Abstract
Objective:
To determine prevalence, clinicoradiological characteristics and outcome of patients with mesenteric panniculitis (MP) in a large hospital-based population.
Methods:
Consecutive abdominal CT examinations of 3820 patients were evaluated for MP. Clinical characteristics, therapy and outcome of patients with MP were evaluated during a 5-year follow-up period. A matched pair analysis was performed to further investigate the relation between MP and malignancy.
Results:
94 (2.5%) patients with MP were identified (mean age, 66.6 ± 11.2 years, 70.2% male). MP coexisted with malignancy (especially prostatic carcinoma) in 48.9% of patients, and this was slightly but significantly higher than in age- and sex-matched control patients (n = 188, 46.3%). In 48 patients, MP was presumed to be idiopathic. The most frequent presenting symptom was pain (54.3%). Laboratory findings revealed increased acute-phase reactants in half of the patients with MP. CT findings included increased density of mesenterial fat (mean, −56.8 ± 10.8 HU), fat ring sign, tumoural pseudocapsule and small soft-tissue nodules. Patients with MP (14.6%) developed significantly more malignancies during a 5-year follow-up than did the control group (6.9%). One patient was treated with prednisone without satisfactory response.
Conclusion:
The prevalence of MP in this study was 2.5%. In most patients, radiologic features included increased mesenteric fat density, fat ring sign and small soft-tissue nodules. MP was associated with a significant higher prevalence of coexisting malignancies and a higher prevalence of future cancer development.
Advances in knowledge:
A more accurate prevalence of MP on CT is demonstrated. An underlying malignancy may play a role.
Mesenteric panniculitis (MP) is a rare, idiopathic disorder characterized by chronic inflammation of the mesenteric fat. Numerous terms have been reported in literature depending on the pre-dominant presentation, including mesenteric lipodystrophy, sclerosing mesenteritis, mesenteric Weber–Christian disease and retractile mesenteritis or mesenteric fibrosis.1–8 It is now considered that these different diagnostics entities represent a spectrum of a single disease characterized by non-specific inflammation of the mesentery fat that may ultimately lead to fibrosis and retraction.1,9,10 The aetiology of MP remains unknown; it may occur independently or in association with other disorders. Various causes have been suggested, including autoimmune disorders, infection, trauma (including recent surgery) and ischaemia of the mesentery.11,12 It has a poorly understood association with underlying malignancy with conflicting results in literature, which suggests that it may be a paraneoplastic condition at least in some patients.13–16
Owing to the increased use of abdominal diagnostic imaging and the identification of specific signs on CT, MP is being recognized with increasing frequency at CT imaging. To date, mainly case reports and a few larger studies on MP have been published with conflicting results. Reported prevalence rates range from 0.16% to 7.80%.13–17 Most studies reported this condition in patients in middle and late adulthood with a slight male predominance. Patients may be entirely asymptomatic or present with non-specific mainly gastrointestinal manifestations, including abdominal pain, constipation or diarrhoea, an asymptomatic abdominal mass, weight loss, fever or chylous ascites.18–21 Depending on the underlying disease, the natural course of MP is often benign and self-limiting, although few data are available on the clinical outcome and response to therapy of patients with MP.
The aim of this study was to gain more insight into the prevalence and natural course of MP using strict criteria. We assessed prevalence, clinicoradiological characteristics and outcome of MP during a 5-year follow-up period in a large hospital-based population with specific focus on the relation between MP and malignancy.
METHODS AND MATERIALS
Patients
The Albert Schweitzer hospital is a 675-bed general teaching hospital in the south-west of the Netherlands and provides general services. The local institutional review board of the Albert Schweitzer Hospital approved the study.
Between January 2006 and 2007, consecutive abdominal CT examinations of 3820 patients were all retrospectively reanalysed for signs of MP. For all 3820 patients, radiology request forms and radiology reports were examined to record the indication of scanning and subsequent diagnosis. In cases of incomplete data on the radiology request forms or radiology reports, the medical records of these patients were reviewed in further detail. In patients fulfilling all the CT criteria for MP, medical records (electronic patient information system and paper records) were reviewed in detail to further determine the patients' medical history, clinical presentation, laboratory data and outcome after a 5-year follow-up period. Office visit notes, pathology reports, scanned documents from general practitioners or outside institutions and follow-up imaging were used.
A (post hoc) matched pair analysis was performed to gain more insight in the association of MP, underlying malignancy and future cancer development. For each subject with MP, two control subjects were selected from the total study population database. Patients were matched for age and sex. The prevalence, type of malignancy and future cancer development during the 5-year follow-up period were documented for both patient groups using information from medical records and follow-up imaging.
Radiological imaging
All CT examinations were performed using a 64-slice, 40-slice (both Philips Brilliance and Philips Medical Systems, Best, Netherlands) or 4-slice scanner (Siemens Somatom Volume zoom; Siemens AG, Erlangen, Germany) with axial 5-mm-thick reconstruction images from diaphragm to pubic symphysis. Patients were examined using our standard scanning protocol with intravenous and, when indicated, oral contrast, except for patients with renal failure and patients referred for kidney stones (no contrast used). In general, patients were scanned in the arterial or venous phase with a start delay of, respectively, 20 and 65 s. Contrast medium (Schering Nederland BV) was administered (120 ml contrast medium with 30 ml saline chaser volume) at a flow rate of 4 ml s−1. In cases of oral contrast, 25 ml of Telebrix Gastro (300 mg Iodium ml−1; Guerbet, Gorinchem, Nederland BV) was mixed with 1 l of water and administered 1 h before the start of the scan.
