Summary
We describe a rare case of aneurysmal bone cysts (ABCs) that occurred in the petrous portion of the temporal bone. The ABCs were treated with preoperative embolization and complete removal of the mass from the adjacent tissue. The technical details suggest that preoperative embolization is a good treatment option for ABCs.
Keywords: bone cysts, aneurysmal, temporal bone, angiography, digital subtraction
Introduction
Aneurysmal bone cysts (ABCs), first described in 1942 by Jaffe and Lichtenstein, are a rare and benign disease showing rapid growth, with osteolysis and cystic lesions such as an expansile arterial aneurysm 1. We describe a case of ABCs in the temporal bone, which were completely removed with the help of preoperative embolization, and were used to reduce intraoperative bleeding. Forty cases of ABCs in the temporal bone have been reported in the literature, but only two cases described the use of preoperative embolization.
Case Report
A 17-year-old female visited our clinic due to headache, nausea, and vomiting. A history revealed a growing mass in the right temporal area for three months. No history of trauma was noted. The general physical examination was unremarkable. However, a fundoscopic examination showed bilateral papilledema and hemorrhage around the left optic disc.
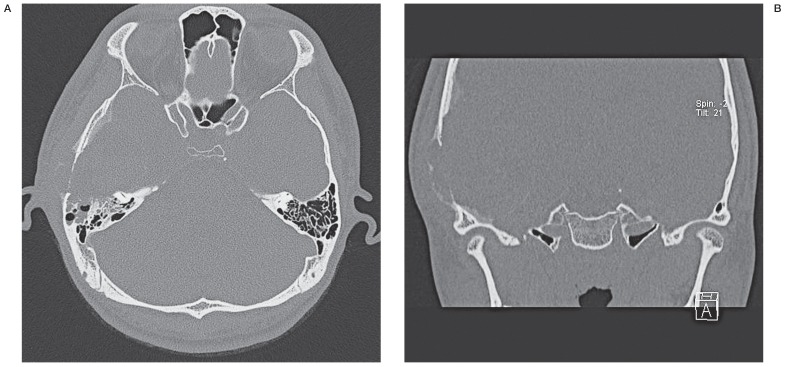
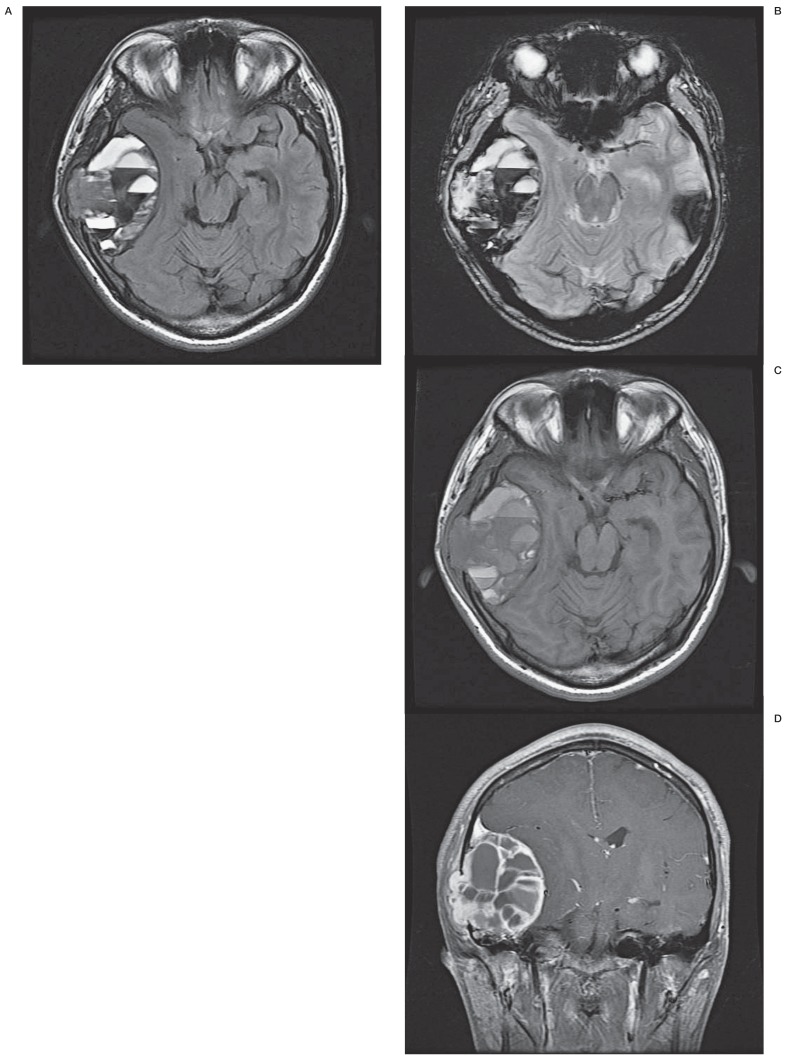
A computed tomography (CT) scan revealed an osteolytic and cystic lesion involving the inner and outer table of the temporal bone as well as the anterior portion of the mastoid air cells (Figure 1). A magnetic resonance image (MRI) revealed a 9 cm-sized multicystic mass with air fluid levels originating from the squamous portion of the temporal bone (Figure 2A). The axial gradient echo image showed that the lower layer of fluid-fluid level demonstrated low signal intensity suggestive of blood (Figure 2B). Low signal intensity was detected in the fibrous capsule and septa (Figure 2C) with homogeneous enhancement (Figure 2D). The mass did not involve the meninges or compress the brain parenchyma due to an indirect mass effect.
