Abstract
Renal transplantation, first performed successfully in the 1950s, is the treatment of choice for most patients with end-stage renal failure. It confers longer term survival and a better quality of life than do both haemodialysis and peritoneal dialysis. The success of renal transplantation is dependent on the preservation of renal graft function and despite the many advances in surgical techniques, immunosuppressive regimens and supportive therapies, many challenges remain including post-operative ureteral obstruction. This complication can pose a risk to graft, and, occasionally, to patient survival. In this pictorial review, we describe the causes of ureteral obstruction following renal transplantation and illustrate the pivotal role of radiology in both diagnosing and managing these complications.
Ureteral obstruction occurs in 2–10% of renal transplant patients post-operatively, usually presenting within the first few weeks, or the first year.1,2 Prompt diagnosis and remedial treatment are vital to prevent graft loss. Here, we review the causes of ureteral obstruction, the diagnostic process and the role of image-guided minimally invasive percutaneous techniques in their management.
CAUSES OF RENAL TRANSPLANT URETERAL OBSTRUCTION
The main causes and risk factors for ureteral obstruction are summarized in Table 1.
Table 1.
Causes of obstruction in renal transplant patients
| Risk factors | Intrinsic obstruction | Extrinsic compression | Others |
|---|---|---|---|
| >65 years old More than two transplant arteries Increased cold ischaemia time Stentless anastomotic transplant |
Oedema Clot Tumour Calculi |
Lymphocele Abscess Haematoma |
Ischaemia Kinking Previously unrecognized PUJ obstruction Misplacement of ureteric anastomosis |
PUJ, pelviureteric junction.
Ureteric ischaemia is the most common cause accounting for around 90% of cases.2,3 The distal ureter close to the ureterovesical junction is invariably involved as this area is particularly vulnerable to ischaemia owing to its anatomical location, being furthest from the renal artery. The proximal ureter is supplied by small branches from the main renal artery, but this can be variable. Indeed, it is now routine practice for the ureter to be stented for the first 4–6 weeks after transplantation as added protection since ureteric ischaemia typically presents early if a stent is not placed at the time of transplant surgery or following stent removal from the site of anastomosis. Causes of ureteric obstruction may be classified as early (<3 months) or late (>3 months) post transplantion.4
Early obstruction is often related to ureteric ischaemia, a narrow anti-reflux tunnel at the ureterovesical anastomosis or external compression by a lymphocoele or haematoma.1 Errors in surgical technique are also a recognized cause. A long ureter increases the risk of early obstruction, as it is more prone to ischaemia and liable to kinking, presenting as intermittent obstruction (Figure 1).
Figure 1.
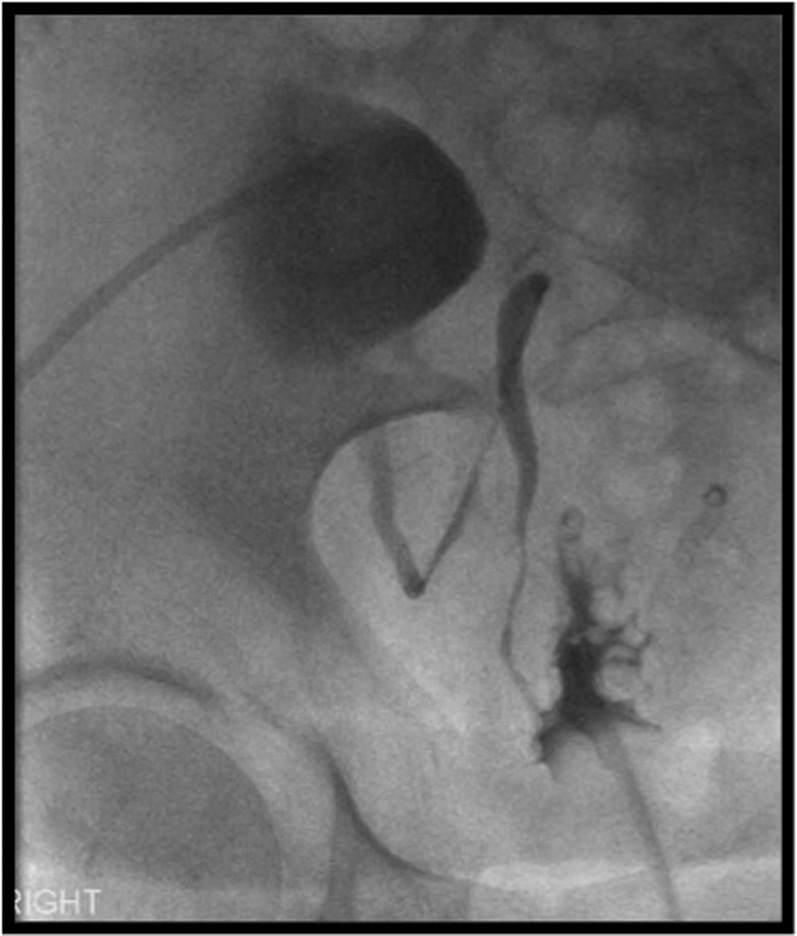
A 59-year-old male with a history of recurrent ureteral obstruction and hydronephrosis, for which he had undergone ureteric reconstruction and transplantation of the native ureter into the transplant kidney. This nephrostogram performed following nephrostomy for hydronephrosis shows opacification of the transplanted ureter, which is long and tortuous, and therefore prone to stricturing and intermittent kinking (see Renal transplant obstruction section for further details).