CT criteria for the diagnosis of MP were (1) a solitary well-defined mass of inhomogeneous fatty tissue at the root of the small bowel mesentery with attenuation values higher than those of the subcutaneous and retroperitoneal fat; (2) engulfment of superior mesenteric vessels without vascular involvement; (3) mass displacing the bowel loops with no evidence of invasion; (4) lymph nodules with a short axis <10 mm. The CT features recorded were size of the mass (maximum transverse diameter), density (Hounsfield units), the presence of calcifications, the presence and size of lymph nodes, the presence of the “fat-ring” sign (preservation of fat nearest to the mesenteric vessels) and the presence of a “tumoural pseudocapsule” (dense stripe in the peripheral region differentiating normal mesentery from the inflammatory process).7,13 Density was measured at three different areas within the fatty mass and compared with the density of retroperitoneal and subcutaneous fat. When fibrosis and retraction predominated in the mesentery, it was defined as retractile mesenteritis.9 During the 5-year follow-up period changes in density, size and extent of the mesentery on available follow-up imaging were compared with the initial CT scan to document regression, progression or stabilization of MP findings.
The term MP is solely reserved for idiopathic inflammation leading to infiltration of the mesentery and must be differentiated from any alternative causes altering density of the mesenteric fat (“misty mesentery”).22,23 Exclusion criteria therefore included (1) mesenteric oedema and massive ascites; (2) haemorrhage (owing to recent trauma or surgery defined as <6 months prior to the CT examination) or mesenteric ischaemia; (3) mesentery fat inflammation and necrosis in acute pancreatitis or other focal inflammatory processes; and (4) neoplasm involving the mesentery including lymphoma, inflammatory pseudotumour, mesenteric cyst or lipomatous tumours, desmoid tumour and carcinoid tumour.
A keyword search in radiology databases may lead to underreporting of the prevalence of MP (the term “mesenteric panniculitis” or “panniculitis” may not have been used in the radiology report or features of MP may not have been identified by the reporting radiologist). Therefore, the CT examinations of all 3820 patients were retrospectively reanalysed by one radiologist (NvP-K). All cases with mesenteric infiltration were then evaluated by a second radiologist (TRH) and a decision was reached by consensus as to which cases fulfilled the MP criteria.
Measurements
Variables included age, sex, ethnicity, clinical signs and symptoms, duration of symptoms, blood pressure, body mass index (BMI) and body surface area (BSA). Ethnicity was divided in following categories: Caucasian (Dutch/Western Europe and from the USA and Canada), North African/Turkish, Black, Asian and other ethnicity. A history of abdominal surgery and inflammatory bowel disease were assessed in each patient. The presence of cardiovascular risk factors and/or cardiovascular disease (CVD) (established coronary, cerebrovascular or peripheral vascular disease) was assessed. Smoking history was defined as never, former and current smoker. Laboratory measurements included erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum creatinine, haemoglobin, haematocrit, mean corpuscular volume, white blood cell count, and serum albumin. All laboratory data within a time window of 6 weeks around the date of the CT examination were used. Findings of CT scanning were documented as described.
Statistical analysis
Descriptive statistics were used to summarize the results. Continuous variables were reported as mean and standard deviation or, in case of skewed distribution, as median and interquartile range. Differences between continuous variables were analysed using (paired) t-test, Mann–Whitney test or Wilcoxon signed rank-sum test depending on their distribution. Categorical variables were expressed as proportions and compared with the χ2 test or Fischer exact test. In the (post hoc) matched pair analysis, the McNemar test (for paired samples) was used to test the differences in prevalence, type of malignancy and future cancer development. A p-value ≤0.05 was considered statistically significant. All data were analysed using SPSS® for Windows (v. 18.0; SPSS Inc, Chicago, IL).
RESULTS
Clinical presentation and diagnosis
Baseline characteristics of the total study population and subgroups of patients with and without MP are shown in Table 1. The main indications for obtaining the initial CT scan in the total study group were evaluation/staging of malignancy (48.4%), abdominal pain (27.3%) and kidney stones (haematuria and/or flank pain, 11.3%). In 120 patients (3.1% of total study population), the abdominal CT scan showed mesenteric infiltration. 26 of these patients (0.7% of total study population) met the exclusion criteria: mesenteric oedema or ascites (n = 9); mesenteric haemorrhage owing to recent surgery (n = 1); mesentery fat inflammation owing to other focal inflammatory processes (n = 4); neoplasm involving the mesentery (malignant lymphoma, n = 11; carcinoid tumour, n = 1). In the remaining 94 patients (2.5% of the total study population), CT findings of mesenteric infiltration met our strict definition of MP. No cases of retractile mesenteritis were found.
Table 1.
Characteristics of the total study population and subgroup of patients with and without mesenteric panniculitis (MP)
| Variable | Study population |
||
|---|---|---|---|
| Total study population | Patients without MP | Patients with MP | |
| Number of patients | 3820 | 3726 | 94 |
| Age (years) | 62.7 ± 16.0 | 62.6 ± 16.1 | 66.6 ± 11.2 |
| Male (sex) | 1891 (49.5) | 1825 (49.0) | 66 (70.2)a |
| Ethnicity | |||
| Caucasian | 3556 (93.1%) | 3463 (92.9%) | 93 (98.9%)b |
| North African/Turkish | 140 (3.7%) | 139 (3.7%) | 1 (1.1%) |
| Black | 35 (0.9%) | 35 (0.9%) | 0 (0%) |
| Asian | 24 (0.6%) | 24 (0.6%) | 0 (0%) |
| Other | 33 (0.9%) | 33 (0.9%) | 0 (0%) |
Values are mean ± standard deviation or number and percentage, where appropriate.
p < 0.001 vs male sex in patients without MP.
