Abstract
Background
We sought to determine the quantitative expression of human leukocyte antigen–DR (HLA-DR) on monocytes in patients with acute intestinal bacterial infections and inflammatory bowel disease (IBD).
Methods
The quantitative expression of HLA-DR on monocytes was determined by fluorescence-activated cell sorting analysis in patients with IBD, patients with acute intestinal bacterial infections (bact.), and healthy subjects (contr.).
Results
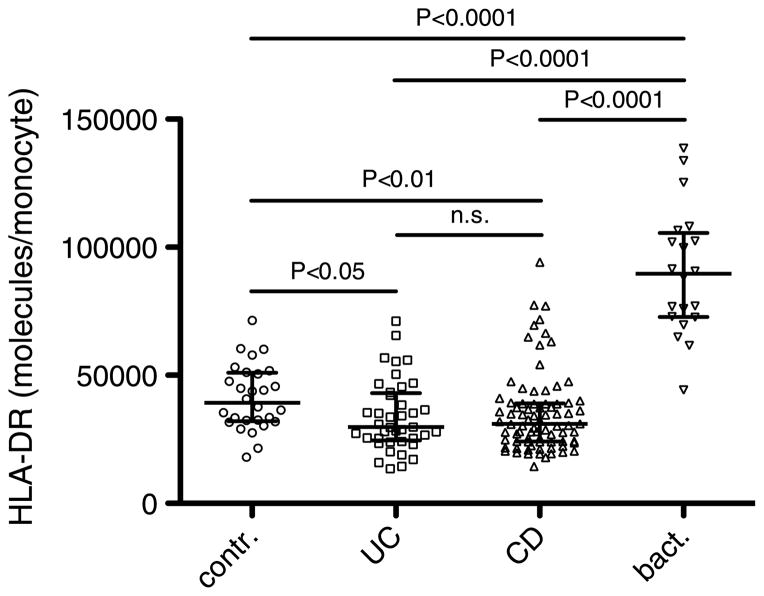
The quantitative expression of HLA-DR in patients with bact. (n = 20; 90,000 molecules per monocyte; confidence interval [CI], 79,000–102,000) was significantly higher than that in patients with ulcerative colitis (n = 40, 30,000; CI, 30,000–38,000; P < 0.0001), Crohn disease (n = 80, 31,000; CI, 32,000–39,000; P < 0.0001), or in contr. (n = 28, 39,000; CI, 36,000–46,000; P < 0.0001). In patients with ulcerative colitis and Crohn disease, HLA-DR expression was significantly decreased, as compared with contr. (P < 0.05 and P < 0.01, respectively). With a cutoff point of 50,000, HLA-DR showed a sensitivity of 95% and a specificity of 92% in discriminating between bact. and active IBD.
Conclusion
The quantitative measurement of HLA-DR expression could serve as a valuable tool to discriminate between bact. and active IBD.
Keywords: Inflammation, monocytes, immune response, Crohn disease, ulcerative colitis
INTRODUCTION
The diagnostic approach to differentiate among patients with diarrheal diseases is a major challenge in clinical practice. As symptoms are often unspecific, differential diagnosis encompasses consideration of a wide range of possible causes, including intestinal infections and chronic inflammatory conditions, such as inflammatory bowel disease (IBD). From a clinician’s standpoint, it is crucial to rapidly distinguish bacterial infections from acute flares of IBD, because patients often present seriously ill, and divergent modalities of treatment are required. Although acute flares of IBD are treated with steroids or tumor necrosis factor α (TNF-α) antagonists, such treatment strategies could be detrimental in patients with intestinal infections as septic complications could occur.
In industrialized regions of the world, bacterial infections account for about one third of all cases of diarrhea. In approximately 15% of these cases, Campylobacter, Salmonella, and Shigella are the underlying pathogens (1). Available laboratory markers such as white blood cell counts, C-reactive protein (CRP), and the stool marker calprotectin are rather unspecific. Serum procalcitonin has been shown to be elevated in self-limited colitis, but not in IBD (2). Therefore, the diagnosis of IBD requires endoscopic, histologic, and radiologic tests. Stool cultures are regarded as the criterion standard for the diagnosis of intestinal bacterial infections. However, a turnaround time of up to 72 h limits its utility for rapid decision-making purposes. Therefore, further biomarkers suitable for accurate diagnosis of intestinal infections with short turnaround times are needed.
