Abstract
Objective:
Dysphagia remains a side effect influencing the quality of life of patients with head and neck cancer (HNC) after radiotherapy. We evaluated the relationship between planned dose involvement and acute and late dysphagia in patients with HNC treated with intensity-modulated radiation therapy (IMRT), after a recontouring of constrictor muscles (PCs) and the cricopharyngeal muscle (CM).
Methods:
Between December 2011 and December 2013, 56 patients with histologically proven HNC were treated with IMRT or volumetric-modulated arc therapy. The PCs and CM were recontoured. Correlations between acute and late toxicity and dosimetric parameters were evaluated. End points were analysed using univariate logistic regression.
Results:
An increasing risk to develop acute dysphagia was observed when constraints to the middle PCs were not respected [mean dose (Dmean) ≥50 Gy, maximum dose (Dmax) >60 Gy, V50 >70% with a p = 0.05]. The superior PC was not correlated with acute toxicity but only with late dysphagia. The inferior PC was not correlated with dysphagia; for the CM only, Dmax >60 Gy was correlated with acute dysphagia ≥ grade 2.
Conclusion:
According to our analysis, the superior PC has a major role, being correlated with dysphagia at 3 and 6 months after treatments; the middle PC maintains this correlation only at 3 months from the beginning of radiotherapy, but it does not have influence on late dysphagia. The inferior PC and CM have a minimum impact on swallowing symptoms.
Advances in knowledge:
We used recent guidelines to define dose constraints of the PCs and CM. Two results emerge in the present analysis: the superior PC influences late dysphagia, while the middle PC influences acute dysphagia.
In the past decade, substantial progress has been made in the treatment of head and neck cancer (HNC). Several reports show that radiotherapy (RT) with concomitant chemotherapy or altered fractionation schedules improve tumour control and survival rate.1,2
However, xerostomia and dysphagia often remain relevant side effects for patients with HNC, compromising their quality of life (QoL), as a consequence of radiation damage to the parotid glands and to the organ at risk (OAR) involved in the swallowing process (SWOARs).3
Intensity-modulated radiation therapy (IMRT) and rotational intensity-modulated techniques, including volumetric-modulated arc therapy (VMAT), allow for a better dose conformation to target structures while reducing the dose.4–8 In comparison with three-dimensional-conformal radiation therapy, several studies have shown that IMRT in HNC treatment reduces overall adverse effects such as xerostomia and dysphagia and thus improves QoL, even when chemotherapy is added.9–13
Regarding tolerance of the parotid glands, several studies have suggested significant recovery when the mean dose is inferior to 26 Gy. Open questions remain for SWOARs, especially with reference to the delineation modalities of the involved structures to the volumes or the dose constraints to be applied.14–18 More authors hypothesized that sparing a portion of the constrictor muscles (PCs), not involved by tumour and not at risk of subclinical disease, might reduce dysphagia.19–21 These studies obtained different results, maybe, owing to a number of methodological issues and to the ambiguous contouring of the PCs. For this purpose, Christianen et al22 recently defined guidelines for SWOARs contouring.
Based on these findings, the aim of this retrospective analysis is to evaluate potential relationships between planned dose–volume parameters and observed incidence of acute and late dysphagia in patients with HNC treated with IMRT or VMAT, after a recontouring of the PCs according to these recently published guidelines.
METHOD AND MATERIALS
Patients
Between December 2011 and December 2013, 56 patients (43 males and 13 females), with a median age of 64 years (range, 24–86 years) and with histologically proven HNC, received radiation treatment with IMRT or VMAT in Sacro Cuore-Don Calabria Hospital, Negrar-Verona, Italy. >10% of the patients had non-squamous histology (carcinoma undifferentiated, lymphoepithelial, sarcomatoid, mucoepidermoid). Patients' characteristics are shown in Table 1.
Table 1.
Patient baseline characteristics and demographics (n = 56)
| Factors | Description |
|---|---|
| Gender | |
| Male | 77% (n = 43) |
| Female | 23% (n = 13) |
| Age | |
| Age (years) | Median, 64; range 24–86 |
| Smoker | |
| Yes | 77% (n = 43) |
| No | 23% (n = 13) |
| Diabetic | |
| Yes | 5% (n = 3) |
| No | 95% (n = 53) |
| Primary site | |
| Rinopharynx | 9% (n = 5) |
| Oropharynx | 30% (n = 17) |
| Oral cavity | 18% (n = 10) |
| Larinx sovraglottic | 9% (n = 5) |
| Larinx glottic | 30% (n = 17) |
| Salivary glands | 4% (n = 2) |
| Histology | |
| Epidemoidal | 88% (n = 49) |
| Others | 13% (n = 7) |
| Grading | |
| Grade 1 | 27% (n = 15) |
| Grade 2 | 46% (n = 26) |
| Grade 3 | 27% (n = 15) |
| Stage | |
| I | 20% (n = 11) |
| II | 13% (n = 7) |
| III | 18% (n = 10) |
| IVA | 46% (n = 26) |
| IVB | 4% (n = 2) |
| Chemotherapy | |
| Cisplatino weekly | 20% (n = 11) |
| Cisplatino 3-weekly | 32% (n = 18) |
| Induction | 2% (n = 1) |
| None | 46% (n = 26) |
| Radiotherapy | |
| Radical | 71% (n = 40) |
| Adjuvant | 29% (n = 16) |
Each patient underwent a pre-treatment evaluation that included a complete history and physical examination, CT and/or MRI scans and/or fluorine-18 fludeoxyglucose–positron emission tomography (18F-FDG-PET) of the head and neck region, and direct flexible fiberoptic endoscopic examination.
