTV margins resulted in a significant 31% reduction in rectal dosage. In a similar study, comparing margin reduction to 4 mm (with a margin of 3-mm posteriorly) with and without fiducial-based image guidance showed that with fiducials, acceptable target coverage could be maintained even at this tight margin, the V98 exceeding 95% in all fractions studied, whereas without image guidance, only 71% of fractions achieved this end point.22 In one of the few studies using clinical outcome data, patients treated with image-guided IMRT using daily three-dimensional ultrasound were stratified according to CTV–PTV margin, which was reduced from 10 to 5 mm during the study period.31 Although margin reduction did not detrimentally impact upon rates of freedom from biochemical failure, no benefit was observed in terms of late rectal toxicity.
Quality of cone beam CT images
Although CBCT image resolution is inferior to that of conventional helical CT and more prone to streaking artefacts, this study demonstrates that both soft-tissue matching and delineation of the prostatic CTV and rectum can be reliably performed using images obtained by CBCT. Other studies have confirmed that, in experienced observers, soft-tissue visualization on CBCT can be performed with a high degree of accuracy.32 However, as for any imaging modality, interobserver variation occurs, which should be incorporated as a source of error when planning a margining strategy.
The CBCT scans were outlined by a single clinician; however, owing to the random selection of cases from the previous year's caseload, it was not possible to ensure that the original plans were also contoured by the same clinician. In order to provide a consistent approach within the department, all plans are outlined using guidelines based on the CHHiP trial protocol, and the same guidelines were applied when contouring the study CBCTs. However, despite the use of guidelines, clinician uncertainty, especially at the level of the prostatic apex, can lead to interobserver variation in delineation on both conventional and CBCT images.33 Similarly, differences in image quality between the planning CT and CBCT images could introduce another source of systemic variation in pelvic structure delineation between the planning and verification contours. Simple geometric volume analysis showed that mean differences in the prostatic CTV2 volumes as contoured on the planning CT compared with those contoured on the CBCTs equated with submillimetre differences in radii of the equivalent sphere indicating a good level of consistency between planning CT and CBCT contours despite the potential interobserver and image quality differences. It is recognized that a more comprehensive assessment of the impact of intra- and interobserver differences may be preferential in future work.
Residual sources of error
IGRT provides the potential for margin reduction but does not reduce systematic errors to zero since no system is perfect and residual sources of error remain. In the present study, it was considered that margin reduction <3 mm was not feasible owing to the presence of uncorrected sources of error, including inaccuracies of the imaging and repositioning system (e.g. limited resolution of couch movement), delineation and matching errors and, probably most significantly, intrafractional organ motion.
Several investigators have demonstrated that motion of the pelvic organs occurs within the time scale of irradiation.34–36 In the present study, it was not possible to assess intrafractional motion directly. Previous work using tracking of implanted fiducials has shown average prostatic displacements during a radiotherapy fraction may be safely incorporated within a margin of 1 mm.35 However, for some patients, much larger displacements of up to 9.5 mm occur. Cine-MRI highlighting rectal filling is an important factor, showing that for patients presenting with an empty rectum, prostatic displacements of <3 mm (90%) could be anticipated, whereas with full rectums, significantly larger displacements occurred.36 Kotte et al34 assessed intrafractional prostatic motion over the entire treatment course of 427 patients with implanted fiducials. A margin of ≥2 mm was recommended on account of intrafractional motion, as although larger excursions may occur during individual fractions, reciprocal motion means that the overall effect over the course of treatment remained small.
Real-time prostate tracking data during IMRT, obtained using an electromagnetic tracking system (Calypso; Calypso Medical Technologies, Seattle, WA), has shown that margins of 3 mm are sufficient to cover the prostate 95.5% of the time for treatments up to 3 min, and 93.1% within 7 min.37 The typical image plus treatment time at our centre is 4 min using CBCT and RapidArc. We therefore consider that a 3-mm CTV2 to PTV2 margin to incorporate intrafractional organ motion is reasonable and safe, especially given that the dose fall-off outside PTV2 but within PTV1 is only to 81%, reducing the potential dose effect of any CTV2 displacements.
Disadvantages of cone beam CT-based image-guided radiotherapy
Additional radiation dose from daily pelvic CBCT can be significant and is dependent upon a number of factors, including beam quality, tube output, scanning geometry, technique setting as well as patient size.38 At our centre, for standard pelvic CBCT, the estimated dose to the isocentre is approximately 25 mGy per scan, which equates to around 7 mSv equivalent dose (ED). Taking account of repeated scans, the average number of CBCTs performed over the course of treatment is 39, resulting in an additional radiation dose to the patient of about 1 Gy or 263 mSv ED.