The aetiology of late ureteral stricture is less well defined.5 It may be owing to ischaemic fibrosis caused by a deficient vascular supply, vasculitis in the context of an acute rejection episode or vasoconstriction caused by immunosuppressant therapy, such as corticosteroids and calcineurin inhibitors. In comparison, ureteric tumours and ureterolithiasis, which are common causes of ureteric obstruction in non-transplant patients, are infrequent in the transplant ureteric stenosis setting. Nevertheless, malignant ureteric stricture should be considered if the stenosis develops late after transplantation, because the incidence of bladder carcinoma is three times higher in the transplant population.6 Ureterolithiasis is infrequent in transplant recipients but data on incidence are sparse.
Some cases of transplant ureteric stenosis may be associated with ureteric leak or necrosis. Whereas stenosis of a native ureter causes colic, and transplant ureteric obstruction is often painless because the ureter is denervated. Alternatively, non-specific pain may be felt over the transplant, especially if there is associated infection, but silent ureteric obstruction and renal graft loss is a risk. The clinical presentation of poor renal function and oliguria is non-specific and may be seen with any cause of renal transplant dysfunction, for example, renal allograft rejection or renal artery stenosis. Therefore, there should be a low threshold for further radiological investigation to rule out obstructive uropathy in patients who experience an acute rise in serum creatinine together with an unexplained reduction in urine output.7
DIAGNOSIS
There are several imaging modalities that can be used to diagnose and assess hydronephrosis, including ultrasonography, CT, MR urography and scintigraphy.
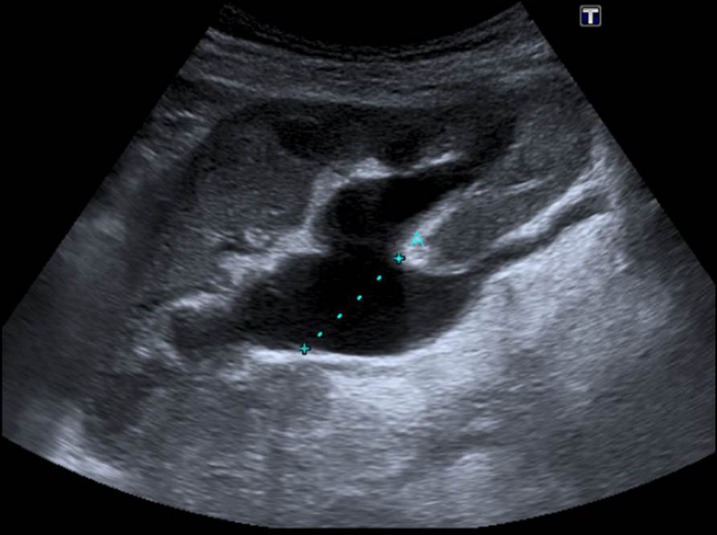
Ultrasonography is the ideal risk-free first-line investigation, providing a sensitive tool for confirming hydronephrosis, excluding periureteric collections and ensuring normal transplant perfusion (Figure 2), but hydronephrosis may only be minimal in the early stages. Furthermore, mild calyceal distension is not an infrequent occurrence in the absence of obstruction, reflecting the dependency of some calyces in the supine position and free ureteric reflux if the bladder is full.2 The transplant kidney is more prone to vesicoureteric reflux for several reasons. Its placement in the pelvis results in loss of the normal submucosal tunnel and oblique orientation of the native ureter, so the valve-like effect is not retained. This is exacerbated by the short length of the transplant ureter. Repeating the ultrasonography examination with an empty bladder is therefore crucial and, if necessary, whilst the patient is standing. Periodic sonographic examination together with close monitoring of renal function by serial serum electrolyte evaluation is necessary in those with minimal calyceal dilatation to direct the need for intervention. Slight pelvic dilation may be seen in the normal transplant kidney owing to increased urine production by the solitary kidney and loss of ureteric tonicity following denervation. In the acute phase after transplantation, the ureter may exhibit impaired peristalsis temporarily. The resulting transient dilation should be minor and confined to the renal pelvis. A further consideration when observing the post-operative ultrasonography as a baseline is the possibility of transient obstruction owing to oedema at the vesicoureteric junction anastomosis. The exception is the finding of low-level echoes in a febrile patient with a dilated pyelocalyceal system, which may indicate pyonephrosis.