Planning
Immobilization of each patient for simulation and during treatment was achieved with a thermoplastic head and shoulder mask (Civco, Orange City, IA). A treatment-planning CT with 3-mm slice thickness and intravenous contrast was acquired in the treatment position and matched with 18F-FDG-PET or MRI in the treatment position to better define the biological target volume and/or clinical target volume (CTV) and OARs.
According to the Radiation Therapy Oncology Group (RTOG) guidelines,23 the following OARs were contoured for the pre-treatment planning: the spinal cord, brain stem, ipsilateral and contralateral parotid glands, oral cavity and larynx (for non-laryngeal cancers). Whenever close to the planning target volume (PTV), the eyes, optic nerves, and optic chiasm were delineated.
In radical setting, gross tumour volume (GTV), high-risk subclinical disease (CTV1) and low-risk subclinical disease (CTV2) were defined on CT scan after simulation procedure. PTV1, PTV2 and PTV3 were generated with an isotropic expansion of 5 mm from CTV1, CTV2 and CTV3, respectively. These volumes were irradiated to a total dose of 70 Gy (33–35 fractions), 59.94–63.00 Gy (33–35 fractions) and 54.45–58.1 Gy (33–35 fractions), respectively, with daily fractions of 2.12/2.00 Gy, 1.80/1.81 Gy and 1.65/1.66 Gy with simultaneous-integrated boost.
In the post-operative setting, two volumes of interest were identified: CTV1 including the tumour bed (primary and involved nodes) and CTV2 including elective lymphatic areas. PTV1 and PTV2 were generated with an isotropic expansion of 5 mm from CTV1 and CTV2, respectively. These volumes were irradiated to a total dose of 60 Gy in 30 fractions and 54 Gy in 30 fractions, respectively, with daily fractions of 2 Gy and 1.8 Gy, respectively.
The dose was prescribed to cover 95% of the PTV. Target dose homogeneity was obtained by maintaining V107% dose prescription (Dp) <3% and a maximum dose (Dmax) <110% Dp.
Planning objectives required PTV coverage of 95–107%. Concerning OARs, they were set as follows: the spinal cord: Dmax 0.1 cc <46 Gy; brain stem: Dmax 0.1 cc <54 Gy; parotid glands: V30 <45%; mean dose (Dmean) <26 Gy; larynx, V40 <50%; and oral cavity (not involved), V40 <50%. All dose distributions were computed with the anisotropic analytical algorithm v. 10.0.28 implemented in the Eclipse™ (Varian, Palo Alto, CA) treatment planning system with a calculation grid resolution of 2.5 mm.
All plans were performed with 9-field sliding window IMRT or 4-arc VMAT (Rapid Arc; Varian Medical System, Palo Alto, CA) and nominal energy of 6 MV.
Treatment procedure
Before each daily fraction, the patients were submitted to image-guided radiotherapy (IGRT) procedure by means of a daily tube potential–cone beam CT (CBCT) to check and correct in real-time set-up errors and to follow anatomical changes of the treated region.
CBCT low-dose head model (80 kVp, 0.4 mAs) was used to generate images; clockwise and anticlockwise 180° gantry rotations were used alternatively to reduce dose to patients.24
All corrections carried out after matching between CBCT and planning CT were recorded and collected.
Chemotherapy
Cisplatinum 100 mg mq−1 was added every 21 days during RT for patients with performance status (PS) Eastern Cooperative Oncology Group (ECOG) = 0–1, age less than 70 years, disease T/N+ or T3–T4/N0; cisplatinum 30 mg mq−1 was added weekly if PS = 2, age less than 70 years, disease T/N+ or T3–T4/N0; induction TCF (docetaxel, cisplatin, 5-floruracile) if PS = 0, age less than 65 years, disease T/N3; and no chemotherapy if PS ECOG >2, disease T1–T2/N0, age over 70 years.25
Toxicity evaluation and follow-up
At baseline, no cases of dysphagia and xerostomia were recorded. Toxicity was evaluated weekly during radiation treatment, and periodically after the end of the treatment: 1 month after RT clinical evaluation, then regular visits every 3 months for the first 2 years.
Toxicities occurring within 3 months from the beginning of radiotherapy were defined as acute, and those occurring after 3 months as late toxicity. Patients were assessed for toxicities by the European Organization for Research and Treatment for Cancer/RTOG radiation morbidity scoring criteria. Clinical data were collected and evaluated for statistical evaluation.
Re-contouring
The compliance to radiation treatment was 100%; no patient interrupted or discontinued the planned IMRT or VMAT schedule; and, for the end point of the study, all clinical and dosimetrical data were retrospectively evaluated.
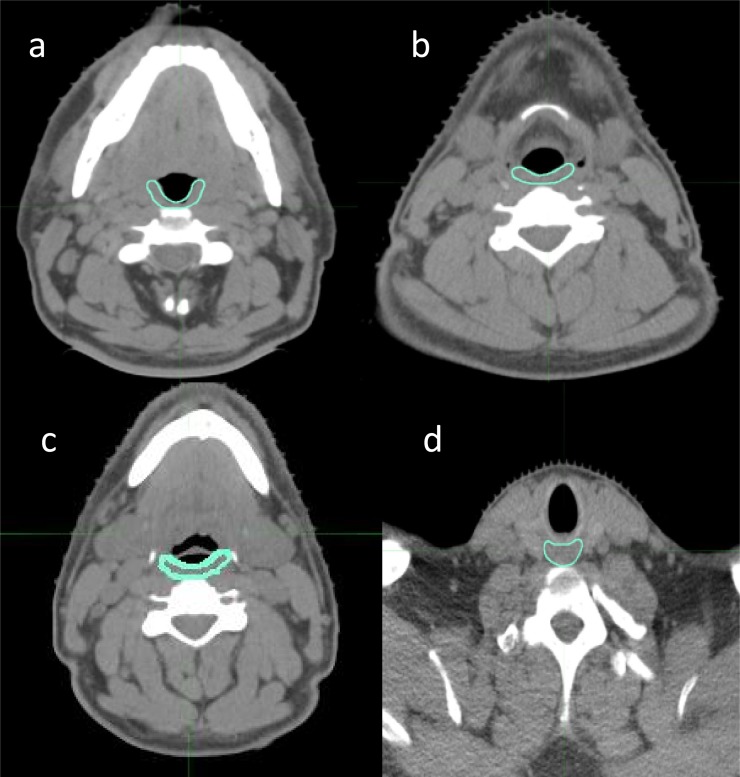
On planning CT scan, the PCs were retrospectively contoured according to Christianen et al22 guidelines by a single observer and subsequently reviewed by another radiation oncologist as shown in Figure 1. The PCs and the cricopharyngeal muscle (CM) were indicated as shown in Table 2.