Human leukocyte antigen–DR (HLA-DR) is a crucial molecule in antigen presentation. Its upregulation by monocytes enables these cells to increase antigen presentation that supports the clearance of pathogens. Expression of HLA-DR on monocytes has therefore been considered as a marker of immune competency of the host, and a decrease in the expression of HLA-DR has been observed not only in critically ill patients (3–5), but also in patients with autoimmune diseases such as systemic lupus erythematosus (6, 7) or rheumatoid arthritis (8). In patients with rheumatoid arthritis, a decrease in the expression of HLA-DR is associated with an aggressive course of the disease (9). Interestingly, decreased HLA-DR expression on peripheral blood monocytes was also observed in patients with active ulcerative colitis (UC) (10–12).
A number of bacterial compounds have been shown to upregulate HLA-DR expression. Among these compounds are bacterial proteins such as Salmonella typhimurium endotoxin-associated protein (13), Leptospira interrogans glycoprotein (14), bacterial cell wall compounds including lipopolysaccharides (15), peptidoglycan, and lipoteichoic acid (16), as well as bacterial DNA (17). Furthermore, inflammatory cytokines including interferon γ (IFN-γ) (18, 19), interleukin 2 (IL-2) (20), IL-4, and granulocyte-macrophage colony-stimulating factor have been shown to upregulate HLA-DR (21). Increased release of IFN-γ is stimulated by various enteric pathogens including Salmonella, Campylobacter (22), Shigella (23), and Clostridium difficile (24).
In the current study, we tested the hypothesis that quantitative measurements of HLA-DR expression on monocytes would allow to discriminate between patients with bacterial intestinal infections and patients with IBD. Using receiver operating characteristic (ROC) curve analysis, we aimed to determine cutoff values that discriminate between these conditions.
METHODS
Patients and blood collection
The study was approved by the local ethics committee (Hietzing Hospital). Venous blood samples were drawn from patients with IBD in the acute phase or in remission. The diagnosis of IBD was established according to clinical, endoscopic, histologic, and radiologic criteria (25, 26). The clinical activity of disease was determined using the Crohn Disease Activity Index (CDAI) and the Colitis Activity Index (CAI). Acute disease was defined as CDAI greater than 150 or CAI greater than 4. The diagnosis of intestinal bacterial infection was based on positive stool cultures. Blood samples were obtained within 48 h after the onset of symptoms was reported. Blood samples were kept at 4°C and stained for flow cytometric analysis of HLA-DR expression within 4 h except for experiments addressing the stability of HLA-DR expression under prolonged storage conditions.
Monoclonal antibodies
For quantitative measurements of HLA-DR expression, we used a phycoerythrin-labeled mouse anti–human HLA-DR antibodies (clone L243 from BD Biosciences, San Jose, Calif). Monocytes were labeled with peridinin-chlorophyll-protein–labeled mouse anti–human CD14 antibodies (clone MΦP9; BD Biosciences) and Cy5.5 cyanine dye-labeled anti-CD64 antibodies (BD Biosciences).
Flow cytometry
The expression of HLA-DR was measured by flow cytometry using a FACSCalibur instrument (BD Biosciences). Monocytes were gated according to their distribution in forward- and side-scatter plots and based on the staining characteristics described above. To determine the number of HLA-DR molecules per monocyte, a Quantibrite phycoerythrin fluorescence quantitation kit (BD Biosciences) was used according to the manufacturer’s instructions. Enumeration of HLA-DR molecules per cell was conducted with QuantCALC software (BD Biosciences), which allows conversion of fluorescence readings to HLA-DR molecules per cell.
Statistical analysis
If not otherwise stated, data are presented as medians and lower/upper 95% confidence intervals (CIs). Data were tested for normality using the Kolmogorov-Smirnov test. The Student t and the Mann-Whitney U tests were used for single comparisons, and the Kruskal-Wallis analysis of variance with Bonferroni posttest was used for multiple comparisons. The associations between clinical (CDAI, CAI) or biochemical (CRP) activity parameters and HLA-DR expression were assessed with Spearman correlation coefficient. To detect diagnostic cutoff values of HLA-DR expression, ROC curves were estimated using a logistic regression model and plotting sensitivity versus 1-specificity. The comparison of the ROC curves was done by a nonparametric approach. Calculations were performed with SAS software (SAS Institute, Cary, NC) and Prism 5 for Mac OS X (GraphPad Software, Inc, La Jolla, Calif).
