Abstract
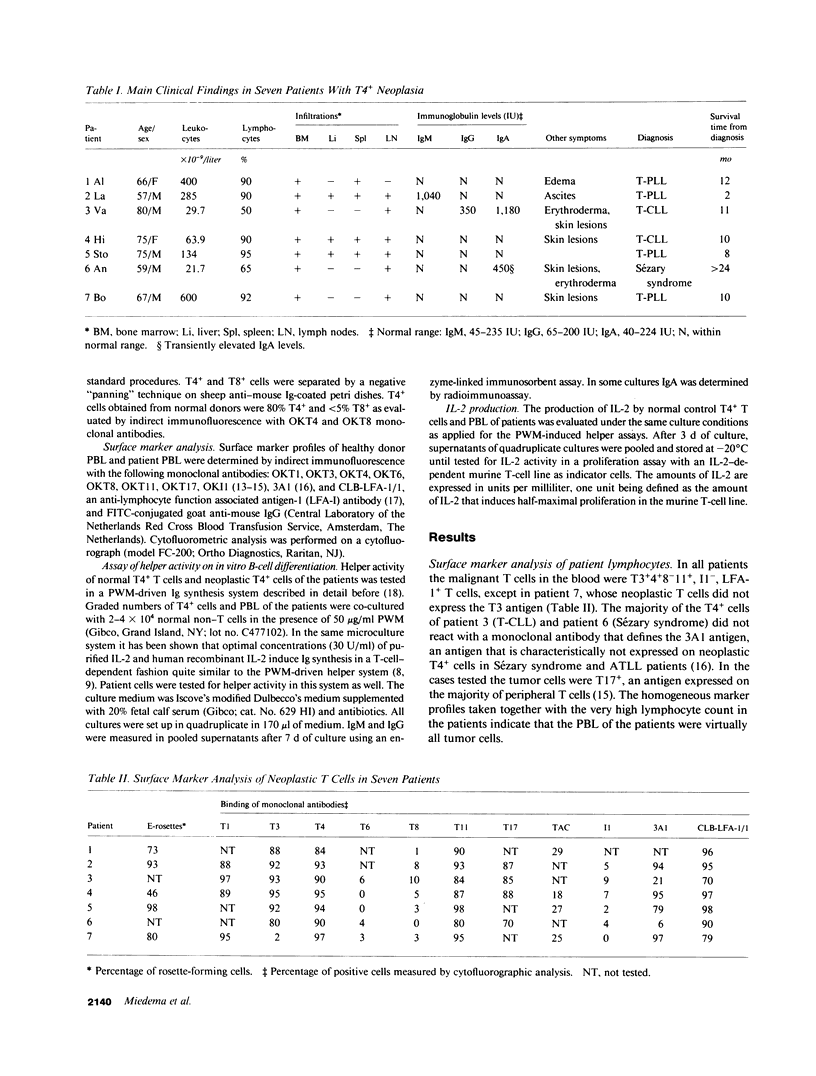
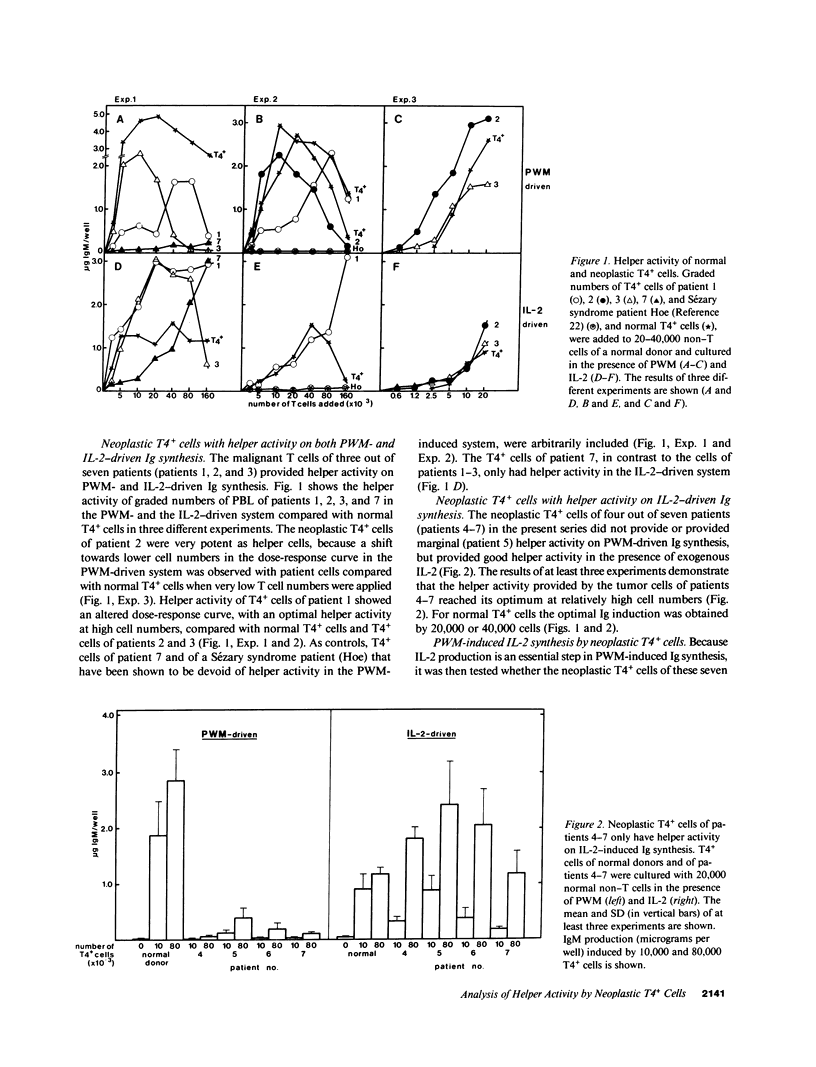
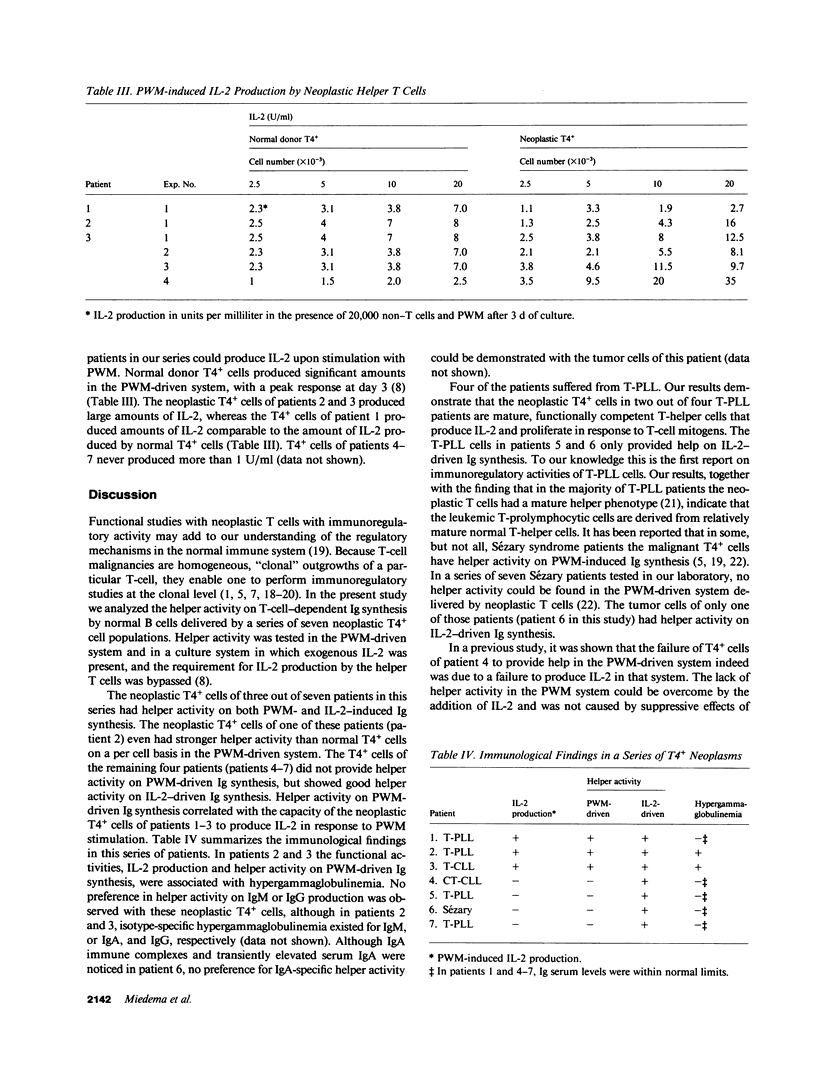
The neoplastic T cells of a series of seven patients with chronic T-cell neoplasia were tested for helper activity on pokeweed mitogen (PWM)-induced and interleukin 2 (IL-2)-induced Ig synthesis. The neoplastic T cells of all patients had a T3+4+8-11+I1- phenotype but differed in expression of the 3A1 antigen. The neoplastic T cells of three patients had helper activity on both PWM- and IL-2-driven Ig synthesis, and in addition produced IL-2 in response to PWM stimulation. Two of these patients had hypergammaglobulinemia. In contrast, the neoplastic T cells in the remaining four patients did not produce IL-2 and did not support PWM-driven Ig synthesis. The T4+ cells of these four patients, however, provided excellent helper activity on IL-2-driven Ig synthesis. These findings emphasize the role of IL-2 in T cell-dependent Ig synthesis and clearly show that IL-2 production is required for helper activity in the PWM-driven system. It is concluded that the combined use of PWM- and IL-2-driven Ig synthesis systems allows separate analysis of IL-2 production and T-helper activity in health and disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broder S., Edelson R. L., Lutzner M. A., Nelson D. L., MacDermott R. P., Durm M. E., Goldman C. K., Meade B. D., Waldmann T. A. The Sézary syndrome: a malignant proliferation of helper T cells. J Clin Invest. 1976 Dec;58(6):1297–1306. doi: 10.1172/JCI108585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Uchiyama T., Waldmann T. A. Neoplasms of immunoregulatory cells. Am J Clin Pathol. 1979 Oct;72(4 Suppl):724–731. [PubMed] [Google Scholar]