Figure 2.
A 69-year-old male cadaveric renal transplant recipient. Ultrasound scan performed 40 days after transplantation to investigate unexplained rising creatinine shows hydronephrosis. Cortical thickness is preserved that indicates this is unlikely to be chronic. The transplant ureter was dilated up to the vesicoureteric junction indicating a distal anastomotic stricture (Figure 5).
CT offers greater anatomical detail to elucidate the cause and extent of hydronephrosis, in addition to pinpointing the site of obstruction. CT imaging can be performed with or without intravenous contrast depending on the renal function and suspected cause of obstruction. Delayed CT studies after intravenous contrast are useful for confirming ureteric leak or necrosis.
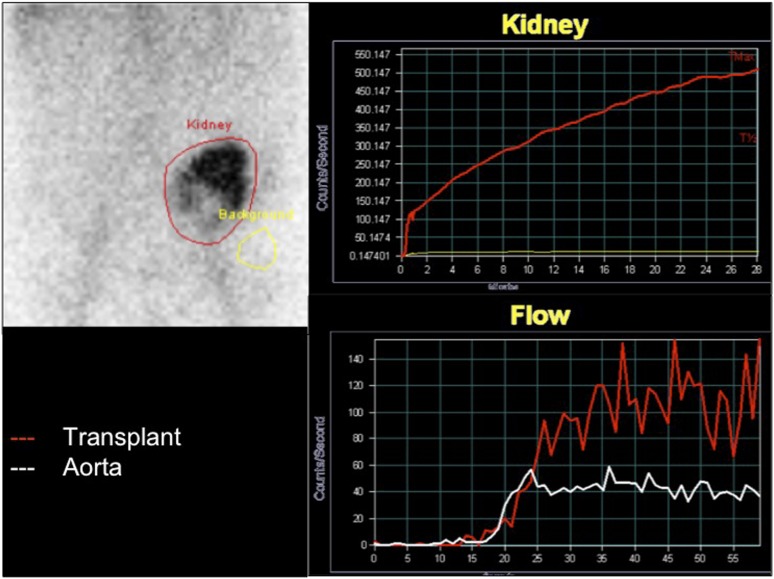
In cases where ultrasonography and CT diagnosis of hydronephrosis is indeterminate or incongruent with the clinical and biochemical findings, scintigraphy with 99mTc mercaptotriglycylglycine (MAG3) may have a role. Besides providing information on renal excretory function, a MAG3 scan is valuable in excluding other causes of diminished renal function, for example, acute tubular necrosis, rejection and drug toxicity. Delayed images obtained 2–4 h after injection of radionuclide can help to not only differentiate between an obstructed collecting system and a dilated but unobstructed system (Figure 3) but may also demonstrate that a pelvic collection noted on ultrasonography or CT is a urinoma secondary to a ureteric leak. Intravenous urography has now been superseded by CT, but may help in selected cases such as suspected obstruction in a patient with a duplex collecting system (Figure 4a). Antegrade pyelography and Whitaker tests are also of historical interest, but may help in the case of suspected non-dilated ureteric obstruction. MR urography can be used in selected cases and offers similar advantages to CT, without the risk of ionizing radiation, but administration of intravenous contrast may be required to obtain suitable images. Additionally, susceptibility artefact from metallic objects such as surgical clips can impede visualization of ureteral segments or create a false representation of ureteral stenosis. In our centre, MR urography is seldom used although it does have a role to play in those with iodinated-contrast allergy, and graft recipients with uroenteric diversions. A heavily weighted T2 sequence can provide intravenous urogram-equivalent quality images superior to non-contrast CT. Serial images help to define peristaltic function. Better soft-tissue differentiation provides the ability to identify intraluminal pathology such as debris, clots or tumour but not calculi.