Ethnicity was missing in 32 patients (all patients without MP).
Baseline characteristics and associated disorders of these patients with MP are shown in Table 2. In 46 patients with MP (48.9%), a previous (58.7%) and/or concurrent malignancy (56.5%), often metastasized (37%), was present. Malignancies included prostatic carcinoma (n = 12), colorectal carcinoma (n = 7), extra-abdominal non-Hodgkin lymphoma (n = 4), urothelial cell carcinoma (n = 3), breast carcinoma (n = 3), stomach carcinoma (n = 2), oesophageal carcinoma (n = 2), skin malignancy (n = 2), Hodgkin lymphoma (n = 1), renal cell carcinoma (n = 1), adenocarcinoma of the duodenum (n = 1) and seminoma testis (n = 1). Seven patients had two malignancies: two patients had a history of prostate cancer and a skin malignancy, one patient had mesothelioma and a skin malignancy, one patient had oesophageal carcinoma and pancreatic cancer, one patient had colorectal carcinoma and prostate cancer, one patient had urothelial cell carcinoma and prostate cancer and one patient had ovarian carcinoma and breast carcinoma. In 17 of the 48 patients with MP without previous and/or concurrent malignancy, a concurrent benign disorder was present that may potentially have accounted for the patients' clinical symptoms at presentation: urinary tract stones (n = 11), retroperitoneal fibrosis (n = 2), gall stones (n = 3) and cholangitis (n = 1). In the remaining 29 patients, MP was an isolated finding.
Table 2.
Characteristics of patients with mesenteric panniculitis (MP) with and without concurrent and/or previous malignancy
| Variable | Patients with MP |
||
|---|---|---|---|
| Overall | Malignancy | No malignancy | |
| Number of patients | 94 | 46 | 48 |
| Age (years) | 66.6 ± 11.2 | 68.5 ± 9.8 | 64.7 ± 12.1 |
| Male (sex) | 66/94 (70.2%) | 34/46 (73.9%) | 32/48 (66.7%) |
| Abdominal surgery | 42/92 (45.7%) | 22/45 (48.9%) | 20/47 (42.6%) |
| Time before CT (years) | 9 (26.5) | 4 (13) | 24 (28.0)a |
| Less than 1 year before CT scan | 3/28 (10.7%) | 3/15 (20%) | 0/13 (0%) |
| Inflammatory bowel disease | 2/91 (2.2%) | 2/45 (4.4%) | 0/46 (0%) |
| Cardiovascular diseaseb | 13/92 (14.1%) | 5/45 (11.1%) | 8/47 (17.0%) |
| Hypertension | 25/92 (27.1%) | 10/45 (22.2%) | 15/47 (31.9%) |
| Number of anti-hypertensive drugs | 0 (1) | 0 (0) | 0 (1) |
| Diabetes mellitus | 8/92 (8.7%) | 5/45 (11.1%) | 3/47 (6.4%) |
| Smoking status | |||
| Current smoker | 17/90 (18.9%) | 6/43 (14.0%) | 11/47 (23.4%) |
| Former smoker | 11/90 (12.2%) | 5/43 (11.6%) | 6/47 (12.8%) |
| Body mass index (kg m−2) | 26.9 ± 3.1 | 26.0 ± 3.0 | 27.7 ± 3.0a |
| Body surface area (m2) | 1.96 ± 0.18 | 1.92 ± 0.18 | 2.01 ± 0.18a |
| Systolic blood pressure (mmHg) | 144 (28) | 140 (21) | 140 (30) |
| Diastolic blood pressure (mmHg) | 80 (14) | 80 (15) | 80 (16) |
Values are mean ± standard deviation; median and interquartile range (25th–75th percentile); or number and percentage, where appropriate.
p ≤ 0.05.
Defined as established coronary, peripheral vascular and/or cerebrovascular disease.
Approximately half of the 94 patients with MP had a history of previous abdominal surgery for several different conditions. This did not differ between patients with or without previous and/or concurrent malignancy. Overall, median time interval from abdominal surgery to the CT examination was 9 years. This time interval was significantly shorter in patients with malignancy than those patients without malignancy (Table 2). In three patients, abdominal surgery was performed within the last year before the CT examination. In two patients, inflammatory bowel disease was recorded (Crohn's disease, n = 1; ulcerative colitis, n = 1). Overall, cardiovascular risk factors and/or established CVD were frequently present (Table 2). Except for BMI and BSA (significantly higher in patients with MP without malignancy), none of these variables differed significantly between the two groups.
The most frequent presenting symptom was pain with 54.3% of patients complaining of lower back, abdominal and/or flank pain (Table 3). Patients with MP without malignancy experienced significantly more pain than patients with MP and malignancy. Conversely, constipation and/or diarrhoea showed a trend to be more frequently present in the group of patients with malignancy (Table 3, p = 0.054). Other frequent symptoms included weight loss, nausea and vomiting, and fever. Laboratory findings revealed increased acute-phase reactants in half of the patients. Median ESR and CRP tended to be higher in patients with MP and coexisting malignancy than in patients with MP without malignancy, albeit not statistically significant (Table 3).
Table 3.