- Catovsky D., Wechsler A., Matutes E., Gomez R., Bourikas G., Cherchi M., Pepys E. O., Pepys M. B., Kitani T., Hoffbrand A. V. The membrane phenotype of T-prolymphocytic leukaemia. Scand J Haematol. 1982 Nov;29(5):398–404. doi: 10.1111/j.1600-0609.1982.tb00614.x. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Naiem F., Saxon A., Stevens R., Gale R. P. Leukemia of T-helper lymphocytes: clinical and functional features. Leuk Res. 1981;5(1):1–10. doi: 10.1016/0145-2126(81)90091-6. [DOI] [PubMed] [Google Scholar]
- Gramatzki M., Dolan M. F., Fauci A. S., Maples J. A., Bonnard G. D., Strong D. M. Immunologic characterization of a helper T-cell lymphoma. Blood. 1982 Apr;59(4):702–708. [PubMed] [Google Scholar]
- Haynes B. F., Bunn P., Mann D., Thomas C., Eisenbarth G. S., Minna J., Fauci A. S. Cell surface differentiation antigens of the malignant T cell in Sezary syndrome and mycosis fungoides. J Clin Invest. 1981 Feb;67(2):523–530. doi: 10.1172/JCI110062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Granelli-Piperno A., Steinman R. M. Dendritic cells induce T lymphocytes to release B cell-stimulating factors by an interleukin 2-dependent mechanism. J Exp Med. 1983 Dec 1;158(6):2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Zanders E. D., Feldmann M., Lake P., Eckels D. D., Woody J. N., Beverley P. C. The dissociation of interleukin-2 production and antigen-specific helper activity by clonal analysis. Immunology. 1983 Nov;50(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Miedema F., Terpstra F. G., Smit J. W., Daenen S., Gerrits W., Hegde U., Matutes E., Catovsky D., Greaves M. F., Melief C. J. Functional properties of neoplastic T cells in adult T cell lymphoma/leukemia patients from the Caribbean. Blood. 1984 Feb;63(2):477–481. [PubMed] [Google Scholar]