Figure 3.
Mercaptotriglycylglycine renogram performed 3 months after transplantation demonstrates central and inferior photopaenic defects likely to represent the dilated renal pelvis. The kidney is moderately well perfused and shows selective uptake of tracer. There is persistent retention of tracer within renal parenchyma, which may be secondary to a combination of obstruction and acute tubular necrosis.
Figure 4.
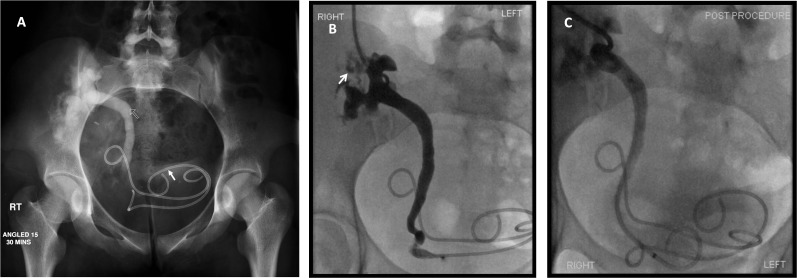
(a) An 18-year-old female live-related renal transplant recipient. Intravenous urogram demonstrates duplex renal transplant with delayed excretion from the upper moiety and a standing column within the ureter (routline arrow). The upper moiety ureteric stent has migrated into the bladder (solid arrow). Normal appearing calyces are seen in relation to the lower moiety, and the ureteric stent to this section of the duplex kidney appears to be in a satisfactory position. There is contrast seen in the bladder, which is likely to represent drainage from the lower moiety. (b) Nephrostogram performed 2 days following nephrostomy placement within the dilated upper moiety shows extravasation from calyces in the upper moiety of the transplant duplex kidney (arrow), in addition to a dilated upper moiety ureter to the level of the distal ureter. (c) 8.5-French, 12-cm ureteric stent placed with the upper moiety ureter in satisfactory position. A temporary covering nephrostomy was also placed.
INTERVENTIONAL RADIOLOGICAL APPROACHES
Once ureteric obstruction is confirmed or strongly suspected, urinary diversion must be undertaken promptly to minimize kidney damage. This is best achieved by percutaneous nephrostomy insertion. Although retrograde stent insertion may be used, this can be technically challenging, as the ureteric anastomosis is routinely performed along the anterolateral bladder wall, making it difficult to access and manipulate with an endoscopic approach.
Once renal function has improved, definitive treatment of the ureteric obstruction is undertaken. Our practice is to carry out percutaneous balloon dilatation if technically feasible, followed by temporary antegrade ureteric stent placement. If the stenosis recurs after stent removal, surgical revision or long-term ureteric stenting is advocated.
Percutaneous nephrostomy insertion poses a risk of bleeding, which should be minimized by ensuring an adequate platelet and haemoglobin count and coagulation time.7,8 We consider a platelet count of >80,000 × 109 per litre, a haemoglobin of >8 g dl−1 and an international normalized ratio of <1.5 to be acceptable. Urine is tested for infection, and use of prophylactic antibiotics is directed by local policy. Imaging should be reviewed for anatomical consideration and procedure planning, to reduce the risk of injury to bowel, peritoneum and adjacent vessels.