Figure 1.
A) Axial temporal bone computed tomography (CT) scan shows bony destruction of the anterior portion of the mastoid air cells resulting in the meniscus sign. B) Coronal temporal bone CT scan shows severe bony thinning and destruction of the inner and outer tables of the temporal bone and a focal area of ground-glass attenuation along the inner table of the temporal bone.
Figure 2.
A) Axial fluid attenuated inversion recovery image shows an expansile extra-axial mass with fluid-fluid levels involving the temporal bone with a multicystic soap bubble appearance. The upper layer of the fluid-fluid level is not suppressed as cerebral spinal fluid. B) The axial gradient-echo image shows that the lower layer of the fluid-fluid level demonstrates low signal intensity suggestive of blood. C) Axial T1-weighted non-contrasted image. D) Coronal T1-weighted image with contrast enhancement. D) A well-enhanced internal septa and a soft tissue attenuated lesion anchoring to the squamous portion of the temporal bone.
The surgeon decided to surgically excise the mass, considering the patient's symptoms, the papilledema secondary to increased intracerebral pressure, and the relatively rapid progression of the mass; preoperative embolization was requested.
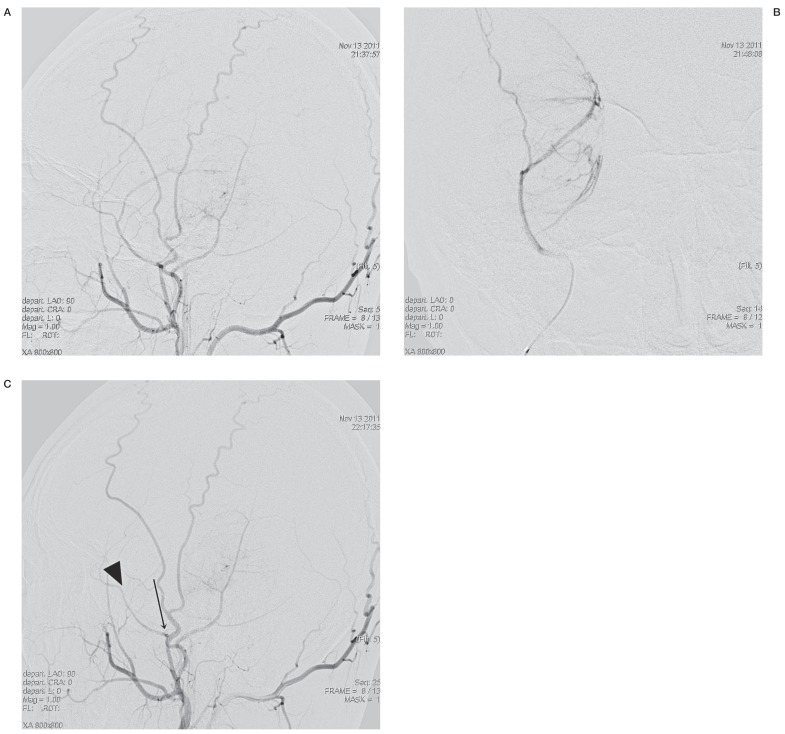
A large mass feeding from the middle meningeal artery and superficial temporal artery was detected on right external carotid angiography. Particle embolization with polyvinyl alcohol 150-250 (Contour, Boston Scientific, Fremont, CA, USA) was done with guidance from a 5 Fr Envoy catheter (Cordis Corp, Miami Lakes, FL, USA) and superselection with an agility 10 soft (Cordis) and Prowler 14 (Cordis).