The estimated life time attributable risk for cancer induction from pelvic CBCT has been calculated as approximately 400 per 10,000 persons if 30 pelvic tube potential CBCT scans are perfomed.39 The lowest possible doses that permit adequate visualization should be used and consideration is given to reducing the frequency of CBCTs. Any additional radiation exposure must be justifiable in terms of patient benefit. Other disadvantages of CBCT include additional time for patient set-up and staff training.12
Study limitations and future work
Although this is a relatively small study, the cohort of 18 patients allows the study of individual data thus enabling recognition of important outcomes that may have been missed in grouped data. There are, however, important limitations to the work, which should be considered when interpreting results.
This study was designed to investigate the impact of margin reduction on the region covered by the synchronous boost to the prostate alone (i.e. the CTV2 to PTV2 margin). PTV2 represents the high-dose region associated with the most severe late effects; the rectal volume receiving ≥60 Gy is frequently associated with the risk of grade ≥2 rectal toxicity or bleeding.21 Reducing the CTV2 to PTV2 margin will spare the rectum from the highest doses, with the potential for achieving the greatest gains in terms of reduced severe complications. However, there is growing recognition of the contribution of lower dose regions (40–50 Gy) to late rectal toxicity,2 and therefore future studies may wish to consider the entire dose distribution to the rectum and ways of reducing the volume receiving even modest doses.
Interfractional motion of the SVs and their impact on margin considerations were not assessed. Earlier work has demonstrated greater inter- and intraobserver variability in contouring SVs than the prostate on helical CT images,40 and subjective assessment of image quality for the purposes SV contouring is rated poor on CBCT.33 It was therefore considered that contouring SVs on CBCT would pose a challenge in terms of reproducibility and reliability. The extent of interfractional motion and deformation of the prostate and SVs is also not uniform, and thus margining considerations may be different.41,42 This may be an area of interest for future work.
In order to generate meaningful results, it was necessary to make several assumptions. Firstly, that sampling one CBCT weekly provides a reasonable surrogate for the mean doses across all 37 fractions. This was considered appropriate since large variations in the dose distribution were not anticipated and, by sampling weekly, it was hoped that any systemic changes would be identified.
CBCT-derived contours were transposed onto the original plan in order to obtain dose estimates for the CTV2 and rectum under the assumption that the original dose distribution is spatially invariant. Whilst, in practice, owing to tissue inhomogeneities and surface curvature, this may not hold true, for deep-seated tumours such as the prostate, the error is small and the assumption therefore considered valid.43
Consensus is lacking regarding the most appropriate parameters for use in radiobiological modelling. To calculate NTCP, the parameters chosen were based on those suggested for grade ≥2 late toxicity or bleeding from the QUANTEC publication,21 which used a combination of meta-analysis and literature review to identify parameters judged to best define the available published data. This measure has been validated clinically44,45 and, for rectal bleeding and proctitis, parameter fits are considered as robust; although for other parameters, such as rectal urgency and stool frequency, a poorer fit is noted.45 In the calculation of TCP, an α/β ratio of 10 Gy for prostate cancer was used. Whilst, in the majority of cancers, an α/β ratio of around 10 Gy is considered accurate, for prostate cancer, this is a more contentious issue with growing recognition that the true figure may be much lower; indeed, values as low as 1.5 Gy have been suggested.46 Given the lack of consensus and in keeping with other authors,47 it was decided to use the original value for α/β ratio of 10 as specified by the BIOPLAN software rather than adopting a lower figure. The TCP values are for plan comparison purposes, and, therefore, although the use of different values of α/β ratio would change the absolute values for TCP, the way in which the TCP alters with changing margins would not, and therefore neither would the aims or conclusions of the study.
For future work, it would be interesting to determine whether the toxicity predicted from the NTCP calculations performed in this study fit with clinical outcome data. Exploration into the feasibility of a reduction in CTV1 to PTV1 margins could also be informative, although may prove more challenging since the dose drop off from PTV1 will be sharper than for PTV2, which is encompassed within the low-dose region of the PTV1.