Major symptoms and signs at presentation and laboratory findings in patients with mesenteric panniculitis (MP)
| Variable | Patients with MP |
|||
|---|---|---|---|---|
| Overall | Malignancy | No Malignancy | ||
| Symptoms and signs |
||||
| Pain | 51/94 (54.3%) |
15/46 (32.6%) | 36/48 (75.0%)a | |
| Lower back | 6/94 (6.4%) |
3/46 (6.5%) | 3/48 (6.3%) | |
| Abdominal | 30/94 (31.9%) |
8/46 (17.4%) | 22/48 (45.8%)a | |
| Flank | 20/94 (21.3%) |
5/46 (10.9%) | 15/48 (31.3%)a | |
| Weight loss | 20/94 (21.3%) |
11/46 (23.9%) | 9/48 (18.8) | |
| Constipation/diarrhea | 4/94 (4.3%) |
4/46 (8.7%) | 0 (0%)a | |
| Nausea/vomiting | 11/94 (11.7%) |
4/46 (8.7%) | 7/48 (14.6%) | |
| Fever/rigors | 6/94 (6.4%) |
1/46 (2.2%) | 5/48 (10.4%) | |
| Laboratory findings |
||||
| ESR (mm h−1) | 18 (42) |
30 (58) | 16 (39) | |
| C-reactive protein (mg l−1) | 11 (30) |
14 (32) | 9 (28) | |
| Increased acute-phase reactants |
||||
| ESR | 10/24 (41.7%) |
6/12 (50.0%) | 4/12 (33.3%) | |
| C-reactive protein | 17/33 (51.5%) |
8/14 (57.1%) | 9/19 (47.4%) | |
| WBC, ×109 per litre | 7.7 (4.2) |
8.1 (4.3) | 7.6 (4.5) | |
| Hb (mmol l−1) | 8.7 ± 1.2 |
8.5 ± 1.2 | 8.9 ± 1.1 | |
| Haematocrit (l l−1) | 0.43 (0.08) |
0.42 (0.05) | 0.43 (0.10) | |
| MCV (fl) | 88.8 ± 5.0 |
88.9 ± 6.0 | 88.6 ± 3.9 | |
| Albumin (g l−1) | 40.3 ± 4.3 |
39.7 ± 3.3 | 40.7 ± 4.9 | |
| Creatinine level (mg dl−1) | 95.5 (25.0) | 98.0 (20.0) | 92.0 (30.0) | |
ESR, erythrocyte sedimentation rate; MCV, mean corpuscular volume; WBC, white blood cells.
Values are mean ± standard deviation, median and interquartile range (25th–75th percentile) or number and percentage, where appropriate.
p ≤ 0.05.
Radiological findings
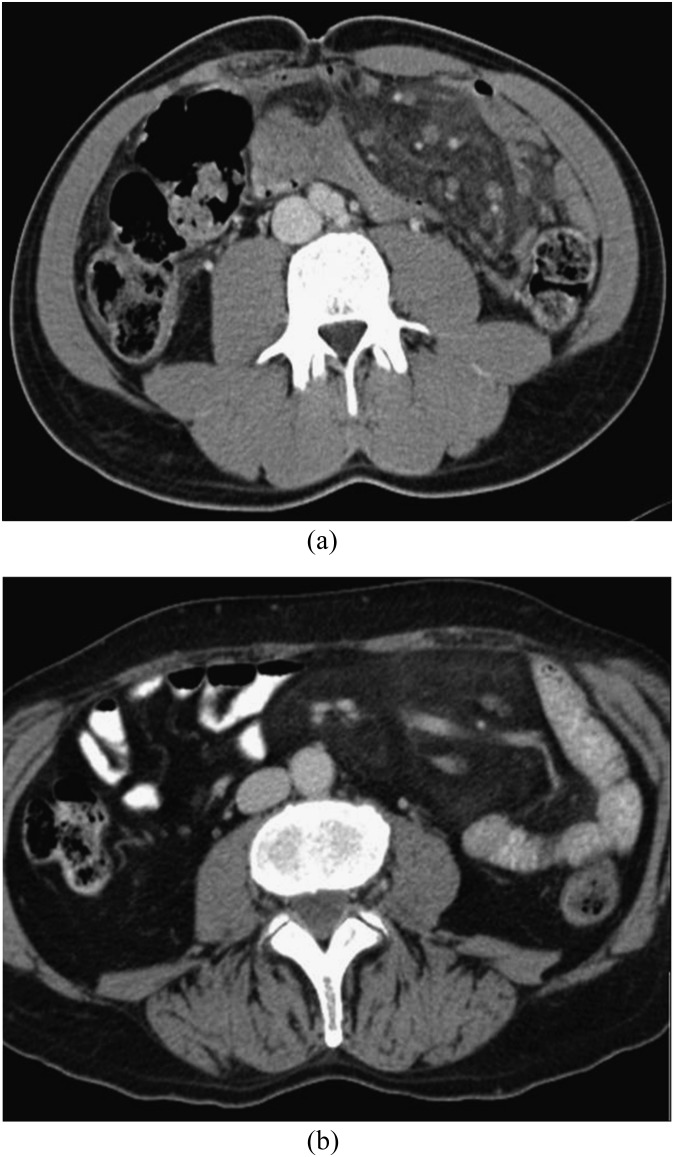
Figure 1 shows two examples of MP. CT findings are summarized in Table 4. Mean maximum transverse diameter was 9.5 cm. The maximum transverse diameter of the mass was directed towards the left abdomen (leftward orientation) in the majority of patients.13 The “fat-ring” sign (Figure 2) was present in most patients. Mean density of the fatty halo around the mesenteric vessels and nodules or lymph nodes was significantly lower than the density of the mass (Table 4). In more than half of patients, a “tumoural pseudo-capsule” was positive (Figure 3). Nodules of soft-tissue density were found in 92 patients, calcifications only in 4 patients.
