The mortality from lung cancer exceeds that from breast, colorectal and pancreatic cancer combined. This is because three-quarters of patients present with late-stage disease when treatment is palliative and survival is short. If detected early, lung cancer can be cured, so screening would seem to be an important intervention. Until the publication of the National Lung Screening Trial (NLST),1 there was no evidence to support the implementation of screening with low-dose CT (LDCT). This publication has sparked a different approach to the subject from asking whether it works to what we still need to know to implement with the least harm and cost. These remaining issues will be reviewed.
DOES LUNG CANCER MERIT A SCREENING PROGRAMME?
Criteria for effective screening programmes have been defined in the USA and UK.2,3 The UK Screening Committee has 23 criteria for an effective national screening programme.3 The first is that the disease should be an important health problem—the annual mortality from lung cancer is 35,000 in the UK and almost 160,000 in the USA, more than that for breast, colorectal and pancreatic cancer combined.4,5 Other criteria sensibly include that the problem exists despite the delivery of optimal prevention, treatment and the consideration of new treatments. Smoking cessation interventions have had a major impact on mortality from lung cancer,6 but despite continued efforts, the rate of reduction of smoking is slowing and there remain many ex-smokers at risk. Three-quarters of patients present with late-stage disease and the treatment at this stage has had little impact on mortality despite significant advances in targeted therapy. There is some evidence that radical treatment rates are increasing, but at only a modest rate and mainly in the older age group.7 Other important criteria are that there should be well-defined risk factors for the disease and a validated, sensitive and acceptable screening test that can be applied effectively to reduce mortality. Until recently, the latter criterion had not been met. Thus, lung cancer is a health problem that clearly meets these disease-specific criteria.
THE EVIDENCE FOR LUNG CANCER SCREENING
Many of the early trials of screening with chest radiographs, sputum cytology and later CT were not designed to minimize the now well-established biases operating in screening trials that result in longer survival but no reduction in mortality.8 Overdiagnosis is a bias that results from cancers being diagnosed that would never limit life expectancy, but once diagnosed by screening improves the overall results of the screening arm in a trial against no screening or a less sensitive screening method. Lead time bias is where, as a result of screening, cancer is diagnosed earlier, and this results in a longer measured survival, even though there is no effect on the eventual date of death. Lag-time bias is the tendency of more indolent tumours to be detected by screening because of their long pre-symptomatic phase, whilst the very aggressive ones tend to present between the screens, so present equally in the screened and control arms. What was needed was a well-designed, adequately powered randomized trial.
The US-based NLST1 randomized 53,454 people aged between 55 and 74 years, who had smoked within 15 years and accumulated a minimum of 30 pack years, to 3 annual screens with either LDCT or chest radiography. The trial recruited between 2002 and 2004, and in October 2010 it was stopped 1 year earlier than planned as the pre-specified lung cancer mortality reduction of 20% had been reached in the LDCT arm. The trial also showed a reduction in all-cause mortality of 6.7%. The number needed to screen (NNS) to prevent 1 lung cancer death was 320 for those who completed at least 1 screen. In the breast mammography trials, for females aged 50–59 years, the NNS was 1139 after 11–20 years follow-up; the NNS for flexible sigmoidoscopy was 817 to prevent 1 colon cancer death.9,10 Other much smaller trials of moderate or low quality have reported on early mortality but none has the statistical power to show a difference, and when combined with the results of NLST in a meta-analysis, there was still a 19% reduction in lung cancer-specific mortality.11,12 Thus, the criteria for a validated and sensitive screening test had finally been met.
During 2011 and 2012, several US professional organizations made recommendations for implementation of screening in people who would have met the NLST entry criteria, with some extensions. Finally, the US Preventive Services Task Force (USPST), following a commissioned independent analysis of the evidence, recommended that lung cancer screening should be offered according to NLST entry criteria but with an extension of the upper age limit to 80 years.11 More recently, the Centers for Medicare and Medicaid national coverage determination panel were not convinced that the benefits of CT screening outweighed the harms and rated the intervention at 2.2 out of 5.0. This was thought especially true in the older age group where there is a higher false-positive rate. However, Pinsky et al13 have shown that people aged 65–75 years benefit as much as those aged under 65 years as their higher prevalence of lung cancer offsets the harms from the greater number of false positives.