- Miedema F., Terpstra F. G., Smit J. W., van der Veen J. P., Melief C. J. T gamma lymphocytosis is clinically non-progressive but immunologically heterogeneous. Clin Exp Immunol. 1985 Aug;61(2):440–449. [PMC free article] [PubMed] [Google Scholar]
- Miedema F., Tetteroo P. A., Hesselink W. G., Werner G., Spits H., Melief C. J. Both Fc receptors and lymphocyte-function-associated antigen 1 on human T gamma lymphocytes are required for antibody-dependent cellular cytotoxicity (killer cell activity). Eur J Immunol. 1984 Jun;14(6):518–523. doi: 10.1002/eji.1830140607. [DOI] [PubMed] [Google Scholar]
- Miedema F., Van Oostveen J. W., Sauerwein R. W., Terpstra F. G., Aarden L. A., Melief C. J. Induction of immunoglobulin synthesis by interleukin 2 is T4+/T8- cell dependent. A role for interleukin 2 in the pokeweed mitogen-driven system. Eur J Immunol. 1985 Feb;15(2):107–112. doi: 10.1002/eji.1830150202. [DOI] [PubMed] [Google Scholar]
- Miedema F., Willemze R., Terpstra F. G., van Vloten W. A., Meijer C. J., Melief C. J. Regulatory activity of neoplastic T cells in Sezary syndrome on in vitro immunoglobulin production. Leuk Res. 1984;8(5):873–884. doi: 10.1016/0145-2126(84)90108-5. [DOI] [PubMed] [Google Scholar]
- Mingari M. C., Gerosa F., Carra G., Accolla R. S., Moretta A., Zubler R. H., Waldmann T. A., Moretta L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature. 1984 Dec 13;312(5995):641–643. doi: 10.1038/312641a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rümke H. C., Miedema F., ten Berge I. J., Terpstra F., van der Reijden H. J., van de Griend R. J., de Bruin H. G., von dem Borne A. E., Smit J. W., Zeijlemaker W. P. Functional properties of T cells in patients with chronic T gamma lymphocytosis and chronic T cell neoplasia. J Immunol. 1982 Jul;129(1):419–426. [PubMed] [Google Scholar]