Figure 1.
(a–d) Definition of the constrictor and cricopharyngeal muscles in axial CT slice.
Table 2.
Anatomic borders of constrictors and cricopharyngeal muscles
| Muscle | Cranial | Caudal | Anterior | Posterior | Lateral | Medial |
|---|---|---|---|---|---|---|
| Superior PCM | Caudal tip of the pterygoid plates (hamulus) | Lower edge of C2 | Hamulus of pterygoid plate; mandibula; base of tongue; pharyngeal lumen | Prevertebral muscle | Medial pterygoid muscle | Pharyngeal lumen |
| Middle PCM | Upper edge of C3 | Lower edge of hyoid bone | Base of tongue; hyoid bone | Prevertebral muscle | Greater horn of hyoid bone | Pharyngeal lumen |
| Inferior PCM | First slice caudal to the lower edge of hyoid bone | Lower edge of the arythenoid cartilages | Soft tissue of supraglottic/glottic larynx | Prevertebral muscle | Superior horn of thyroid cartilage | Pharyngeal lumen |
| Cricopharyngeal muscle | First slice caudal to the arytenoid cartilages | Lower edge of the cricoid cartilages | Posterior edge of cricoid cartilage | Prevertebral muscle | Thyroid cartilage, fatty tissue, thyroid gland |
C2, second cervical vertebra; C3, third cervical vertebra; PCM, pharyngeal constrictor muscle.
Statistical analysis
Descriptive statistics were used to analyse the data. End points were analysed using univariate logistic regression and contingence tables with Fisher's exact test for the association between acute/late dysphagia, dose–volume and clinical parameters.
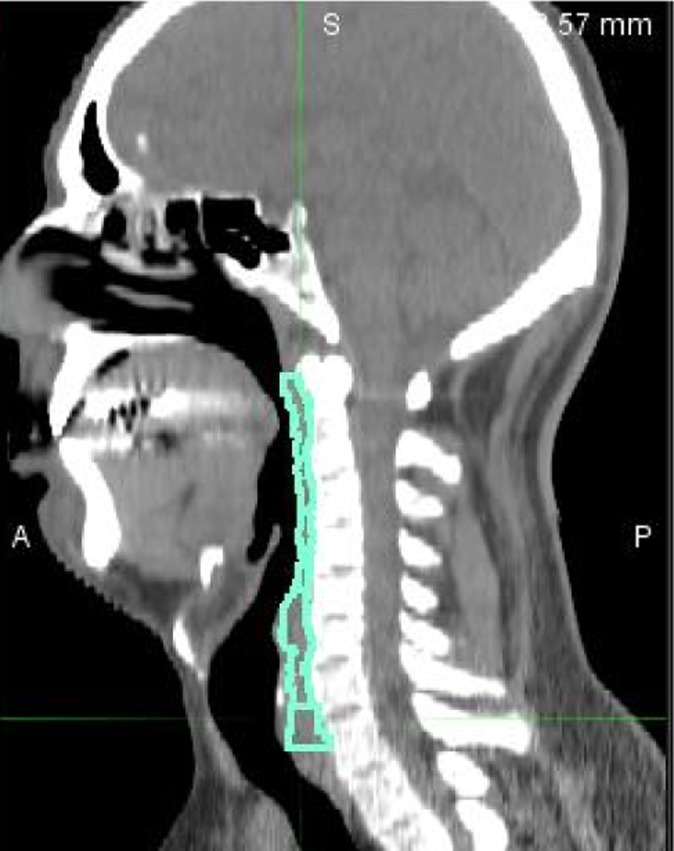
Dosimetric parameters for each PC and CM were related to acute and late toxicities (during RT, at 3, 6 and 12 months from RT). We evaluated a sort of “constraints-escalation” from V30 increasing every 5 Gy until maximum dose was reported in the dose–volume histogram (DVH) of each structure (Figure 2).
Figure 2.
Definition of the constrictor and cricopharyngeal muscles in sagittal projection. A, anterior; P, posterior; S, superior.
The dosimetric parameters for ipsilateral and contralateral parotid glands were Dmean >26 Gy and V30 >50%.
p ≤ 0.05 was considered significant. Data were analysed using R-software (Varian, Palo Alto, CA).
Locoregional control and overall survival were estimated using the Kaplan–Meier method. Time to recurrence and overall survival were calculated from the date of diagnosis to the date of relapse and the date of death or last follow-up, respectively.
RESULTS
Acute and late toxicity
During RT, acute dysphagia and xerostomia were registered as follows: Grade (G) 0–1 in 10 patients (18%), G2 in 36 patients (64%), G3 in 10 patients (18%); and G0–1 in 25 patients (45%), G2 in 30 patients (54%), G3 in 1 patient (1%), respectively. No case of G4 toxicity was registered.