Outside the USA, there have been international consensus statements that have made recommendations for further research and analysis of the existing randomized trials in Europe.14,15 The Dutch–Belgium NELSON trial16 is due to report on mortality in 2015–16, and results of all of the European trials may be pooled around the same time. These data will answer important questions about how better nodule management and more sophisticated radiology influences the efficacy of screening. There is considerable debate about whether the results of NELSON should be awaited before screening programmes are implemented.
THE REMAINING ISSUES AND POTENTIAL BARRIERS TO IMPLEMENTATION
So what are the remaining questions and potential barriers to implementation of LDCT screening in the UK? The answer relates to important remaining questions about selection, recruitment, the level of harm, optimal clinical pathways and cost effectiveness, all of which are criteria set by the UK National Screening Committee (Table 1). The recommendations made by the USPST may have gone too far, not by extending screening to the older age group, but by including younger people without a more sophisticated estimate of the risk of lung cancer. More recent publications have suggested that by better selection using a risk prediction model, there might have been more lives saved with reduction in the NNS to 270.17 If screening were to have been restricted to the subjects with a >1.25% risk of lung cancer death over 5 years, this falls further to 166.18 Better selection methods are available and validated, and their use may go some way to placate the recently expressed reservations about CT screening from Medicare and Medicaid.
Table 1.
Barriers to lung cancer screening implementation and proposed solutions
| Key problem | What is the barrier to implementation? | What is the solution? |
|---|---|---|
| 1. NNS | • Higher false-positive rates and decreased cost effectiveness if the inclusion criteria are too broad | • Use of risk prediction models rather than just age and smoking cut-offs to better guide who should be screened (UKLS) |
| 2. Radiation exposure | • Estimates suggest 1 radiation-induced lung cancer for every 22 lung cancer deaths prevented • Positron emission tomography-CT in the investigation of false-positive lesions increases radiation dose |
• Low-dose CT reduces radiation dose to around one-fifth of conventional CT • Clear selection criteria for screening and robust nodule management guidelines will reduce false positives |
| 3. False-positive scans | • In NLST, there were around 25 benign lesions for every cancer detected, with psychological and possible physical harm from further investigations | • Volumetric nodule assessment employed by NELSON and UKLS to better assess nodules and reduce false positives • Risk assessment models to guide who should be screened |
| 4. Overdiagnosis | • Estimates suggest 10–20% overdiagnosis with screening again with associated physical and psychological harm | • Clear nodule guidelines, with a cautious approach to subsolid nodules (more likely to represent more indolent tumours) |
| 5. Smoking cessation | • Concern regarding false reassurance with screening leading to continued/new uptake of smoking | • Combination of screening with smoking cessation programmes • Somewhat reassuring smoking cessation results from NLST (but substantially higher smoking cessation in both arms than in background rates) |
| 6. Cost effectiveness | • Some models based on NLST are too expensive | • Careful and clear guidance regarding management of positive/indeterminate CT results • Risk profiling of the screened population to reduce the NNS • Multiple health interventions including smoking cessation |
| 7. Hard-to-access groups | • Work suggests that those at highest risk of developing lung cancer are least likely to participate in/complete screening programmes, with consequent cost effectiveness implications | • Research is ongoing to determine how best to engage and retain these high-risk and hard-to-access groups |
NLST, National Lung Screening Trial; NNS, number needed to screen; UKLS, United Kingdom Lung Screen.