- Sauerwein R. W., Van der Meer W. G., Dräger A., Aarden L. A. Interleukin 2 induces T cell-dependent IgM production in human B cells. Eur J Immunol. 1985 Jun;15(6):611–616. doi: 10.1002/eji.1830150615. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Shen H. H., Talle M. A., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. V. Suppressor cells within the activated OKT4+ population belong to a distinct subset. J Immunol. 1982 Mar;128(3):1386–1390. [PubMed] [Google Scholar]
- Vyth-Dreese F. A., van der Reijden H. J., de Vries J. E. Phorbol-ester-mediated induction and augmentation of mitogenesis and interleukin-2 production in human T-cell lymphoproliferative disease. Blood. 1982 Dec;60(6):1437–1446. [PubMed] [Google Scholar]
- Waldmann T. A., Greene W. C., Sarin P. S., Saxinger C., Blayney D. W., Blattner W. A., Goldman C. K., Bongiovanni K., Sharrow S., Depper J. M. Functional and phenotypic comparison of human T cell leukemia/lymphoma virus positive adult T cell leukemia with human T cell leukemia/lymphoma virus negative Sézary leukemia, and their distinction using anti-Tac. Monoclonal antibody identifying the human receptor for T cell growth factor. J Clin Invest. 1984 Jun;73(6):1711–1718. doi: 10.1172/JCI111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y. Phenotypic and functional analysis of leukemic cells from 16 patients with adult T-cell leukemia/lymphoma. Blood. 1983 Jan;61(1):192–199. [PubMed] [Google Scholar]
- van Oers M. H., Zeijlemaker W. P., Schellekens P. T. Separation and properties of EA-rosette-forming lymphocytes in humans. Eur J Immunol. 1977 Mar;7(3):143–150. doi: 10.1002/eji.1830070306. [DOI] [PubMed] [Google Scholar]