At 3 months from the end of RT, toxicity was reported as follows: G0–1 dysphagia in 33 patients (59%), G2 in 18 patients (32%), G3 in 5 patients (9%); G0–1 xerostomia in 30 patients (54%) and G2 in 26 patients (46%).
At 6 months from RT, G0–1 dysphagia was recorded in 43 patients (77%) and G2 in 13 patients (23%), while G0–1 xerostomia was registered in 39 patients (70%) and G2 in 17 patients (30%), respectively. No G3 toxicity occurred.
At 12 months from RT, toxicity was: G0-1 dysphagia in 44 patients (91%), G2 in 4 patients (9%); G0–1 xerostomia in 41 patients (85%), G2 in 7 patients (15%). No G3 toxicity occurred.
In five patients with oropharynx disease and in two patients with supraglottic disease feeding tubes [percutaneous endoscopy gastrostomy (PEG)] were placed. In detail, in two patients they were placed prophylactically by surgeon, and in the other case for G3 acute dysphagia, only in two patients was the treatment stopped for 7 days. Five patients maintained PEG for 6 months after the end of treatment, and in two patients, PEG was removed after 3 months for symptom resolution.
Clinical outcomes
At a median follow-up of 24 months (range, 10–36 months), 2 years actuarial overall survival (OS) was 100% and 2 years actuarial local control was 96.3%. 3 years OS was 88.9% (we registered one death), while 3 years local control was 57%; in detail, we registered four local failures (all patients with locally advanced disease treated without concomitant chemotherapy). All patients received their chemotherapy as planned.
Dosimetric parameters for superior constrictor muscle
For acute dysphagia ≥G2, no dosimetric parameters showed a statistical correlation.
At 3 months from RT, Dmean ≥ 50 Gy, Dmax ≥ 60 Gy, V50 and V55 ≥70% increased the risk of toxicity (p < 0.01, p < 0.05, p < 0.01 and p < 0.01, respectively). No statistical correlation was found with the other constraints.
For late dysphagia ≥G2 (at 6 months from RT), the dosimetric parameters that showed major correlations were V50, V55 and V60 ≥70% with an increasing risk of toxicity from three to nine times (p < 0.05). For late dysphagia ≥G2 (at 12 months from RT), no dosimetric parameters showed a statistical correlation.
Dosimetric parameters for middle constrictor muscle
For acute dysphagia ≥G2, Dmean ≥ 50 Gy and 55 Gy, and V50 ≥70% increased the risk of toxicity (p < 0.05, p < 0.05 and p < 0.01, respectively). No statistical correlation was found with other constraints.
At 3 months from RT, Dmean ≥ 50 Gy, Dmax ≥ 60 Gy and V50 ≥70% increased the risk of toxicity (p < 0.01, p < 0.05 and p < 0.01, respectively). No statistical correlation was found with other constraints.
For late dysphagia ≥G2 (at 6 and 12 months from RT), no dosimetric parameters showed a statistical correlation.
Dosimetric parameters for inferior constrictor muscle
No dosimetric parameter showed a statistical correlation for acute/late dysphagia ≥G2 (during treatment and at 3, 6 and 12 months from RT).
Dosimetric parameters for cricopharyngeal muscle
During treatment, only Dmax > 60 Gy showed a correlation with dysphagia ≥G2 (p < 0.05), while no dosimetric parameter was related to acute/late toxicity.
Dosimetric parameters for ipsilateral parotid gland
Dmean > 26 Gy and V30 >50% are statistically related with acute xerostomia ≥G2 (p < 0.0001; odds ratio: 1.06; 95% CI: 1.03–1.10) with an increasing risk of 1.06 times for every Gray over 26 Gy and xerostomia at 6 and 12 months from RT [p < 0.05; odds ratio: 1.04; 95% confidence interval (CI): 1.01–1.07], with an increasing risk of 1.04 times for every Gray over 26 Gy, at 6 and 12 months.
Dosimetric parameters for contralateral parotid gland
Dmean > 26 Gy and V30 >50% are statistically related with acute xerostomia ≥G2 (p < 0.001; odds ratio: 1.21; 95% CI: 1.10–1.33) with an increasing risk of 1.2 times for every Gray over 26 Gy and xerostomia at 6 and 12 months from RT (p < 0.05; odds ratio: 1.10; 95% CI: 1.03–1.17), with an increasing risk of 1.1 times for every Gray over 26 Gy, at 6 and 12 months.
All dosimetric data of PCs are shown in Table 3.
Table 3.