CONCLUSIONS
The use of daily online IGRT with CBCT allows reduction in the CTV2 to PTV2 margin to be achieved safely, without compromising tumour control, and is beneficial in terms of reduced predicted rectal toxicity. This alludes to the possibility of dose escalation with the potential for improved tumour control. Care must be taken to account for residual sources of error and caution is urged against arbitrary use of margin reduction without prior thorough assessment.
REFERENCES
- 1.Office for National Statistics Mortality Statistics. Deaths registrered in 2010, England and Wales. London, UK: National Statistics; 2011. [Google Scholar]
- 2.Gulliford SL, Foo K, Morgan RC, Aird EG, Bidmead AM, Critchley H, et al. Dose-volume constraints to reduce rectal side effects from prostate radiotherapy: evidence from MRC RT01 Trial ISRCTN 47772397. Int J Radiat Oncol Biol Phys 2010; 76: 747–54. doi: 10.1016/j.ijrobp.2009.02.025 [DOI] [PubMed] [Google Scholar]
- 3.Geinitz H, Zimmermann FB, Thamm R, Erber C, Müller T, Keller M, et al. Late rectal symptoms and quality of life after conformal radiation therapy for prostate cancer. Radiother Oncol 2006; 79: 341–7. [DOI] [PubMed] [Google Scholar]
- 4.Aubry JF, Beaulieu L, Girouard LM, Aubin S, Tremblay D, Laverdière J, et al. Measurements of intrafraction motion and interfraction and intrafraction rotation of prostate by three-dimensional analysis of daily portal imaging with radiopaque markers. Int J Radiat Oncol Biol Phys 2004; 60: 30–9. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Lee RJ, Handrahan D, Sause WT. Intensity-modulated radiotherapy using implanted fiducial markers with daily portal imaging: assessment of prostate organ motion. Int J Radiat Oncol Biol Phys 2007; 68: 912–19. [DOI] [PubMed] [Google Scholar]
- 6.Bylund KC, Bayouth JE, Smith MC, Hass AC, Bhatia SK, Buatti JM. Analysis of interfraction prostate motion using megavoltage cone beam computed tomography. Int J Radiat Oncol Biol Phys 2008; 72: 949–56. doi: 10.1016/j.ijrobp.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Chandra A, Dong L, Huang E, Kuban DA, O'Neill L, Rosen I, et al. Experience of ultrasound-based daily prostate localization. Int J Radiat Oncol Biol Phys 2003; 56: 436–47. [DOI] [PubMed] [Google Scholar]
- 8.Ten Haken RK, Foreman JD, Heimburger DK, Gerhardsson A, McShan DL, Perez-Tamayo C, et al. Treatment planning issues related to prostate movement in response to differential filling of the rectum and bladder. Int J Radiat Oncol Biol Phys 1991; 20: 1317–24. [DOI] [PubMed] [Google Scholar]
- 9.Schallenkamp JM, Herman MG, Kruse JJ, Pisansky TM. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys 2005; 63: 800–11. [DOI] [PubMed] [Google Scholar]
- 10.Mohan DS, Kupelian PA, Willoughby TR. Short-course intensity-modulated radiotherapy for localized prostate cancer with daily transabdominal ultrasound localization of the prostate gland. Int J Radiat Oncol Biol Phys 2000; 46: 575–80. [DOI] [PubMed] [Google Scholar]
- 11.Burnet NG, Adams EJ, Fairfoul J, Tudor GS, Hoole AC, Routsis DS, et al. Practical aspects of implementation of helical tomotherapy for intensity-modulated and image-guided radiotherapy. Clin Oncol (R Coll Radiol) 2010; 22: 294–312. doi: 10.1016/j.clon.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Barney BM, Lee RJ, Handrahan D, Welsh KT, Cook JT, Sause WT. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys 2011; 80: 301–5. doi: 10.1016/j.ijrobp.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 13.Boda-Heggemann J, Lohr F, Wenz F, Flentje M, Guckenberger M. kV cone-beam CT-based IGRT: a clinical review. Strahlenther Onkol 2011; 187: 284–91. doi: 10.1007/s00066-011-2236-4 [DOI] [PubMed] [Google Scholar]