Figure 1.
(a, b) Mesenteric panniculitis: the mesenteric fat is hyperdense compared with the subcutaneous or retroperitoneal fat and displaces the surrounding small bowel loops.
Table 4.
CT findings in patients with mesenteric panniculitis (MP)
| Variable | Patients with MP |
|---|---|
| Number of patients | 94 |
| Transverse diameter (cm) | 9.5 ± 1.9 |
| Orientation transverse diameter | |
| Leftward | 91 (96.8%) |
| Rightward/central | 3 (3.2%) |
| Density mesenteric fat (HU) | −56.8 ± 10.8 |
| Density retroperitoneal fat (HU) | −105.0 (8)a |
| Density subcutaneous fat (HU) | −109.2 ± 6.7a |
| Fat ring sign | 88 (93.6%) |
| Density (HU) | −105.5 (12)a |
| Stripe or pseudocapsule | 53 (56.4%) |
| Lymph nodes | |
| None | 2 (2.1%) |
| <5 mm | 81 (86.2%) |
| 5–10 mm | 11 (11.7%) |
| Calcifications | 4 (4.3%) |
Values are mean ± standard deviation, median and interquartile range or number and percentage, where appropriate.
p < 0.001 vs mean density mesenteric fat.
Figure 2.

Mesenteric panniculitis with the characteristic “fat-ring” sign (arrow). The mesenteric vessels, which are surrounded by normal fat, are enveloped by hyperdense mesenteric fat.
Figure 3.

Mesenteric panniculitis with “tumoural pseudo-capsule” (arrow), a dense stripe in the peripheral region differentiating normal mesentery from the inflammatory process.
5-year follow-up
Patient records were reviewed extensively 5 years after MP was documented on the initial CT scan. Follow-up data are summarized in Table 5. Four patients (4.3%) were lost to follow-up. 27 patients had died, mainly owing to their co-existing malignancy. CT findings of MP stabilized or disappeared in the majority of patients. None of the cases progressed to fibrosis. Pain symptoms diminished spontaneously in the majority of patients. Regarding treatment, only one patient with MP without malignancy was treated with prednisone without satisfactory response.
Table 5.
Follow-up data (5-year) in patients with mesenteric panniculitis (MP)
| Variable | Patients with MP |
||
|---|---|---|---|
| All patients | Patients with malignancy | Patients without malignancy | |
| Number of patients | 94 | 46 | 48 |
| Lost to follow-up | 4 (4.3) | 1 (2.2) | 3 (6.3)a |
| Died | 27 (28.7) | 22 (47.8) | 5 (10.4)a |
| Follow-up CT examination | |||
| Number of patients | 37 (39.4) | 22 | 15 |
| Number of examinations | 2 (2) | 2 (2) | 2 (3) |
| Stabilization of findings | 26 | 17 | 9 |
| Regression/normalization of findings | 10 | 5 | 5 |
| Progression of findings | 1 | 0 | 1 |
| Pain symptoms | |||
| Persistent pain | 9/51 (17.6) | 3/15 (20.0) | 6/36 (16.7) |
| Regression of pain | 42/51 (82.4) | 12/15 (80.0) | 30/36 (83.3) |
Values are median and interquartile range (25th–75th percentile) or number and percentage, where appropriate.
p ≤ 0.05.
Seven patients with MP without a malignancy at presentation developed a malignancy after initial diagnosis of MP. These malignancies included skin malignancy (n = 2), prostate cancer, bladder cancer, lung cancer, appendiceal cancer and chronic lymphocytic leukaemia. The duration between initial diagnosis of MP and development of malignancy ranged from 2 months to 4 years and 4 months with a mean of 19 months. In retrospect, no minimal signs of malignancy were found on the initial CT examinations for these patients.
Association between mesenteric panniculitis and malignancy: matched pair analysis
To further unravel the relation of MP and malignancy a (post hoc) matched pair analysis was performed regarding type, prevalence of malignancy and future cancer development (Table 6). The prevalence of previous and/or concurrent malignancy was significantly higher in patients with MP than in the control group. This was particularly true for the prevalence of prostatic carcinoma. Colorectal carcinoma prevalence was significantly lower in patients with MP. Other cancer types did not significantly differ between the two patient groups. Metastases were found significantly more frequently in patients with MP at the time of the initial CT scan, and during the 5-year follow-up time, metastases developed significantly more frequently in the control patients. In the patients with MP, the chance of future cancer development was significantly higher than in the control group (Table 6).
Table 6.
Relation of mesenteric panniculitis (MP) with malignancy: matched pair analysis
| Variable | Patients with MP | Control patients |
|---|---|---|
| Number of patients | 94 | 188 |
| Age (years) | 66.6 ± 11.2 | 66.6 ± 11.1 |
| Male (sex) | 66 (70.2%) | 132 (70.2%) |
| Malignancy at initial CT scan | ||
| Previous and/or concurrent malignancy | 46 (48.9%) | 87 (46.3%)a |
| Metastasis | 17/46 (37.0%) | 23/87 (26,4%)a |
| Prostatic carcinoma | 16/46 (34,8%) | 23/87 (26.3%)a |
| Colorectal carcinoma | 8/46 (17.4%) | 18/87 (20.7%)a |
| 5-year follow-up | ||
| Lost to follow-up | 4 (4.3%) | 14 (7.4%)a |
| Died | 27/90 (30.0%) | 69/174 (39.7%) |
| Malignancy during follow-up | 7/48 (14.6%) | 7/101 (6.9%)a |
| Metastasis during follow-up | 2/29 (6.9%) | 13/63 (20.6%)a |
McNemar test for paired samples; p < 0.05.