Dosimetric factors for constrictors: univariate logistic regression analysis
| Structures | Dysphagia | Constraints | Volume (cm3) and median (range) | Univariate analysis |
||
|---|---|---|---|---|---|---|
| OR | p-value | 95% CI | ||||
| SPC | Acute 3 months 6 months |
Dmax 60 Dmean 50 V50 V55 V65 Dmax 60 Dmean 50 V50 V55 V65 Dmax 60 Dmean 50 V50 V55 V65 |
11.3 (10.8–12.4) | 3.18 1.875 1.55 2.16 1.05 12.52 9.6 6.78 10 2.25 1.86 9.33 NA NA 4.37 |
0.197 0.450 0.702 0.449 1.00 0.01 0.006 0.007 0.001 0.304 0.65 0.03 0.027 0.01 0.117 |
0.38–11.6 0.41–8.51 0.34–7.02 0.46–10.16 0.18–5.97 1.48–105.58 1.89–48.6 1.64–28.04 2.38–12.01 0.60–8.42 0.33–10.41 0.99–87.38 NA NA 0.74–25.8 |
| MPC | Acute 3 months 6 months |
Dmax 60 Dmean 50 V50 V55 V65 Dmax 60 Dmean 50 V50 V55 V65 Dmax 60 Dmean 50 V50 V55 V65 |
2.02 (1.70–2.64) | 3.18 8.5 13.25 9.1 NA 12.52 7.52 4.2 2.71 2.36 1.86 3.26 4.77 5.75 0.51 |
0.197 0.01 0.008 0.05 0.08 0.009 0.013 0.039 0.144 0.204 0.65 0.39 0.204 0.189 1 |
0.68–14.88 1.50–47.96 1.48–116.26 1.03–80.08 NA 1.48–105.58 1.48–38.07 1.13–15.49 0.81–90 0.66–8.35 0.33–10.41 0.35–30.7 0.51–44.32 0.61–53.42 0.054–4.89 |
| IPC | Acute 3 months 6 months |
Dmax 60 Dmean 50 V40 V50 V55 V65 Dmax 60 Dmean 50 V40 V50 V55 V65 Dmax 60 Dmean 50 V40 V50 V55 V65 |
5.4 (4.2–5.8) | 2.06 1.42 2.53 1.09 1.09 2.30 1.14 0.81 0.86 0.58 0.58 0.4 1.07 1.44 2.41 0.86 0.86 NA |
0.43 0.711 0.24 1.00 1.00 0.44 1 0.772 1 0.39 0.39 0.23 1 1 0.65 1 1 NA |
0.45–9.41 0.31–6.40 0.55–11.69 0.24–4.89 0.24–4.89 0.42–12.67 0.34–3.83 0.25–2.59 0.25–2.97 0.18–1.87 0.18–1.87 0.11–1.40 0.17–6.54 0.23–8.73 0.25–22.66 0.15–4.80 0.15–4.80 NA |
| Crico | Acute 3 months 6 months |
Dmax 60 Dmean 50 V40 V50 V55 V65 Dmax 60 Dmean 50 V40 V50 V55 V65 Dmax 60 Dmean 50 V40 V50 V55 V65 |
1.4 (1.2–1.8) | NA 1.66 3.18 2.75 3.73 NA 0.43 0.66 1.13 0.59 0.43 0.46 NA 0.95 0.86 1.15 0.37 NA |
0.04 0.7 0.197 0.277 0.411 0.176 0.35 0.56 1 0.55 0.35 0.33 0.159 1 1 1 0.64 0.314 |
NA 0.35–7.8 0.68–14.88 0.50–15.07 0.42–33.07 NA 0.11–1.63 0.21–2.11 0.31–4.02 0.18–1.92 0.11–1.63 0.1–1.95 NA 0.17–5.26 0.14–5.33 0.2–6.35 0.039–3.49 NA |
CI, confidence interval; Crico, cricopharyngeal muscle; Dmax, maximum dose; Dmean, mean dose; IPC, interior constrictor muscle; MPC, middle constrictor muscle; NA, OR not estimable; OR, odds ratio; SPC, superior constrictor muscle; V40, volume structure receiving ≥40 Gy; V50, volume structure receiving ≥50 Gy; V55, volume structure receiving ≥55 Gy; V60, volume structure receiving ≥60 Gy.
p-value estimated with Fisher’s exact test.
Correlations between clinical factors and dysphagia
Univariate logistic regression analysis for clinical parameters showed a significant correlation with oropharynx primary site (p < 0.05) and acute/late dysphagia. No correlations with sex, smoke of cigarette, diabetes, stage of disease and chemotherapy, when added, were shown. Moreover, late xerostomia ≥G2 is statistically related with dysphagia ≥G2 (p < 0.05).
DISCUSSION
IMRT and VMAT achieved an excellent dose distribution, especially in a concave-shaped target volume and for patients with HNC; they have shown a reduction in RT toxicity.4–8 For this reason, in the past few years, the evaluation of OARs, in terms of variation in volume, geometry or contouring methods, was analysed by several authors to improve therapeutic ratio reducing the risk of toxic effects.17–21,26–28
Swallowing dysfunction after radiotherapy is correlated with compromised QoL and can lead to life-threatening complications. Limiting the radiation dose to the crucial SWOARs is expected to decrease the incidence and severity of radiation-induced dysphagia.
Based on the findings of video fluoroscopy, Eisbruch et al20 were the first to identify the dysfunction of PCs and other structures crucial for long-term dysphagia and aspiration in HNC after concurrent chemoradiotherapy. No distinction was made in this study among the various levels of the PCs, so they were outlined as a single structure for dose assessment purpose. In their analysis, mean dose to the PCs and larynx >50 Gy both correlated significantly with the occurrence of late dysphagia and aspiration, respectively.
Levendag et al21 assessed the relationship between RT dose received by the muscular components of the PCs and dysphagia related to QoL in oropharyngeal cancer. For late dysphagia, ≥G3 significant relationships were found between a Dmean > 50 Gy for superior and middle PC. The probability of PC disorders increased significantly with dose (±19% per 10 Gy after 55 Gy) for the superior and middle PCs. In the multivariate analysis, concomitant chemotherapy was not an influencing factor.
Caglar et al29 evaluated early dysfunction of SWOARs after IMRT with or without chemotherapy and attempted to determine the clinical and/or dosimetric factors correlating with swallowing toxicity. They did not find any correlation with the superior PC dose and early dysphagia, whereas the Dmean ≥ 50 Gy to the larynx and inferior PC was a significant predictor for aspiration.