RESULTS
Patient characteristics
A total of 29 healthy controls and 140 patients with confirmed Crohn disease (CD; n = 80), UC (n = 40), or bacterial infections (bact.; n = 20) were enrolled. Of the bact. patients, stool cultures were positive for Campylobacter (n = 11), Salmonella (n = 8), or Shigella (n = 1). More detailed patient characteristics are given in Tables 1 and 2.
Table 1.
Patient characteristics I
| CD (n = 80) | UC (n = 40) | |
|---|---|---|
| Sex, male/female, n | 44/36 | 22/18 |
| Age, y | 36.5 (11.8) | 43.6 (15.4) |
| CDAI | 121 (106.4) | |
| CAI | 3 (3.3) | |
| Duration of disease, y | 5.8 (7.0) | 4.8 (4.5) |
| Behavior (CD), n (%) | ||
| Nonpenetrating, nonstricturating | 42 (53) | |
| Stricturating | 12 (15) | |
| Penetrating | 26 (33) | |
| Location (CD), n (%) | ||
| Terminal ileum | 19 (24) | |
| Colon | 18 (23) | |
| Ileocolonic | 33 (41) | |
| Upper gastrointestinal tract | 10 (13) | |
| Location (UC), n (%) | ||
| Extensive colitis | 28 (70) | |
| Left-sided colitis | 10 (25) | |
| Proctitis | 2 (5) | |
| Concomitant medication, n (%) | ||
| Steroids | 21 (26) | 12 (30) |
| Azathioprine | 13 (16) | 9 (23) |
| Methotrexate | 1 (1) | 0 (0) |
| TNF-α antagonists | 9 (11) | 0 (0) |
Data of age, CDAI, CAI, and duration of disease are given as mean (SD).
Table 2.
Patient characteristics
| n | Sex, Male/Female, n | Age, y | WBC, G/I | CRP, mg/dL | |
|---|---|---|---|---|---|
| CD active | 25 | 17/8 | 35 (12) | 10.31 (3.39) | 5.19 (7.00) |
| CD remission | 55 | 27/28 | 37 (12) | 8.70 (3.56) | 1.20 (2.40) |
| UC active | 14 | 9/5 | 42 (13) | 9.56 (3.08) | 3.93 (4.00) |
| UC remission | 26 | 13/13 | 44 (17) | 7.05 (2.63) | 0.75 (0.78) |
| Bact. | 20 | 18/2 | 35 (16) | 8.25 (2.51) | 9.42 (5.29) |
| Contr. | 28 | 13/15 | 38 (10) |
Data are given as mean (SD).
WBC indicates white blood cell count.
Quantitative HLA-DR expression
Human leukocyte antigen–DR expression on the cell surface of monocytes was significantly higher in patients with bacterial intestinal infections (median, 90,000; CI, 79,000–102,000 HLA-DR molecules per monocyte) than in healthy controls (contr.: median, 39,000; CI, 36,000–46,000; P < 0.0001) or in patients with IBD with UC (median, 30,000; CI, 30,000–38,000; P < 0.0001) or CD (median, 31,000; CI, 32,000–39,000; P < 0.0001; Fig. 1). In patients with IBD, HLA-DR expression was lower than that in healthy controls (UC vs. contr.: P < 0.05; CD vs. contr.: P < 0.01). The comparison of HLA-DR expression in patients with CD and UC revealed no statistically significant difference between these two patient populations (P > 0.85).
Fig. 1. Human leukocyte antigen–DR is highly upregulated in bacterial intestinal infections but downregulated in patients with IBD.
Quantitative HLA-DR expression on peripheral monocytes in healthy subjects (contr.) and patients with UC, CD, and bacterial intestinal infections (bact.). The error bars show medians with interquartile ranges.
When comparing patients with active (n = 39; median, 28,000; CI, 27,000–36,000) versus inactive IBD (n = 81; median, 31,000; CI, 33,000–40,000), we observed a tendency of lower HLA-DR expression in patients with active disease; this difference, however, failed the statistical significance test (P > 0.055).