One key remaining issue is recruitment of people into screening programmes. Lung cancer is much more common in less economically advantaged groups, who also represent a hard-to-access group. NLST recruited patients who were better educated and more affluent than the US census average1 and United Kingdom Lung Screen (UKLS) noted that the lower socioeconomic groups were less likely to participate despite being at higher overall risk.19 The answer to this issue is not known; it is a topic of ongoing research projects and will be an important factor to monitor during implementation.
Physical harm may result from radiation and further investigation of abnormal findings, and psychological harm may result from the resulting anxiety. LDCT reduces the radiation dose to approximately one-fifth of a typical thoracic CT, and this equates to less than half a person's annual background radiation dose. Based on modelling, the USPST estimated that annual CT screening for lung cancer would cause 1 radiation-induced lung cancer death for every 22 prevented lung cancer deaths.11 Further improvements in technology may improve this figure.
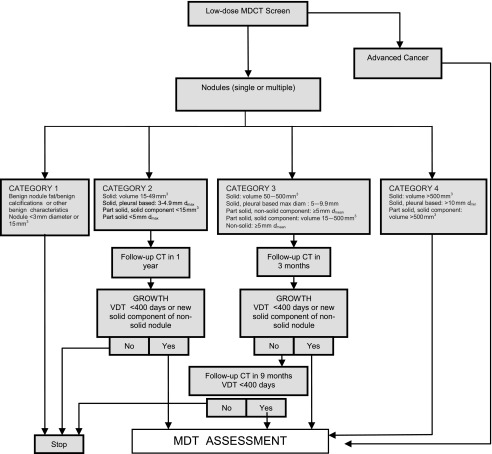
CT detects many benign nodules and masses (about 25 for every cancer detected). In the NLST, 59/26,722 (0.2%) of participants underwent a CT-guided lung biopsy for a benign lesion and 7 (0.03%) had a major complication.1 Clinical management pathways were not pre-specified in NLST, and they found that, where data were available, 2.2% of participants underwent positron emission tomography thus receiving a much higher radiation dose. The Dutch–Belgian NELSON trial and the UKLS pilot trial both employ LDCT follow-up by using volume measurements rather than diameter to detect growth.16,20 This reduces the number of false-positive screens and defines more accurately the need for invasive procedures. Optimal screening follow-up pathways are becoming clear and will limit false-positive tests and the harm from further investigation. The nodule management algorithm for UKLS is shown in Figure 1.
Figure 1.
United Kingdom Lung Screen nodule care pathway management protocol. diam, diameter; dmax, maximum diameter; dmean, mean diameter; dmin, minimum diameter; max, maximum; MDCT, multidetector CT; MDT, multidisciplinary team; VDT, volume doubling time.
People who are overdiagnosed do not benefit from early diagnosis but are subject to the same harm as others from investigations, treatment and the psychological impact of being told they have cancer. They may, however, benefit from health interventions that are provided with screening such as smoking cessation advice. Estimates based on the excess observed in NLST and in lead time estimation from a variety of sources suggest that 10–20% of screen-detected cancers are overdiagnosed (a maximum 18.5% in NLST).21,22 Thus, the number of cases overdiagnosed is similar to the number of lives saved; this is similar to estimates for breast cancer screening, where recent reviews suggest between 0.5 and 3.0 overdiagnosed cases per life saved.23,24 However, overdiagnosis is far more likely where the final diagnosis was bronchoalveolar cell carcinoma (now lepidic predominant pattern adenocarcinoma). Thus, a cautious approach to subsolid nodules found during screening may limit the harm from overdiagnosis.