Similar to these studies, in our series a possible correlation of dose to middle PC was noted, with an increasing risk to develop acute toxicity when constraints are not respected. Superior PC was not correlated with acute toxicity, but statistical analysis showed a probable pathogenetic role in late dysphagia.
Dirix et al30 wanted to establish a relationship between late dysphagia and RT doses to the SWOARs correlating clinical parameters such as the impact of tumour site, tumour stage and pre-treatment swallowing problem. The SWOARs identified were PCs, base of the tongue, supraglottic larynx, glottic larynx and CM. At univariate analysis, a mean dose ≥50 Gy to middle PC, inferior PC and to supraglottic larynx significantly correlated with late dysphagia.
Only one study31 did not find any relationship between dose to the PCs and late dysphagia.
In the study of Mortensen et al,32 65 patients were examined for PC disorders with modified barium swallow (MBS). Similar to our analysis, PCs were delineated as described by Christianen et al22, and the DVHs of OARs were analysed. Late dysphagia correlated with the dose to superior and middle PCs (all p < 0.04). Dmean to the superior PC <60 Gy correlated with low risk of aspiration (<30%) and Dmean to the middle PC <60 Gy correlated with low risk of high MBS score (<30%).
With regard to our results, several considerations could be performed. In our series, only ten patients developed G3 acute dysphagia; at 3 months, five patients developed G3 dysphagia; and no case of G3 developed dysphagia at 6 months and 12 months. We decided to homogenize the toxicity in two classes: G0–1 and ≥G2, because no significantly statistical result was found for G3 toxicity owing to limited cases.
The inferior PC seems not to be correlated with acute or late dysphagia, for CM only Dmax > 60 Gy is correlated with dysphagia during RT ≥G2. Thus, these structures seem to have a minimum or not impact for swallowing symptoms. On the contrary, the superior PC seems to have a major role, being correlated with dysphagia at 3 and 6 months, while middle PC maintains this correlation only until 3 months from the beginning of RT, and it seems to not have an influence on late dysphagia.
The studies available in literature retrieved different results, this may be owing to a number of methodological issues and the unambiguous contouring of swallowing structures. With these uncertainties, it is necessary to standardize these aspects.
In our analysis, using Christianen guidelines to define PMs and CM, interesting results for the readers have been reported. The first finding was that when changing limits of the structures, in particular of the superior PC, using Christianen definition, results are similar to data reported in literature. It means that, maybe, the volume of the structures did not influence the constraints. Another interesting finding was that the middle pharyngeal constrictor was related to acute dysphagia, while the superior pharyngeal constrictor influenced late dysphagia. A clear definition of different structures (and an evaluation of their involvement) could influence differently acute and late settings of toxicities.
Future evaluations on the impact of volumes of the critical structures on coverage and homogeneity of the target are needed.
Starting from this background, we decided to propose and apply in our department the following constraints for PCs, with a minor priority with respect to PTV coverage: Dmean ≤ 50 Gy, Dmax ≤ 60, V50 <70% for middle PC; Dmax ≤ 60 Gy, V50 <70% for superior PC; Dmax ≤ 60 Gy for CM.
Regarding to the dose constraints for parotid glands, our analysis showed that for ipsi and contralateral parotid glands, Dmean > 26 Gy and V30 >50% are statistically related with acute and late xerostomia ≥G2, as reported in literature.33 Moreover the presence of late xerostomia >G2 is statistically related with dysphagia ≥G2 (p < 0.05), showing a close relationship between salivation and swallowing.
We are conscious that the results of the present study are influenced by several limitations. The main limitation regards the lack of methodology due to the retrospective approach. For this reason we will use the previously described constraints in clinical practice in a prospective way, before validating their value definitively. The second limitation regards the population of study: first of all the sample size is small, and moreover, we analysed in the same group radical and adjuvant treated patients, with different primary disease sites in head and neck region and subsequently with different treated volume. However, these limitations are quite similar to the other reports previously published as shown in Table 4,29–32 where several results seems to be close to ours and furthermore, despite the declared limitations, the results about parotid glands confirmed the literature data.
Table 4.
Studies assessing dose–volume analyses for late dysphagia
| Study | Patients | Site | Dosimetric factors correlated with late dysphagia | Limits | Anatomical borders |
|||
|---|---|---|---|---|---|---|---|---|
| SPC | MPC | IPC | Crico | |||||
| Feng34 | 36 | OP/NP | PCs (mean dose, V50, V60, V65) | Cranial Caudal |
Caudal tips of pterygoid plates Upper edge hyoid bone |
Upper edge of the hyoid bone Lower edge of the hyoid bone |
Below the hyoid bone Inferior edge of the cricoid |
Not mentioned Not mentioned |
| Levendag21 | 56 | OP | SPC, MPC (mean dose) | Cranial Caudal |
Mild C2 Upper C3 |
Upper C3 Upper C4 |
Upper C5 Mid C6 |
Mild C6 First ring of trachea |
| Jensen35 | 25 | PH | SL (mean dose, V60, V65) | Cranial Caudal |
Lower part transverse process C2 Top of cricoid cartilage |
Lower part transverse process C2 Top of cricoid cartilage |
Lower part transverse process C2 Top of cricoid cartilage |
Not mentioned Not mentioned |