Previous studies investigating HLA-DR expression in IBD patients used median channel fluorescence (MCF) values as readout. For comparison, we expressed our results in MCF values. In patients with bacterial intestinal infections, MCF values were significantly higher (median, 2,100; CI, 1,100–2,600) than those in healthy controls (contr.: median 800; CI, 700–1,100; P < 0.005) and in patients with IBD (UC: median, 600; CI, 500–700; P = 0.0005; CD: median 500; CI, 500–600; P < 0.0001). In patients with IBD, we found that MCF values were significantly lower than those in healthy controls (UC: P < 0.01; CD: P < 0.005).
Correlation of quantitative HLA-DR expression with clinical disease activity scores
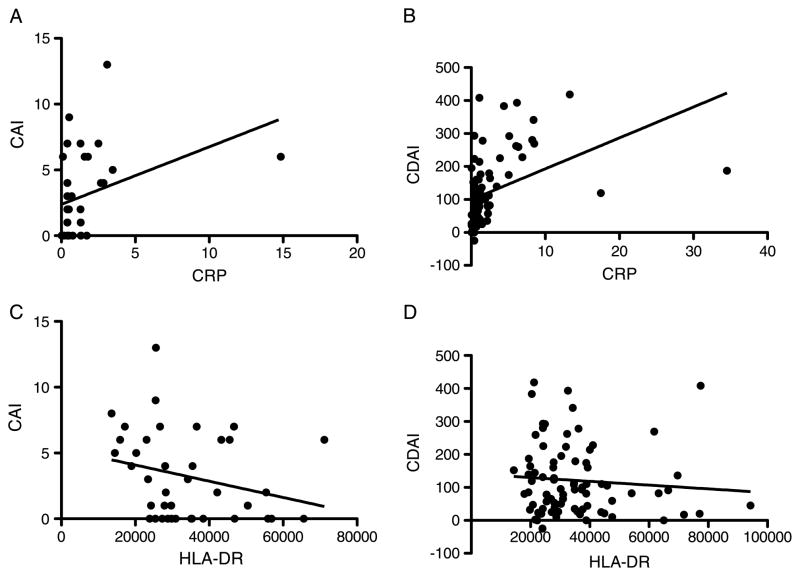
Whereas CRP levels showed a positive correlation with disease activity of both diseases (CAI: r = 0.58, P = 0.002; CDAI: r = 0.65, P < 0.0001; Fig. 2A&B), HLA-DR expression levels showed a significant negative correlation with CAI (r = −0.33, P < 0.05) but no clear correlation with CDAI (r = −0.12, P = 0.28; Fig. 2, C and D).
Fig. 2. Correlations between CRP or the quantitative expression of HLA-DR on monocytes and clinical activity indices in patients with IBD.
A, Correlation between CRP and the CAI in patients with UC. B, Correlation between CRP and the CDAI in patients with CD. C, Correlation between HLA-DR and CAI in patients with UC. D, Correlation between HLA-DR and CDAI in patients with CD.