DISCUSSION
This study represents one of the few larger series of patients with MP with detailed information on prevalence, clinicoradiological characteristics, associated diseases and outcome after 5 years of follow-up. Few studies reported data on the prevalence of MP. One study reported a prevalence of 0.6% with a female predominance;13 more recently, a study was published in 613 patients with a very high prevalence ranging from 3.4% to 7.8% depending on the CT criteria used, with a slight female predominance.17 Other studies reported a lower prevalence ranging from 0.16% to 0.60%;14–16 however, these studies were all based on a keyword search instead of actually reanalysing all the CT scans for signs of MP. In our view, this may lead to under-reporting as the specific term “mesenteric panniculitis” or “panniculitis” may not have been used in the radiology report or the features of MP may not have been identified by the reporting radiologist. In our large study population using very strict CT criteria and exclusion criteria, we found a prevalence of 2.5% and a 1.4-to-1.0 male-to-female ratio. The male predominance is in agreement with most other studies.1,3,4,17 The increasing prevalence of MP may be owing to the increased use of abdominal diagnostic imaging, the technical revolution in CT scanning, better identification and knowledge of specific signs and radiologist awareness. The very high prevalence reported by Coulier17 in a smaller population may suggest overestimation or selection bias. In contrast to the study of Coulier, we did not include patients with intra-abdominal lymphoma, which may in part explain our lower prevalence.
The pathophysiology of MP remains unknown, and several mechanisms have been postulated. MP has a poorly understood association with underlying malignancy with conflicting results in literature, which suggests that it may at least in some patients be a paraneoplastic condition.13–16 Several studies reported data on MP and coexisting malignancy, but, except for the study of Gögebakan et al,16 none of these studies included a control group with matching for potential confounding variables such as age and sex. We found in accordance with other studies that patients with MP were older and more likely to be male, which would inevitably increase the likelihood of cancer development in general and prostatic cancer specifically. In our matched pair analyses, we, however, found a significant higher prevalence of malignancy and metastasis in patients with MP at the time of the initial CT scan than in the control group. Prostatic carcinoma was the most frequent coexisting malignancy that has only been reported by Coulier.17 In addition, the chance of future cancer development during the 5-year follow-up period was significantly higher in patients with MP than in the control group. These findings are in contrast with the study of Gögebakan et al,16 who did not find any relation between MP and malignancy. It is, however, unclear whether extensive follow up of the control group was performed in this study. In addition, follow-up was available in only 35 of 77 patients with MP based on follow-up imaging. We performed follow-up of both patients with MP and the control patients during a 5-year period using information from follow-up imaging and medical records. Kipfer et al4 suggested that MP is a non-specific response to an underlying abdominal malignancy, which may in part explain our findings. Coulier suggested that a high prevalence of MP explains the spontaneous association with numerous and probably unrelated clinical situations/disorders found in the literature. This, in our view, however, does not explain the significant higher prevalence of both malignancies in general and prostatic carcinoma in particular in patients with MP than in the control group in the matched pair analysis. MP therefore is thought to be relevant in terms of clinical predictivity of an associated neoplasm, particularly for prostatic carcinoma, but these findings need to be confirmed in a large prospective study.
Another potential important finding of the present study is that 50% of patients in this study underwent previous abdominal surgery for several conditions with a median time interval of 9 years. Akram et al20 suggested that the use of powdered surgical gloves before the mid-1980s might have a role in the development of fibrosis and adhesions in some cases. This was, however, not compared with a group of control patients and therefore it is uncertain whether this is more than expected in a normal population.
In our study, the most frequent symptom was abdominal pain and flank pain. This was most frequent in patients with presumed idiopathic MP. Some of these patients had a benign disease, which may in part have explained the patient's clinical symptoms at presentation. This may also in part be the explanation for the significantly higher frequency of reported pain symptoms in this group of patients. Most signs and symptoms may be caused by direct mechanical effect of the mesenteric mass on surrounding structures. Laboratory findings are usually non-specific and include elevated sedimentation rate and leukocytes as well as anaemia.13 We found increased acute-phase reactants in half of the patients.
Regarding the radiological findings, the hallmark of MP is the increased density of the mesenteric fat compared with retroperitoneal and subcutaneous fat, which was confirmed in our study. An additional significant sign on CT is the “fat-ring” sign, which has been reported in 75–85% of patients with MP.10,13 We found a somewhat higher percentage of 92.7%. Another important feature includes the tumoural pseudocapsule, which was found in 56.4%. This is in agreement with earlier reported studies.10,13
MP must be differentiated from any alternative causes altering the density of mesenteric fat.22,23 We used a wide range of conditions as exclusion criteria, which resulted in the exclusion of 26 patients. Several studies, however, have included patients with intra-abdominal non-Hogkin lymphoma.1,4,13,17,20 Typically, non-Hogkin lymphoma causes bulky lymphadenopathy with shrinkage of mesenteric lymphadenopathy after treatment, which may result in residual scarring mimicking MP. Incidentally, a positive “fat-ring” sign has been reported in patients with treated non-Hogkin lymphoma.24 We are therefore convinced that including patients with intra-abdominal non-Hogkin lymphoma will lead to substantial misdiagnosis. Two patients with MP were also diagnosed with inflammatory bowel disease. The “misty mesentery” in these cases fulfilled all the criteria of MP, were located at the jejunal mesentery and not adjacent to thickened bowel walls. Patients were therefore not excluded. However, mesenteric stranding owing to inflammatory bowel disease (especially Crohn's disease) might be difficult to differentiate with certainty from MP. Excluding these two patients did not change our results substantially.