| Caglar29 | 96 | M | IPC (mean dose, V50, V60) | Cranial Caudal |
Pterygoid plates Upper edge of the hyoid bone |
Upper edge of the hyoid bone Lower edge of the hyoid bone |
Inferior edge hyoid bone Lower edge cricoid |
Not mentioned Not mentioned |
| Dirix30 | 53 | M | MPC (mean dose,V50) | Cranial Caudal |
Caudal tip of the pterygoid plates Upper edge hyoid bone |
Upper edge of the hyoid bone Lower edge of the hyoid bone |
Inferior edge hyoid bone Lower edge cricoid |
Lower edge cricoid Upper edge of trachea |
| Bhide31 | 37 | M | No correlations | Cranial Caudal |
Base of the skull Superior end hyoid bone |
Superior end of the hyoid bone Caudal end of the cartilage cricoid |
Inferior edge hyoid bone Lower edge cricoid |
Not mentioned Not mentioned |
| Caudell36 | 83 | M | IPC (V60, V65) | Cranial Caudal |
Pterygoid plates Upper edge of the hyoid bone |
Upper edge of the hyoid bone Lower edge of the hyoid bone |
Inferior edge hyoid bone Lower edge cricoid |
Not mentioned Not mentioned |
| Mortensen32 | 65 | M | SPC, MPC (mean dose) | Cranial Caudal |
Caudal tip of the pterygoid plates Lower edge of C2 |
Upper edge of C3 Lower edge of hyoid bone |
First slice caudal to the lower edge of hyoid bone Lower edge of the arytenoid cartilages |
First slice caudal to the arytenoid cartilages Lower edge of the cricoid cartilages |
| Current study | 56 | M | SPC (Dmax <60,V50 | Cranial Caudal |
Caudal tip of the pterygoid plates Lower edge of C2 |
Upper edge of C3 Lower edge of hyoid bone |
First slice caudal to the lower edge of hyoid bone Lower edge of the arytenoid cartilages |
First slice caudal to the arytenoid cartilages Lower edge of the cricoid cartilages |
C2, second cervical vertebra; C3, third cervical vertebra; C4, fourth cervical vertebra; C5, fifth cervical vertebra; C6, sixth cervical vertebra; Crico, cricopharyngeal muscle; D60, minimum dose received by 60% of a structure; Dmax, dose maximum; IPC, inferior constrictor muscle; M, miscellaneous; MPC, middle constrictor muscle; NP, nasopharynx; OP, oropharynx; PCs, all constrictors; PCS, pharyngeal constrictor muscle; PH, pharynx; SL, supraglottic larynx; SPC, superior constrictor muscle; V50, volume of a structure receiving 50 Gy; V60, volume of a structure receiving 60 Gy; V65, volume of a structure receiving 65 Gy; V70, volume of a structure receiving 70 Gy.
Another criticism of the present analysis is that it was focused only to PCs and CM, with a lack of evaluation of other SWOARs, including base of tongue, supraglottic larynx, upper oesophagus. The rationale of this choice depended on the mixed population of study composed by several cases of oropharynx and larynx diseases. In our opinion, in these cases, due to being involved with the diseases, it was not possible to consider them as SWOARs and for this reason we restricted the analysis only to role of PCs in swallowing disorders.
CONCLUSION
Based on Christianen guidelines, dose constraints to the superior and middle PCs seem to play a role as dosimetric predictors of early/late swallowing disturbances.
A common contouring is needed to define structures that are involved in the swallowing process to suggest dose–volume constraints. In this scenario, prospective trials could be activated to elucidate the importance of doses to the pharyngeal PCs. Functional and anatomic treatment-related disorders and QoL assessment, including dedicated questionnaires, will be further evaluated in a prospective way in future analysis.
REFERENCES
- 1.Pignon JP, le Maitre A, Maillard E, Bourhis J; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4–14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 2.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. ; Meta-Analysis of Radiotherapy in Carcinomas of Head and neck (MARCH) Collaborative Group. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368: 843–54. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol 2009; 19: 35–42. doi: 10.1016/j.semradonc.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 4.Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity modulated arc therapy versus conventional IMRT in head and neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys 2009; 74: 252–9. doi: 10.1016/j.ijrobp.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 5.Vanetti E, Clivio A, Nicolini G, Fogliata A, Ghosh-Laskar S, Agarwal JP, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009; 92: 111–17. doi: 10.1016/j.radonc.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 6.Scorsetti M, Fogliata A, Castiglioni S, Bressi C, Bignardi M, Navarria P, et al. Early clinical experience with volumetric modulated arc therapy in head and neck cancer patients. Radiat Oncol 2010; 5: 93. doi: 10.1186/1748-717X-5-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alongi F, Bignardi M, Garassino I, Pentimalli S, Cavina R, Mancosu P, et al. Prospective Phase II trial of cetuximab plus VMAT-SIB in locally advanced head and neck squamous cell carcinoma. Feasibility and tolerability in elderly and chemotherapy-ineligible patients. Strahlenther Onkol 2012; 188: 49–55. doi: 10.1007/s00066-011-0006-y [DOI] [PubMed] [Google Scholar]
- 8.Doornaert P, Verbakel WF, Bieker M, Slotman BJ, Senan S. RapidArc planning and delivery in patients with locally advanced head-and-neck cancer undergoing chemoradiotherapy. Int J Radiat Oncol Biol Phys 2011; 79: 429–35. doi: 10.1016/j.ijrobp.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 9.Roe JW, Carding PN, Dwivedi RC, Kazi RA, Rhys-Evans PH, Harrington KJ, et al. Swallowing outcomes following intensity modulated radiation therapy (IMRT) for head & neck cancer—a systematic review. Oral Oncol 2010; 46: 727–33. doi: 10.1016/j.oraloncology.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 10.Van der Laan HP, Van de Water TA, Van Herpt HE, Christianen ME, Bijl HP, Korevaar EW, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: a planning comparative study. Acta Oncol 2013; 52: 561–9. doi: 10.3109/0284186X.2012.692885 [DOI] [PubMed] [Google Scholar]