Psychological harm has been shown to be minimal in screening trials with a transient increase in anxiety and distress in subjects with positive or indeterminate findings, returning to baseline after the second screen.25 Distress and fear of cancer decreased in subjects with negative results compared with those at baseline. Whether people might be falsely reassured and continue or even start smoking is not known; smoking cessation rates were 14.5% in the LDCT arm compared with 19.1% in the control arm; both of these were higher than the 6–7% background rate.26
The cost effectiveness of a programme will be strongly influenced by the frequency of screening, duration of screening programme, risk profile of the screened population, uptake of screening in hard-to-reach groups and smoking cessation. The range of reported incremental cost effectiveness ratios (ICERs) remains considerable. A US simulation of the annual screening of ever-smokers using national epidemiological and Mayo Clinic CT trial data estimated the ICERs to be $110,000–$160,000 per quality-adjusted life year (QALY) gained. A US stage-shift model using the Early Lung Cancer Action Program (ELCAP) protocols and outcomes data, Medicare tariffs and national survival rates by stage-produced ICER estimates <$19,000.27 The model was re-estimated for annual screening and yielded baseline estimates of $28,000 or $47,000 depending on whether the model's cancer stage predictions derived from the ELCAP- or the NLST-reported stage shift;28 costs fell further with smoking cessation to $16,000 or $23,000, respectively. The lowest ICER estimated is around $1464 per QALY gained, reported for Israel.29
A POTENTIAL WAY FORWARD
Since the publication of NLST, there has been much international discussion about the issues outlined here and recommendations made for further work to be performed to support implementation.14,15 The USA has gone the furthest in recommending national implementation of screening, and work is ongoing to answer important remaining questions. The solution will vary according to the individual country's healthcare system and may increase the work of radiologists and radiographers. The efficient use of nodule management algorithms and trained non-radiologist readers in combination with computer-aided detection software may mitigate this.12 Private healthcare providers are offering CT screening in many countries and the concern is that this is not regulated. The importance of adherence to standards that serve to minimize harm and maximize benefit has been emphasized.30 The Committee on Medical Aspects of Radiation Exposure will publish a report in 2014 further emphasizing the need for an expert approach in the independent sector.31 However, the most important concern with private provision of screening is that it does nothing for the majority of those at risk, as lung cancer is more common in the less advantaged. Those of us who care for patients with lung cancer want screening to be introduced as soon as possible for all those at risk, but it has to be on the basis that it is cost effective because of the high total cost. To ensure effective implementation, it may be best to start with a programme that targets those most at risk and employs pathways that minimize risk of harm, including those due to overdiagnosis, and then expand as the essential unanswered issues are clarified. A biennial or annual screen from the age of 60 years in those with a risk of lung cancer in excess of 1% per annum, with clear protocols for the management of abnormal findings would fit, with ongoing research into the best methods for selection and recruitment.12
REFERENCES
- 1.National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Available from: http://www.cancer.gov/cancertopics/pdq/screening/overview/HealthProfessional#Section_10
- 3.UK National Screening Committee. Programme appraisal criteria. Available from: http://www.screening.nhs.uk/criteria
- 4.Cancer Research UK. Cancer mortality in the UK in 2012. Available from: http://publications.cancerresearchuk.org/downloads/Product/CS_REPORT_MORTALITY.pdf
- 5.America Cancer Society. Cancer facts and figures 2014. Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
- 6.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000; 321: 323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riaz SP, Linklater KM, Page R, Peake MD, Møller H, Lüchtenborg M. Recent trends in resection rates among non-small cell lung cancer patients in England. Thorax 2012; 67: 811–14. doi: 10.1136/thoraxjnl-2012-201768 [DOI] [PubMed] [Google Scholar]