MP has generally been reported as a self-limiting disease, and the overall prognosis is good. Significant morbidity and a chronic debilitating disease has, however, been described in up to 20% of patients with even one fatal case.20,25 Daskalogiannaki et al13 and Coulier17 both found a great stability of CT findings in MP, this was confirmed in our study. None of our patients showed progression to mesenteric fibrosis (retractile mesenteritis), which was also found in other studies.13–15 This may suggest that MP is not in the same spectrum of disease as rectractile mesenteritis. There is no consensus on treatment for symptomatic cases of MP, and treatment decisions are based on small case series.26–29 In advanced or progressive cases, treatment with anti-inflammatory, immunomodulatory and antifibrotic agents has been advocated. Akram et al20 reported a beneficial outcome in 63% of symptomatic patients treated with a combination of Tamoxifen and prednisone with a mean duration of treatment of 2–30 months. In our study, only one patient was treated with prednisone without satisfactory response.
Some limitations of this study should be considered. A limitation is the lack of histological proof as none of the patients underwent needle aspiration or core biopsy and diagnosis was purely based on CT findings. In our view, the incidental and self-limiting nature of MP and the well-recognized CT characteristics of MP extensively described in the literature did not justify biopsy.9,13,19,30,31 Another limitation is the lack of data on treatment, as only one patient was treated mainly because physicians may be reluctant to start empiric therapy owing to unfamiliarity with the disorder. Finally, limitations of this study are those inherent to retrospective studies where data have been collected in a clinical context. Larger prospective studies are necessary to confirm our findings, to identify high-risk patients for the development of malignancy and to make proper recommendations for follow-up imaging (CT currently is the method of choice for evaluation of mesenteric abnormalities, but it is well known that this contributes to an increased risk of malignancy owing to the radiation dose). This motivated us to start an observational prospective study in patients with MP with specific focus on the relation with malignancy.
CONCLUSIONS
The prevalence of MP in our large study population using very strict CT criteria and exclusion criteria was 2.5%, which is likely to be more accurate than earlier reported prevalence. It may be responsible for the clinical manifestations in a substantial percentage of patients. More importantly, patients with MP had a significantly higher prevalence of both malignancies in general and prostatic carcinoma in particular as well as a higher chance of future cancer development. MP may be relevant in terms of clinical predictivity of an associated neoplasm and should not be disregarded by the radiologist and clinician. Larger prospective studies with follow-up are necessary to confirm our findings, to identify high-risk patients for the development of malignancy and to make proper recommendations for follow-up imaging taking into account the potential risk of follow-up CT imaging and subsequent (cumulative) radiation dose.
REFERENCES
- 1.Emory TS, Monihan JM, Carr NJ, Sobin LH. Sclerosing mesenteritis, mesenteric panniculitis and mesenteric lipodystrophy: a single entity? Am J Surg Pathol 1997; 21: 392–8. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JK, Hwang WS. Idiopathic retractile (sclerosing) mesenteritis and its differential diagnosis. Am J Surg Pathol 1989; 13: 513–21. [DOI] [PubMed] [Google Scholar]
- 3.Durst AL, Freund H, Rosenmann E, Birnbaum D. Mesenteric panniculitis: review of the leterature and presentation of cases. Surgery 1977; 81: 203–11. [PubMed] [Google Scholar]
- 4.Kipfer RE, Moertel CG, Dahlin DC. Mesenteric lipodystrophy. Ann Intern Med 1974; 80: 582–8. [DOI] [PubMed] [Google Scholar]
- 5.Ogden WW, Bradburn DM, Rives JD. Panniculitis of the mesentery. Ann Surg 1960; 151: 659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden WW, Bradburn DM, Rives JD. Mesenteric panniculitis: review of 27 cases. Ann Surg 1965; 161: 864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulier B. Mesenteric panniculitis. Part 1: MDCT—pictorial review. JBR-BTR 2011; 94: 229–40. [DOI] [PubMed] [Google Scholar]
- 8.Lawler LP, McCarthy DM, Fishman EK, Hruban R. Sclerosing mesenteritis: depiction by multidetector CT and three-dimensional volume rendering. AJR Am J Roentgenol 2002; 178: 97–9. [DOI] [PubMed] [Google Scholar]