- 11.Van der Laan HP, Christianen ME, Bijl HP, Schilstra C, Langendijk JA. The potential benefit of swallowing sparing intensity modulated radiotherapy to reduce swallowing dysfunction: an in silico planning comparative study. Radiother Oncol 2012; 103: 76–81. doi: 10.1016/j.radonc.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Nutting CM. Intensity modulated radiotherapy (IMRT) in head and neck cancers—an overview. Gulf J Oncol 2012; 12: 17–26. [PubMed] [Google Scholar]
- 13.Wang X, Hu C, Eisbruch A. Organ-sparing radiation therapy for head and neck cancer. Nat Rev Clin Oncol 2011; 8: 639–48. doi: 10.1038/nrclinonc.2011.106 [DOI] [PubMed] [Google Scholar]
- 14.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. ; PARSPORT Trial Management Group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): Phase 3 multicentre randomised controlled trial. Lancet Oncol 2011; 12: 127–36. doi: 10.1016/S1470-2045(10)70290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta T, Agarwal J, Jain S, Phurailatpam R, Kannan S, Ghosh-Laskar S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol 2012; 104: 343–8. doi: 10.1016/j.radonc.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007; 25: 4873–9. [DOI] [PubMed] [Google Scholar]
- 17.Fiorentino A, Cozzolino M, Caivano R, Pedicini P, Chiumento C, Oliviero C, et al. Cone-beam computed tomography dose monitoring during intensity-modulated radiotherapy in head and neck cancer: parotid glands. Clin Transl Oncol 2013; 15: 412–15. doi: 10.1007/s12094-012-0946-4 [DOI] [PubMed] [Google Scholar]
- 18.Fiorentino A, Caivano R, Metallo V, Chiumento C, Cozzolino M, Califano G, et al. Parotid gland volumetric changes during intensity-modulated radiotherapy in head and neck cancer. Br J Radiol 2012; 85: 1415–19. doi: 10.1259/bjr/30678306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiorentino A, Cozzolino M, Caivano R, Pedicini P, Oliviero C, Chiumento C, et al. Head and neck intensity modulated radiotherapy parotid glands: time of re-planning. Radiol Med 2014; 119: 201–7. doi: 10.1007/s11547-013-0326-3 [DOI] [PubMed] [Google Scholar]
- 20.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemo-radiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 2004; 60: 1425–39. [DOI] [PubMed] [Google Scholar]
- 21.Levendag PC, Teguha DN, Voet P, van der Esta H, Noever I,de Kruijfa WJ, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol 2007; 85: 64–73 [DOI] [PubMed] [Google Scholar]
- 22.Christianen ME, Langendijk JA, Westerlaan HE, van de Water TA, Bijl HP. Delineation of organs at risk involved in swallowing for radiotherapy treatment planning. Radiother Oncol 2011; 101: 394–402. doi: 10.1016/j.radonc.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 23.Radiation Therapy Oncology Group. Head and Neck Atlases. Available from: http://www.rtog.org/CoreLab/ContouringAtlases/HNAtlases.aspx
- 24.Ding GX, Munro P, Pawlowski J, Malcom A, Coffey CW. Reducing radiation exposure to patients from kv-CBCT imaging. Radiother Oncol 2010; 97: 585–92. doi: 10.1016/j.radonc.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 25.AIOM. Scientific product—guidelines. Available from: http://www.aiom.it/area+pubblica/area+medici/Prodotti+scientifici/linee+guida/1,1,333
- 26.Ricchetti F, Wu B, McNutt T, Wong J, Forastiere A, Marur S, et al. Volumetric change of selected organs at risk during IMRT for oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2011; 80: 161–8. doi: 10.1016/j.ijrobp.2010.01.071 [DOI] [PubMed] [Google Scholar]
- 27.Russi EG, Corvò R, Merlotti A, Alterio D, Franco P, Pergolizzi S, et al. Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: review and recommendations of the supportive task group of the Italian Association of Radiation Oncology. Cancer Treat Rev 2012; 38: 1033–49. doi: 10.1016/j.ctrv.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 28.Pedicini P, Nappi A, Strigari L, Jereczek-Fossa BA, Alterio D, Cremonesi M, et al. Correlation between EGFr expression and accelerated proliferation during radiotherapy of head and neck squamous cell carcinoma. Radiat Oncol 2012; 7: 143. doi: 10.1186/1748-717X-7-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caglar HB, Tishler RB, Othus M, Burke E, Li Y, Goguen L, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008; 72: 1110–18. doi: 10.1016/j.ijrobp.2008.02.048 [DOI] [PubMed] [Google Scholar]
- 30.Dirix P, Abbeel S, Vanstraelen B, Hermans R, Nuyts S. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose–effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2009; 75: 385–92. doi: 10.1016/j.ijrobp.2008.11.041 [DOI] [PubMed] [Google Scholar]
- 31.Bhide SA, Gulliford S, Kazi R, El-Hariry I, Newbold K,Harrington KJ, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol 2009; 93: 539–44. doi: 10.1016/j.radonc.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 32.Mortensen HR, Jensen K, Aksglæde K, Behrens M, Grau C. Late dysphagia after IMRT for head and neck cancer and correlation with dose-volume parameters. Radiother Oncol 2013; 107: 288–94. doi: 10.1016/j.radonc.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 33.Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010; 76: S10–19. doi: 10.1016/j.ijrobp.2009.07.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng FY, Kim HM,Lyden TH, Haxer MJ,Feng M, Worden FP, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effectrelationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2007; 68: 1289–98. [DOI] [PubMed] [Google Scholar]
- 35.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiother Oncol 2007; 85: 74–82. [DOI] [PubMed] [Google Scholar]
- 36.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2010; 76: 403–9. [DOI] [PubMed] [Google Scholar]