Procedure details
Patients are positioned supine.8 Conscious sedation is achieved using titrated sedoanalgesia, whilst continuously monitoring blood pressure, pulse and oxygen saturation. Transplant nephrostomy is usually carried out as a combined ultrasound and fluoroscopic-guided procedure, but CT-guided nephrostomy may also be performed. The ideal calyx for renal access is the most superior and lateral, as this will be well away from the peritoneal reflections and bowel, as well as the renal vessels. At times, the interpolar or lower pole calyx may be more appropriate. In any case, a lateral entry should be ensured with judicious ultrasound examination performed, to avoid bowel injury. The skin, soft tissue and renal capsule in the expected trajectory are then anaesthetized with 1% lidocaine with adrenaline.8 Under ultrasonography guidance, the entry needle (we prefer 18-G/4-French needle/sheath system but any standard nephrostomy entry system can be used) is directed into the centre of the chosen calyx. Once entry into the calyx is established, some urine is aspirated and reserved for bacteriological culture. Iodinated contrast is injected to visualize the renal collecting system using fluoroscopy and to confirm safe calcyceal entry, but overdistension is avoided to minimize the risks of intravasation of possibly infected urine resulting in urosepsis. After correct positioning is confirmed, a guidewire is advanced into the renal pelvis and ideally into the ureter. Following serial dilatation, a nephrostomy catheter is inserted. We favour an 8-French locking pigtail catheter, as this ensures good external urine diversion, even in the presence of purulent urine. Catheter advancement can sometimes be difficult through the tough perinephric fibrosis that develops around the renal transplant. Stiff guidewires (e.g. Amplatz-type guidewire) and dilatation of the track 1-French size larger than the chosen drainage catheter is helpful as is insertion of the catheter using its metal, rather than plastic, trocar. The position of the catheter is confirmed, the catheter is attached to a drainage bag and tethered to the overlying skin with a suture.
Once renal function has improved (typically this will be within 2–3 days), definitive treatment of the ureteric obstruction is undertaken. An initial nephrostogram identifies the site and extent of the ureteral obstruction, along with exclusion of any leak from the collecting system (Figure 4b). A urinary leak from around the stricture site may occur during attempted passage through the stenosis, but this is usually minor. A small leak does not preclude ureteric manipulation, but if large or if the ureter is frankly necrosed, further external drainage is continued to permit the leak to reduce. In our unit, a ureteric stricture, with or without a small ureteric leak, is initially treated with balloon dilatation and stent placement (Figure 4c).
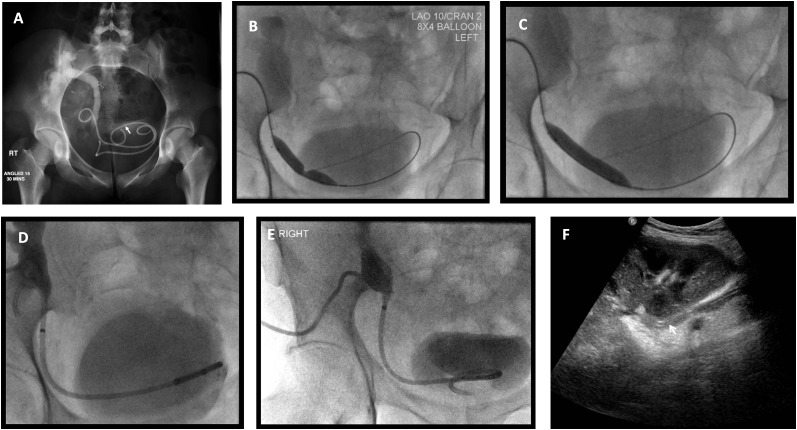
The ureteric stricture is negotiated with a 0.035-inch hydrophilic wire assisted by an appropriately shaped steerable catheter (e.g. hockey tip shape or Cobra shape) (Figure 5a). A tighter stenosis may require a wire of finer gauge and occasionally other catheter shapes. Once crossed, the stricture is dilated. We favour an 8-mm, 4-cm-long high pressure balloon as strictures may be tight (Figure 5b). Elastic recoil is commonly seen with transplant ureteric strictures, and so we maintain balloon dilatation for at least 5 min. However, there are no data to show that either balloon size or prolonged dilatation are important. Nevertheless, in all cases, we aim to abolish the waist (Figure 5c). Occasionally, cutting balloons may be deployed to abolish the waist. Dilatation with a high pressure balloon or cutting balloon rarely results in ureteric rupture and extravasation, but when it does occur, it tends to be minor with no clinical consequences, as the dilated area will be splinted by antegrade ureteric stent insertion. A 6- to 8-French double-J plastic stent is placed and left in situ for 4–6 weeks (Figure 5d). A temporary covering nephrostomy is left in place following ureteric stenting in order to maintain transplant access in case there is poor early stent function. The nephrostomy is clamped the next morning for 4 h. If good bladder urine output continues and stent drainage is confirmed on nephrostography, the nephrostomy catheter is removed (Figure 5e,f). Ultrasonography can confirm satisfactory decompression of the renal collecting system and stent position (Figure 5f). Renal function is carefully monitored over the next 4–6 weeks, and if this remains stable, the ureteric stent is removed under endoscopic guidance.