Analysis of subgroups
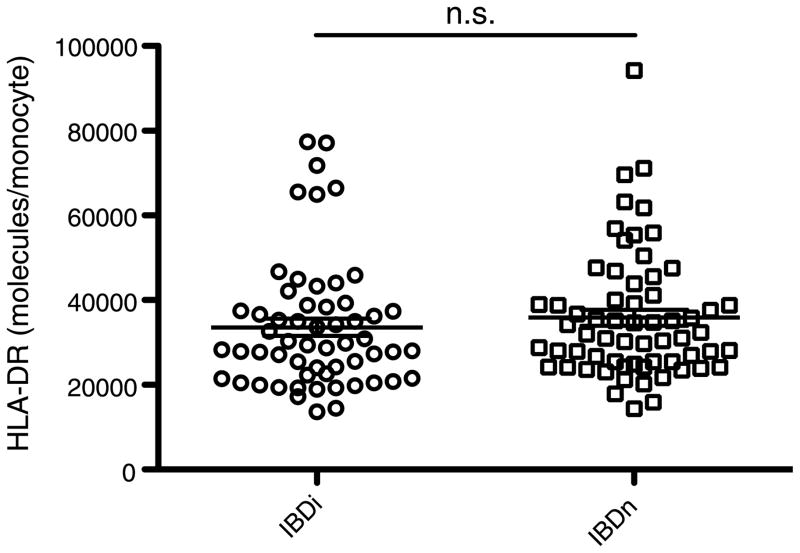
To account for possible effects of therapeutic interventions on HLA-DR expression, we analyzed HLA-DR expression levels in patient subgroups stratified according to the medications these patients received (Fig. 3). The expression of HLA-DR in the group of IBD patients who received immunomodulating drugs (IBDi; n = 57) such as steroids, azathioprine, methotrexate, or TNF-α antagonists (median, 29,000; CI, 30,000–38,000) did not differ from that in patients who did not receive such treatments (IBDn, n = 63; median, 32,000; CI, 32,000–40,000; P > 0.225). Furthermore, in both patient populations, HLA-DR expression was significantly lower than that in healthy controls (contr. vs. IBDi: P < 0.01; contr. vs. IBDn: P < 0.05) and in patients with bacterial infections (bact. vs. IBDi: P < 0.0001; bact. vs. IBDn: P < 0.0001).
Fig. 3. Expression of HLA-DR in patients with IBD is not influenced by medical treatment.
Quantitative expression of HLA-DR in subgroups of patients with IBD: patients with immunmodulatory treatment (ie, steroids, azathioprine, methotrexate, or TNF-α antagonists), designated as IBDi, and patients without such treatments (IBDn). The error bars show medians with interquartile ranges.
ROC curve analysis
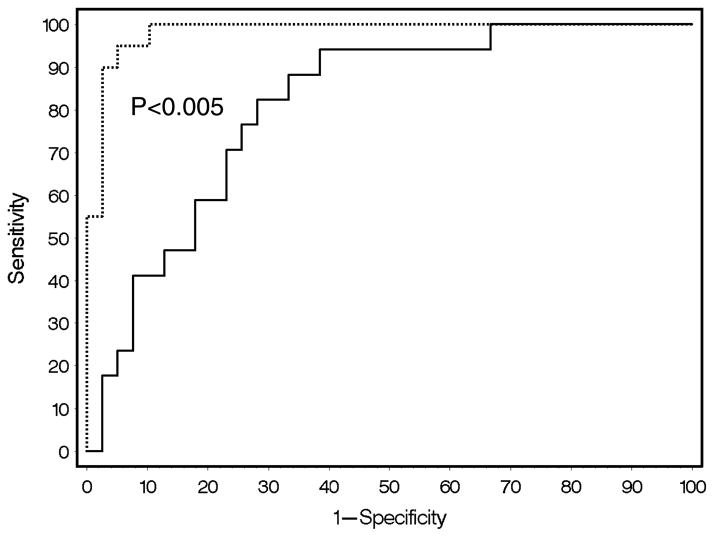
In patients with bacterial intestinal infections, HLA-DR expression was significantly higher than that in patients with active IBD (active UC: median, 26,000; CI, 21,000–40,000; P < 0.0001; active CD: median, 30,000; CI, 26,000–38,000; P < 0.0001). Receiver operating characteristic curves yielded an HLA-DR expression cutoff value of 50,000, which allows discrimination between bacterial infections and active IBD with a sensitivity of 95% and a specificity of 92%. C-reactive protein measurements yielded a cutoff level of 6.07 which indicates a comparatively low sensitivity and specificity (82% and 71%, respectively). The ROC curves are shown in Figure 4. The difference between the ROC curves was highly significant (P < 0.0036).
Fig. 4. Human leukocyte antigen–DR discriminates between patients with active IBD and patients with acute bacterial intestinal infections.
Receiver operating characteristic curves for HLA-DR (dotted line) and CRP (solid line) are shown.
