Abstract
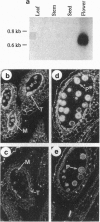
Male fertility in flowering plants is dependent on production of viable pollen grains within the anther. Genes expressed exclusively in the anther are likely to include those that control male fertility. On the basis of their tissue specificity, such genes have been isolated, yet in none of them has this function been demonstrated. Here we report that one such gene, Bcp1, is active in both diploid tapetum and haploid microspores and is required for pollen fertility. Perturbation of this gene in either tapetum or microspores prevents production of fertile pollen in transgenic Arabidopsis plants. When tapetum expression of this gene is perturbed, mature anthers contain dead shriveled pollen. On the other hand, when microspore expression is perturbed, anthers show 1:1 segregation of viable/aborted pollen. These findings identify a class of sporophytic/gametophytic genes controlling male fertility and, hence, reproduction in flowering plants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarts M. G., Dirkse W. G., Stiekema W. J., Pereira A. Transposon tagging of a male sterility gene in Arabidopsis. Nature. 1993 Jun 24;363(6431):715–717. doi: 10.1038/363715a0. [DOI] [PubMed] [Google Scholar]
- Alexander M. P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969 May;44(3):117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray R. G., Grierson D. Molecular genetics of tomato fruit ripening. Trends Genet. 1993 Dec;9(12):438–443. doi: 10.1016/0168-9525(93)90108-t. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J., Heslop-Harrison Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970 May;45(3):115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- McCormick S. Male Gametophyte Development. Plant Cell. 1993 Oct;5(10):1265–1275. doi: 10.1105/tpc.5.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D., Lemieux B., Yen G., Davis R. W. A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev. 1993 Jun;7(6):974–985. doi: 10.1101/gad.7.6.974. [DOI] [PubMed] [Google Scholar]
- Regan S. M., Moffatt B. A. Cytochemical Analysis of Pollen Development in Wild-Type Arabidopsis and a Male-Sterile Mutant. Plant Cell. 1990 Sep;2(9):877–889. doi: 10.1105/tpc.2.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theerakulpisut P., Xu H., Singh M. B., Pettitt J. M., Knox R. B. Isolation and developmental expression of Bcp1, an anther-specific cDNA clone in Brassica campestris. Plant Cell. 1991 Oct;3(10):1073–1084. doi: 10.1105/tpc.3.10.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D., Yamaguchi J., McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development. 1990 Jul;109(3):705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne P., Delvallee I., Dumas C. Rapid assessment of microspore and pollen development stage in wheat and maize using DAPI and membrane permeabilization. Stain Technol. 1987 Sep;62(5):299–304. doi: 10.3109/10520298709108014. [DOI] [PubMed] [Google Scholar]
- Xu H., Davies S. P., Kwan B. Y., O'Brien A. P., Singh M., Knox R. B. Haploid and diploid expression of a Brassica campestris anther-specific gene promoter in Arabidopsis and tobacco. Mol Gen Genet. 1993 May;239(1-2):58–65. doi: 10.1007/BF00281601. [DOI] [PubMed] [Google Scholar]