Figure 5.
Management of the case presented in Figure 2. (a) Nephrostogram following nephrostomy placement indicates tight distal ureteric stricture (arrow). (b) The stricture was crossed with a hydrophilic Terumo™ wire (Terumo Corporation, Somerset, NJ) and dilated with an 8 × 40 -mm balloon. Waisting of the balloon confirms the presence of a stricture. (c) Abolishment of the balloon waist indicating satisfactory dilation of the stricture. (d) An 8.5-French antegrade double-J stent is sited and a locked covering nephrostomy left in situ. (e) Following contrast administration via the indwelling nephrostomy, there is prompt drainage into the urinary bladder. The nephrostomy was consequently removed under fluorosocopy over a standard wire. (f) Ultrasound performed 7 days following nephrostomy removal demonstrated satisfactory appearance of the transplant kidney with no residual hydronephrosis and ureteric stent in situ (arrow).
Continuing surveillance of renal function after stent removal is important as a rising serum creatinine level and/or diminishing urine output may indicate recurrent ureteric stenosis, which warrants surgical ureteric reconstruction. If this is not technically possible, for example owing to a long length of stricture or poor patient health, an antegrade ureteric stent is reinserted and a policy of long-term ureteric stenting instituted, with plastic or metal stents.
Surgical management
Surgical intervention is indicated if minimally invasive procedures fail. Options include ureteroneocystostomy with excision of the stenotic segment and reimplantation, ureteroureterostomy using the recipient ipsilateral ureter (pyeloureterostomy between the donor renal pelvis and recipient ureter), or utilization of a Boari flap (Figure 6). This involves bladder flap substitution of the distal ureter.
Figure 6.
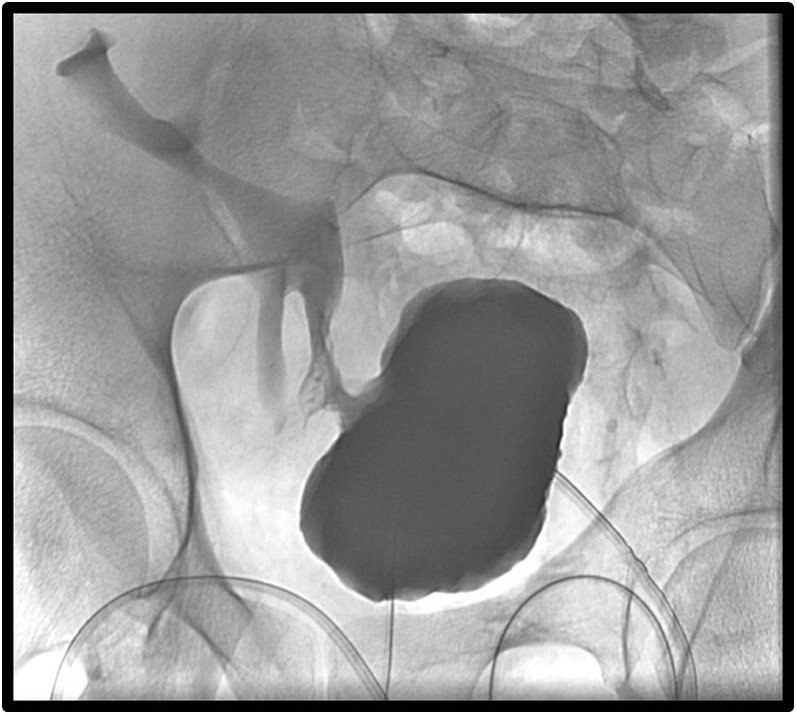
Tubogram acquisition performed via indwelling Salem catheter placed intraoperatively following Boari flap reconstruction for distal transplant ureteric stricture. It shows prompt opacification of the Boari flap and collecting system of the transplant kidney with no stricture or extraluminal leak of contrast. Where excision of a part of the ureter is necessary, a Boari flap of the bladder can be created to bridge the gap for an anastomosis either to transplant ureter or to transplant renal pelvis as in this case.