Surgical excision of the mass was uneventful. Blood loss was about 200 ml, most of which occurred during the craniotomy. After the operation, the patient's symptoms and fundoscopic findings resolved, and no local recurrence has been detected after two years of follow-up.
Figure 3.
A,B) Pre-embolization angiograms. A) Global external carotid artery injection. B) Selective injection of the middle meningeal artery (MMA). The angiograms show the mass, which is mainly fed by the MMA. C) The post-embolization angiogram shows occluded middle (arrowhead) and posterior (arrow) branches of the right MMA with minimal residual tumor staining from the right superficial temporal artery and posterior auricular artery.
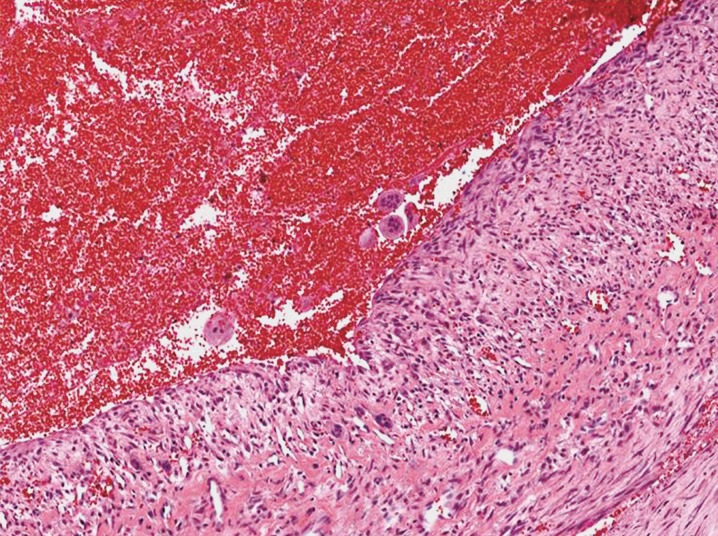
The gross examination of the specimen revealed multiple honeycomb-like cystic lesions partially filled with non-coagulated blood. The microscopic examination showed that the septa of the mass comprised fibroblasts, multinuclear giant cells in the form of osteoclasts, granulation tissue, macrophages filled with hemosiderin, and extravasated red blood cells (Figure 4). Thus, ABCs of the temporal bone were confirmed pathologically.
Figure 4.
The microphotograph of the aneurysmal bone cyst shows cystic spaces including red blood cells which are separated by septa including spindle shaped cells and scattered multinucleate giant cells.
Discussion
Aneurysmal bone cysts (ABCs) are benign but relatively rapidly growing vascular bone lesions, representing about 1% of primary bone tumors 2.
ABCs occur mostly in patients ≤ 20-years-of-age, and the mean age of occurrence is 13 years, but cases of patients aged from one to 59 years at the time of diagnosis have been reported 2,3. ABCs shows a slight female predominance (male: female = 1:1.04-1.8) 2,3. About 52% of ABCs involve the metaphyseal region of long bones such as the femur, tibia, fibula, or humerus, 20% involve the spine, and only 3-6% involve the skull 2,4. About 70% of ABCs are primary lesions and the others are secondary, representing other bone tumors such as giant cell tumors, hemangiomas, and chondroblastomas 5.
The pathogenesis of ABCs is controversial, and their exact cause remains unknown. Recent studies show a genetic aspect to ABCs, and it is accepted that they are a tumor rather than a reactive disease. Oliveira et al. revealed that 69% of primary ABCs are influenced by gene rearrangements localized to t (16,17) in which the ubiquitin-specific protease 6 oncogene is located under the regulatory influence of the highly active cadherin-11 promoter. However, such translocations are not detected in secondary ABCs 6. Leithner et al. showed that insulin-like growth factor-I (IGF-1) or mRNA coding for this growth factor is usually localized in multinucleate giant cells in all specimens. Normal human bone tissue does not show significant levels of IGF-1 expression 2.