- 14.Moseley DJ, White EA, Wiltshire KL, Rosewall T, Sharpe MB, Siewerdsen JH, et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 2007; 67: 942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tudor GS, Rimmer YL, Nguyen TB, Cowen MA, Thomas SJ. Consideration of the likely benefit from implementation of prostate image-guided radiotherapy using current margin sizes: a radiobiological analysis. Br J Radiol 2012; 85: 1263–71. doi: 10.1259/bjr/27924223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoo VS, Dearnaley DP. Question of dose, fractionation and technique: ingredients for testing hypofractionation in prostate cancer—the CHHiP trial. Clin Oncol (R Coll Radiol) 2008; 20: 12–14. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Nieto B, Nahum AE. BIOPLAN: software for the biological evaluation of radiotherapy treatment plans. Med Dosim 2000; 25: 71–6. [DOI] [PubMed] [Google Scholar]
- 18.Hanks GE, Martz KL, Diamond JJ. The effect of dose on local control of prostate cancer. Int J Radiat Oncol Biol Phys 1988; 15: 1299–305. [DOI] [PubMed] [Google Scholar]
- 19.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res 1985; 104: S13–19. [PubMed] [Google Scholar]
- 20.Kutcher GJ, Burman C. Calculation of complication probability factors for non uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys 1988; 16: 1623–30. [DOI] [PubMed] [Google Scholar]
- 21.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 2010; 76(Suppl. 3): S123–9. doi: 10.1016/j.ijrobp.2009.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawlowski JM, Yang ES, Malcolm AW, Coffey CW, Ding GX. Reduction of dose delivered to organs at risk in prostate cancer patients via image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2010; 76: 924–34. doi: 10.1016/j.ijrobp.2009.06.068 [DOI] [PubMed] [Google Scholar]
- 23.Hatton JA, Greer PB, Tang C, Wright P, Capp A, Gupta S, et al. Does the planning dose-volume histogram represent treatment doses in image-guided prostate radiation therapy? Assessment with cone-beam computerised tomography scans. Radiother Oncol 2011; 98: 162–8. doi: 10.1016/j.radonc.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Kupelian PA, Langen KM, Zeidan OA, Meeks SL, Willoughby TR, Wagner TH, et al. Daily variations in delivered doses in patients treated with radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2006; 66: 876–82. [DOI] [PubMed] [Google Scholar]
- 25.Sripadam R, Stratford J, Henry AM, Jackson A, Moore CJ, Price P. Rectal motion can reduce CTV coverage and increase rectal dose during prostate radiotherapy: a daily cone-beam CT study. Radiother Oncol 2009; 90: 312–17. doi: 10.1016/j.radonc.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 26.Lebesque JV, Bruce AM, Kroes AP, Touw A, Shouman RT, van Herk M. Variation in volumes, dose–volume histograms, and estimated normal tissue complication probabilities of rectum and bladder during conformal radiotherapy of T3 prostate cancer. Int J Radiat Oncol Biol Phys 1995; 33: 1109–19. [DOI] [PubMed] [Google Scholar]
- 27.Engels B, Tournel K, Soete G, Storme G. Assessment of rectal distension in radiotherapy of prostate cancer using daily megavoltage CT image guidance. Radiother Oncol 2009; 90: 377–81. doi: 10.1016/j.radonc.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 28.Anderson NS, Yu JB, Peschel RE, Decker RH. A significant decrease in rectal volume and diameter during prostate IMRT. Radiother Oncol 2011; 98: 197–91. doi: 10.1016/j.radonc.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 29.Stasi M, Munoz F, Fiorino C, Pasquino M, Baiotto B, Marini P, et al. Emptying the rectum before treatment delivery limits the variations of rectal dose–volume parameters during 3DCRT of prostate cancer. Radiother Oncol 2006; 80: 363–70. [DOI] [PubMed] [Google Scholar]
- 30.Hammoud R, Patel SH, Pradhan D, Kim J, Guan H, Li S, et al. Examining margin reduction and its impact on dose distribution for prostate cancer patients undergoing daily cone-beam computed tomography. Int J Radiat Oncol Biol Phys 2008; 71: 265–73. doi: 10.1016/j.ijrobp.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 31.Crehange G, Mirjolet C, Gauthier M, Martin E, Truc G, Peignaux-Casasnovas K, et al. Clinical impact of margin reduction on late toxicity and short-term biochemical control for patients treated with daily on-line image guided IMRT for prostate cancer. Radiother Oncol 2012; 103: 244–6. doi: 10.1016/j.radonc.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 32.White EA, Brock KK, Jaffray DA, Catton CN. Inter-observer variability of prostate delineation on cone beam computerised tomography images. Clin Oncol (R Coll Radiol) 2009; 21: 32–8. doi: 10.1016/j.clon.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 33.Lütgendorf-Caucig C, Fotina I, Stock M, Pötter R, Goldner G, Georg D. Feasibility of CBCT-based target and normal structure delineation in prostate cancer radiotherapy: multi-observer and image multi-modality study. Radiother Oncol 2011; 98: 154–61. doi: 10.1016/j.radonc.2010.11.016 [DOI] [PubMed] [Google Scholar]