- 9.Horton KM, Lawler LP, Fishman EK. CT findings in sclerosing mesenteritis (panniculitis): spectrum of disease. Radiographics 2003; 23: 1561–7. [DOI] [PubMed] [Google Scholar]
- 10.Sabate JM, Torrubia S, Maideu J, Franquet T, Monill JM, Perez C. Sclerosing mesenteritis: imaging findings in 17 patients. AJR Am J Roentgenol 1999; 172: 625–9. [DOI] [PubMed] [Google Scholar]
- 11.Parra-Davila E, McKenney MG, Sleeman D, Hartmann R, Rao RK, McKenney K, et al. Mesenteric panniculitis: case report and literature review. Am Surg 1998; 64: 768–71. [PubMed] [Google Scholar]
- 12.Schaffler A, Scholmerich J, Buchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue—emerging role in intestinal and mesenteric diseases. Nat Clin Pract Gastroenterol Hepatol 2005; 2: 103–11. [DOI] [PubMed] [Google Scholar]
- 13.Daskalogiannaki M, Voloudaki A, Prassopoulos P, Magkanas E, Stefanaki K, Apostolaki E, et al. CT evaluation of mesenteric panniculitis: prevalence and associated diseases. AJR Am J Roentgenol 2000; 174: 427–31. [DOI] [PubMed] [Google Scholar]
- 14.Wilkes A, Griffin N, Dixon L, Dobbs B, Frizelle FA. Mesenteric panniculitis: a paraneoplastic phenomenon? Dis Colon Rectum 2012; 55: 806–9. doi: 10.1097/DCR.0b013e318252e286 [DOI] [PubMed] [Google Scholar]
- 15.Smith ZL, Sifuentes H, Deepak P, Ecanow DB, Ehrenpreis ED. Relationship between mesenteric abnormalities on computed tomography and malignancy: clinical findings and outcomes of 359 patients. J Clin Gastroenterol 2013; 47: 409–14. doi: 10.1097/MCG.0b013e3182703148 [DOI] [PubMed] [Google Scholar]
- 16.Gögebakan O, Albrecht T, Osterhoff MA, Reimann A. Is mesenteric panniculitis truely a paraneoplastic phenomenon? A matched pair analysis. Eur J Radiol 2013; 82: 1853–9. doi: 10.1016/j.ejrad.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 17.Coulier B. Mesenteric panniculitis. Part 2: prevalence and natural course: MDCT prospective study. JBR-BTR 2011; 94: 241–6. [DOI] [PubMed] [Google Scholar]
- 18.Hemaidan A, Vanegas F, Alvarez OA, Arroyo MA, Lee M. Mesenteric lipodystrophy with fever of unknown origin and mesenteric calcifications. South Med J 1999; 92: 513–16. [DOI] [PubMed] [Google Scholar]
- 19.Katz ME, Heiken JP, Glazer HS, Lee JK. Intraabdominal panniculitis: clinical, radiographic, and CT features. AJR Am J Roentgenol 1985; 145: 293–6. [DOI] [PubMed] [Google Scholar]
- 20.Akram S, Pardi DS, Schaffner JA, Smyrk TC. Sclerosing mesenteritis: clinical features, treatment, and outcome in ninety-two patients. Clin Gastroenterol Hepatol 2007; 5: 589–96. [DOI] [PubMed] [Google Scholar]
- 21.Ehrenpreis ED, Boiskin I, Schaefer K. Chylous ascites in a patient with mesenteric panniculits. J Clin Gastroenterol 2008; 42: 327–8. doi: 10.1097/MCG.0b013e31802c346d [DOI] [PubMed] [Google Scholar]
- 22.Seo BK, Ha HK, Kim AY, Kim TK, Kim MJ, Byun JH, et al. Segmental misty mesentery: analysis of CT features and primary causes. Radiology 2003; 226: 86–94. [DOI] [PubMed] [Google Scholar]
- 23.van Breda Vriesman AC, Schuttevaer HM, Coerkamp EG, Puylaert JB. Mesenteric panniculitis: US and CT features. Eur Radiol 2004; 14: 2242–8. [DOI] [PubMed] [Google Scholar]
- 24.Valls C. Fat-ring sign in sclerosing mesenteritis. AJR Am J Roentgenol 2000; 174: 259–60. [DOI] [PubMed] [Google Scholar]
- 25.Katsanos KH, Ioachim E, Michail M, Price AC, Agnantis N, Kappas A, et al. A fatal case of sclerosing mesenteritis. Dig Liver Dis 2004; 36: 153–6. [DOI] [PubMed] [Google Scholar]
- 26.Mazure R, Fernandez MP, Niveloni S, Pedreira S, Vazquez H, Smecuol E, et al. Successful treatment of retractile mesenteritis with oral progesterone. Gastroenterology 1998; 114: 1313–17. [DOI] [PubMed] [Google Scholar]
- 27.Bala A, Coderre SP, Johnson DR, Nayak V. Treatment of sclerosing mesenteritis with corticosteroids and azathioprine. AJR Am J Roentgenol 2001; 15: 533–5. [DOI] [PubMed] [Google Scholar]
- 28.Genereau T, Bellin MF, Wechsler B, Le TH, Bellanger J, Grellet J, et al. Demonstration of efficacy of combining corticosteroids and colchicine in two patients with idiopathic sclerosing mesenteritis. Dig Dis Sci 1996; 41: 684–8. [DOI] [PubMed] [Google Scholar]
- 29.Bush RW, Hammar SP, Jr, Rudolph RH. Sclerosing mesenteritis. Response to cyclophosphamide. Arch Invest Med 1986; 146: 503–5. [PubMed] [Google Scholar]
- 30.Mata JM, Inaraja L, Martin J, Olazabal A, Castilla MT. CT features of mesenteric panniculitis. J Comput Assist Tomogr 1987; 11: 1021–3. [DOI] [PubMed] [Google Scholar]
- 31.Kopecky KK, Lappas JC, Baker MK, Madura JA. Mesenteric panniculitis: CT appearance. Gastrointest Radiol 1988; 13: 273–4. [DOI] [PubMed] [Google Scholar]