The usual clinical presentation is tenderness and/or externally visible mass effects at the involved site. Imaging studies are very important for the diagnosis and to make treatment decisions. Simple skull radiography shows the so-called “soap bubble”-like expansible osteolytic lesion. CT shows more details on the relationship between normal bone and ABC lesions and also demonstrates an expansive multiloculated osseous lesion invading the inner and outer tables of the skull. The CT image may show a fluid-fluid level of different attenuations. MRI shows a characteristic fluid-fluid level of different signal intensities. Contrast enhancement in the peripheral capsule and internal septation are detected on enhanced T1-weighted images. The osteolytic lesion is surrounded by inner and outer tables. The dependent portion of the fluid shows low signal intensity on a gradient echo image, due to non-coagulated hemorrhage, and this is an important finding of ABCs. However these findings are not characteristic, and they may be seen in telangiectatic osteosarcomas, giant cell tumors, secondary aneurysmal bone cysts, and simple bone cysts combined with fracture. Hence, pathological confirmation is needed. Fine needle biopsy is insufficient, and surgical excisional biopsy is mandatory. Pathological examination reveals typical hemorrhagic tissues divided with fibrous septa comprising spindle cells, some giant cells, and inflammatory cells 7.
Complete surgical excision of the mass is the treatment of choice because of the high recurrence rate of up to 59% 8. The mass itself is a highly vascular tumor and liable to bleed during surgery. Hence, endovascular embolization is used as additional support during complete removal of the mass. In 1975, Feldman performed endovascular embolization of a bone tumor as a preoperative procedure, which enabled complete removal of the mass from adjacent tissue by thrombosis of the feeding artery, and necrosis and shrinkage of the mass with reduced perioperative bleeding 9. Preoperative angiography reveals the vascularity and hemodynamic status of the tumor. Furthermore, other researchers have shown the effectiveness of endovascular embolization with N-2-butyl cyanoacrylate. They enrolled 55 cases of extremity ABCs and demonstrated that 94% of cases were effectively treated with this method, without recurrence during 0.9−5 years of follow-up 10. However, no reports on embolization involving the skull as the sole treatment modality have been published.
Conclusions
Preoperative embolization appears to be a useful method to treat skull ABCs.
References
- 1.Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44(6):1004–1025. doi: 0.1001/archsurg.1942.01210240043003. [Google Scholar]
- 2.Leithner A, Windhager R, Lang S, et al. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;363:176–179. doi: 10.1097/00003086-199906000-00023. [PubMed] [Google Scholar]
- 3.Zehetgruber H, Bittner B, Gruber D, et al. Prevalence of aneurysmal and solitary bone cysts in young patients. Clin Orthop Relat Res. 2005;439:136–143. doi: 10.1097/01.blo.0000173256.85016.c4. doi: 10.1097/01.blo.0000173256.85016.c4. [DOI] [PubMed] [Google Scholar]
- 4.Ameli N, Abbassioun K, Azod A, et al. Aneurysmal bone cyst of the skull. Can J Neurol Sci. 1984;11(4):466–471. doi: 10.1017/s0317167100046023. [DOI] [PubMed] [Google Scholar]
- 5.Cottalorda J, Kohler R, Sales de Gauzy J, et al. Epidemiology of aneurysmal bone cyst in children: a multicenter study and literature review. J Pediatr Orthop B. 2004;13(6):389–394. doi: 10.1097/01202412-200411000-00008. doi: 10.1097/01202412-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira AM, Perez-Atayde AR, Dal Cin P, et al. Aneurysmal bone cyst variant translocations upregulate USP6 transcription by promoter swapping with the ZNF9, COL1A1, TRAP150, and OMD genes. Oncogene. 2005;24(21):3419–3426. doi: 10.1038/sj.onc.1208506. doi: 0.1038/sj.onc.1208506. [DOI] [PubMed] [Google Scholar]
- 7.Dabska M, Buraczewski J. Aneurysmal bone cyst. Pathology, clinical course and radiologic appearances. Cancer. 1969;23(2):371–389. doi: 10.1002/1097-0142(196902)23:2<371::aid-cncr2820230213>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni T, Goel A, Desai K, et al. Massive aneurysmal bone cyst of the anterior cranial fossa floor--case report. Neurol Med Chir (Tokyo) 2001;41(12):615–619. doi: 10.2176/nmc.41.615. doi: 10.2176/nmc.41.615. [DOI] [PubMed] [Google Scholar]
- 9.Feldman F, Casarella WJ, Dick HM, et al. Selective intra-arterial embolization of bone tumors. A useful adjunct in the management of selected lesions. Am J Roentgenol Radium Ther Nucl Med. 1975;123(1):130–139. doi: 10.2214/ajr.123.1.130. doi: 10.2214/ajr.123.1.130. [DOI] [PubMed] [Google Scholar]
- 10.Rossi G, Rimondi E, Bartalena T, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal Radiol. 2010;39(2):161–167. doi: 10.1007/s00256-009-0757-z. doi: 10.1007/s00256-009-0757-z. [DOI] [PubMed] [Google Scholar]