- 8.Black WC. Computed tomography screening for lung cancer: review of screening principles and update on current status. Cancer 2007; 110: 2370–84. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 137: 347–60. [DOI] [PubMed] [Google Scholar]
- 10.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012; 366: 2345–57. doi: 10.1056/NEJMoa1114635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013; 159: 411–20. doi: 10.7326/0003-4819-159-6-201309170-00690 [DOI] [PubMed] [Google Scholar]
- 12.Field JK, Hansell DM, Duffy SW, Baldwin DR. CT screening for lung cancer: countdown to implementation. Lancet Oncol 2013; 14: e591–600. doi: 10.1016/S1470-2045(13)70293-6 [DOI] [PubMed] [Google Scholar]
- 13.Pinsky PF, Gierada DS, Hocking W, Patz EF, Jr, Kramer BS. National Lung Screening Trial findings by age: medicare-eligible versus under-65 population. Ann Intern Med Sep 2014. doi: 10.7326/M14-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field JK, Aberle DR, Altorki N, Baldwin DR, Dresler C, Duffy SW, et al. The International Association Study Lung Cancer (IASLC) Strategic Screening Advisory Committee (SSAC) response to the USPSTF recommendations. J Thorac Oncol 2014; 9: 141–3. doi: 10.1097/JTO.0000000000000060 [DOI] [PubMed] [Google Scholar]
- 15.Field JK, Smith RA, Aberle DR, Oudkerk M, Baldwin DR, Yankelevitz D, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol 2012; 7: 10–19. doi: 10.1097/JTO.0b013e31823c58ab [DOI] [PubMed] [Google Scholar]
- 16.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009; 361: 2221–9. doi: 10.1056/NEJMoa0906085 [DOI] [PubMed] [Google Scholar]
- 17.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013; 368: 728–36. doi: 10.1056/NEJMoa1211776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013; 369: 245–54. doi: 10.1056/NEJMoa1301851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McRonald FE, Yadegarfar G, Baldwin DR, Devaraj A, Brain KE, Eisen T, et al. The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res (Phila) 2014; 7: 362–71. doi: 10.1158/1940-6207 [DOI] [PubMed] [Google Scholar]
- 20.Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax 2011; 66: 308–13. doi: 10.1136/thx.2010.152066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Independent UKPoBCS. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380: 1778–86. doi: 10.1016/S0140-6736(12)61611-0 [DOI] [PubMed] [Google Scholar]
- 22.Duffy SW, Field JK, Allgood PC, Seigneurin A. Translation of research results to simple estimates of the likely effect of a lung cancer screening programme in the United Kingdom. Br J Cancer 2014; 110: 1834–40. doi: 10.1038/bjc.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patz EF, Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174: 269–74. doi: 10.1001/jamainternmed.2013.12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puliti D, Duffy SW, Miccinesi G, de Koning H, Lynge E, Zappa M, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen 2012; 19(Suppl. 1): 42–56. [DOI] [PubMed] [Google Scholar]
- 25.van den Bergh KA, Essink-Bot ML, Borsboom GJ, Scholten ET, van Klaveren RJ, de Koning HJ. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J 2011; 38: 154–61. doi: 10.1183/09031936.00123410 [DOI] [PubMed] [Google Scholar]
- 26.van der Aalst CM, van den Bergh KA, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch–Belgian randomised controlled lung cancer screening trial. Thorax 2010; 65: 600–5. doi: 10.1136/thx.2009.133751 [DOI] [PubMed] [Google Scholar]
- 27.Pyenson BS, Sander MS, Jiang Y, Kahn H, Mulshine JL. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff (Millwood) 2012; 31: 770–9. doi: 10.1377/hlthaff.2011.0814 [DOI] [PubMed] [Google Scholar]
- 28.Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost–utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One 2013; 8: e71379. doi: 10.1371/journal.pone.0071379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shmueli A, Fraifeld S, Peretz T, Gutfeld O, Gips M, Sosna J, et al. Cost-effectiveness of baseline low-dose computed tomography screening for lung cancer: the Israeli experience. Value Health 2013; 16: 922–31. doi: 10.1016/j.jval.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Field JK, Baldwin D, Brain K, Devaraj A, Eisen T, Duffy SW, et al. CT screening for lung cancer in the UK: position statement by UKLS investigators following the NLST report. Thorax 2011; 66: 736–7. doi: 10.1136/thoraxjnl-2011-200351 [DOI] [PubMed] [Google Scholar]
- 31.Department of Heath. Justification of computed tomography (CT) for individual health assessment. Expert Working Party Report. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/326572/IHA_-_June_Report.pdf