Outcome of percutaneous treatment of renal transplant ureteric stenosis
National and international practice guidelines suggest that the technical success rate for transplant nephrostomy should be >98%.9 The major complication rates after transplant nephrostomy are expected to be in the same range as those for native nephrostomy. Regarding complications related to ureteric intervention, Fontaine et al10 reported no immediate complications employing this percutaneous intervention in a large cohort. The reported success rate of percutaneous ureteric dilatation and stenting is between 62% and 86%.11,12 Existing data suggest that outcome is better in those who develop stenosis within 3 months of transplantation.10 Poorer long-term outcome in those with late-onset obstruction (>3 months after transplantation) may be owing to established or irreversible renal damage. Open surgical revision for ureteral stenosis carries potential risk to the graft and the patient and is reserved for strictures not amenable to stenting. Indeed, a European group, where percutaneous techniques are first line, found that in a total of 566 transplant recipients, only 5.7% required surgical revision for leakage or stenosis.13
CONCLUSIONS
Obstruction of the renal transplant should be promptly diagnosed and treated. Percutaneous nephrostomy is a safe and effective method for immediate relief of ureteric obstruction. Balloon dilatation and ureteric stent insertion has a good technical success rate and outcome.
REFERENCES
- 1.Berger PM, Diamond JR. Ureteral obstruction as a complication of renal transplantation: a review. J Nephrol 1998; 11: 20–3. [PubMed] [Google Scholar]
- 2.Sandhu C, Patel U. Renal transplantation dysfunction: the role of interventional radiology. Clin Radiol 2002; 57: 772–83. [PubMed] [Google Scholar]
- 3.Duty BD, Conlin MJ, Fuchs EF, Barry JM. The current role of endourologic management of renal transplantation complications. Adv Urol 2013; 2013: 246520. doi: 10.1155/2013/246520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonardou P, Gioldasi S, Pappas P. Percutaneous management of ureteral stenosis of transplanted kidney: technical and clinical aspects. Urol Int 2011; 87: 375–9. doi: 10.1159/000331897 [DOI] [PubMed] [Google Scholar]
- 5.Karam G, Hetet JF, Maillet F, Rigaud J, Hourmant M, Soulillou JP, et al. Late ureteral stenosis following renal transplantation: risk factors and impact on patient and graft survival. Am J Transplant 2006; 6: 352–6. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant 2004; 4: 905–13. [DOI] [PubMed] [Google Scholar]
- 7.Akbar SA, Jafri SZ, Amendola MA, Madrazo BL, Salem R, Bis KG. Complications of renal transplantation. Radiographics 2005; 25: 1335–56. [DOI] [PubMed] [Google Scholar]
- 8.Patel U, Hussain FF. Percutaneous nephrostomy of nondilated renal collecting systems with fluoroscopic guidance: technique and results. Radiology 2004; 233: 226–33. [DOI] [PubMed] [Google Scholar]
- 9.Ramchandani P, Cardella JF, Grassi CJ, Roberts AC, Sacks D, Schwartzberg MS, et al. Quality improvement guidelines for percutaneous nephrostomy. J Vasc Interv Radiol 2003; 14: S277–81. [PubMed] [Google Scholar]
- 10.Fontaine AB, Nijjar A, Rangaraj R. Update on the use of percutaneous nephrostomy/balloon dilation for the treatment of renal transplant leak/obstruction. J Vasc Interv Radiol 1997; 8: 649–53. [DOI] [PubMed] [Google Scholar]
- 11.Lojanapiwat B, Mital D, Fallon L, Koolpe H, Raja R, Badosa F, et al. Management of ureteral stenosis after renal transplantation. J Am Coll Surg 1994; 179: 21–4. [PubMed] [Google Scholar]
- 12.Bachar GN, Mor E, Bartal G, Atar E, Goldberg N, Belenky A. Percutaneous balloon dilatation for the treatment of early and late ureteral strictures after renal transplantation: long-term follow-up. Cardiovasc Intervent Radiol 2004; 27: 335–8. [DOI] [PubMed] [Google Scholar]
- 13.Slagt IK, Ijzermans JN, Visser LJ, Weimar W, Roodnat JI, Terkivatan T. Independent risk factors for urological complications after deceased donor kidney transplantation. PLoS One 2014; 9: e91211. doi: 10.1371/journal.pone.0091211 [DOI] [PMC free article] [PubMed] [Google Scholar]