- 34.Kotte AN, Hofman P, Lagendijk JJ, van Vulpen M, van der Heide UA. Intrafraction motion of the prostate during external-beam radiation therapy: analysis of 427 patients with implanted fiducial markers. Int J Radiat Oncol Biol Phys 2007; 69: 419–25. [DOI] [PubMed] [Google Scholar]
- 35.Nederveen AJ, van der Heide UA, Dehnad H, van Moorselaar RJ, Hofman P, Lagendijk JJ. Measurements and clinical consequences of prostate motion during a radiotherapy fraction. Int J Radiat Oncol Biol Phys 2002; 53: 206–14. [DOI] [PubMed] [Google Scholar]
- 36.Ghilezan MJ, Jaffray DA, Siewerdsen JH, Van Herk M, Shetty A, Sharpe MB, et al. Prostate gland motion assessed with cine-magnetic resonance imaging (cine-MRI). Int J Radiat Oncol Biol Phys 2005; 62: 406–17. [DOI] [PubMed] [Google Scholar]
- 37.Curtis W, Khan M, Magnelli A, Stephans K, Tendulkar R, Xia P. Relationship of imaging frequency and planning margin to account for intrafraction prostate motion: analysis based on real-time monitoring data. Int J Radiat Oncol Biol Phys 2013; 85: 700–6. doi: 10.1016/j.ijrobp.2012.05.044 [DOI] [PubMed] [Google Scholar]
- 38.Spezi E, Downes P, Jarvis R, Radu E, Staffurth J. Patient-specific three-dimensional concomitant dose from cone beam computed tomography exposure in image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2012; 83: 419–26. doi: 10.1016/j.ijrobp.2011.06.1972 [DOI] [PubMed] [Google Scholar]
- 39.Kim DW, Chung WK, Yoon M. Imaging doses and secondary cancer risk from kilovoltage cone-beam CT in radiation therapy. Health Phys 2013; 104: 499–503. doi: 10.1097/HP.0b013e318285c685 [DOI] [PubMed] [Google Scholar]
- 40.Fiorino C, Reni M, Bolognesi A, Cattaneo GM, Calandrino R. Intra- and inter-observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol 1998; 47: 285–92. [DOI] [PubMed] [Google Scholar]
- 41.Roeske JC, Forman JD, Mesina CF, He T, Pelizzari CA, Fontenla E, et al. Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder, and rectum during a course of external beam radiation therapy. Int J Radiat Oncol Biol Phys 1995; 33: 1321–9. [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Wu Q, Yan D. The role of seminal vesicle motion in target margin assessment for online image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2009; 73: 935–43. doi: 10.1016/j.ijrobp.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craig T, Battista J, van Dyk J. Limitations of a convolution method for modeling geometric uncertainties in radiation therapy. I. The effect of shift invariance. Med Phys 2003; 30: 2001–11. [DOI] [PubMed] [Google Scholar]
- 44.Liu M, Moiseenko V, Agranovich A, Karvat A, Kwan W, Saleh ZH, et al. Normal tissue complication probability (NTCP) modeling of late rectal bleeding following external beam radiotherapy for prostate cancer: a test of the QUANTEC-recommended NTCP model. Acta Oncol 2010; 49: 1040–4. doi: 10.3109/0284186X.2010.509736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gulliford SL, Partridge M, Sydes MR, Webb S, Evans PM, Dearnaley DP. Parameters for the Lyman Kutcher Burman (LKB) model of normal tissue complication probability (NTCP) for specific rectal complications observed in clinical practise. Radiother Oncol 2012; 102: 347–51. doi: 10.1016/j.radonc.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 46.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002; 52: 6–13. [DOI] [PubMed] [Google Scholar]
- 47.Jensen I, Carl J, Lund B, Larsen EH, Nielsen J. Radiobiological impact of reduced margins and treatment technique for prostate cancer in terms of tumor control probability (TCP) and normal tissue complication probability (NTCP). Med Dosim 2011; 36: 130–7. doi: 10.1016/j.meddos.2010.02.004 [DOI] [PubMed] [Google Scholar]