Stability of HLA-DR expression in blood samples
In the clinical routine, it may be necessary to keep blood samples stored for prolonged periods until they can be processed for flow cytometric analysis, which may not be available on an around-the-clock basis. Initial studies have shown that HLA-DR increases when blood samples are kept at room temperature, possibly because of endogenous IFN-γ production. The stability of HLA-DR expression is thus a serious concern when using it as a readout marker of disease. Therefore, we addressed the stability of this readout by measuring HLA-DR expression in blood samples from consecutive, unselected outpatients. Blood samples were kept at 4°C, and measurements of HLA-DR expression were performed immediately after the blood draw and after storage in a refrigerator at 4°C to 6°C for 24 h (group A), 48 h (group B), or 72 h (group C). In stored blood samples, no significant changes in HLA-DR expression levels were observed compared with samples that were freshly processed after blood collection and immediately subjected to flow cytometric analysis (Table 3).
Table 3.
Stability of HLA-DR in blood samples
| n | Baseline | 24 | 48 h | 72 h | P | |
|---|---|---|---|---|---|---|
| Group A | 10 | 46,103 (24,636) | 51,130 (23,539) | 0.178 | ||
| Group B | 6 | 33,270 (17,795) | 41,197 (13,429) | 0.085 | ||
| Group C | 6 | 51,316 (39,959) | 52,297 (36,628) | 0.527 |
HLA-DR measurement was performed at baseline and 24 h (group A), 48 h (group B), and 72 h (group C). The statistical analysis was performed by t test. Data are given as mean (SD).
DISCUSSION
This study is the first to demonstrate that HLA-DR expression is significantly increased in the acute phase of intestinal bacterial infections. Our study indicates that in patients presenting with acute diarrhea and HLA-DR expression of more than 50,000 molecules per monocyte, bacterial infection is more likely the cause than active IBD. Conversely, we were able to show that HLA-DR is downregulated in patients with IBD and that low expression is indicative of a diagnosis of IBD. Applying this dichotomous relationship to the clinical setting could facilitate the differentiation between the two clinical conditions and aid the diagnosis of acute diarrheal diseases.
The finding of HLA-DR downregulation in IBD seems somewhat surprising as IFN-γ, which is regarded as an important proinflammatory mediator in IBD (27, 28), is a potent stimulator of HLA-DR expression. Thus, other cytokines capable of downregulating HLA-DR such as IL-1 (29) and IL-10 (30) seem to override the stimulatory effect of IFN-γ. Another possible mechanism controlling the expression of HLA-DR on monocytes of IBD patients could be repeated endotoxin exposure. Wolk et al. (31) have demonstrated that initial exposition of monocytes to endotoxin (lipopolysaccharide) provokes a massive proinflammatory response of these cells. However, repeated exposure to lipopolysaccharide results in a state of endotoxin tolerance that is paralleled by a dramatic downregulation of HLA-DR expression. In several studies, the incidence rate of endotoxinemia in IBD patients has been reported to range from 17% to 38% in patients with UC and between 31% and 94% in patients with CD, presumably because of increased intestinal permeability in these patients (32, 33). Accordingly, in the study by Pastor Rojo et al. (33), it was shown that serum lipoprotein-binding protein correlates with markers of clinical activity in patients with active IBD.
Greater than 90% of circulating monocytes are positive for CD14 and negative for CD16. However, according to the findings of Ziegler-Heitbrock et al. (34), a subpopulation of monocytes can be delineated that is positive for CD16 and considered “proinflammatory” with increased production of inflammatory cytokines. In addition, these CD14+CD16+ monocytes show an increased HLA-DR expression, which is associated with heightened capacity in antigen presentation. As shown by Blumenstein et al. (35), the CD14+CD16+ subpopulation expands after bacteremia. It is possible that the differences of monocytic HLA-DR expression we have observed are due to changes in the numbers of CD14+/CD16+ monocytes. This seems, however, unlikely because CD14+/CD16+ monocytes are negative for CD64 and were excluded from analysis because of the properties of the monocyte assay kit we used. This kit consisted of antibodies that recognize CD14 and CD64 simultaneously, and therefore our measurements were restricted to CD64+ monocytes (ie, CD14+CD16− monocytes). Nevertheless, understanding the response of CD14+/CD16+ monocytes to inflammatory intestinal diseases will certainly require further studies.
A drawback of our current study is that patients with an established diagnosis of IBD and complicating bacterial superinfection were not investigated because of a low incidence of such conditions in our study population. In the last decade, an alarming increase in bowel disease associated with C. difficile has been observed. Patients with IBD experiencing infections with C. difficile are particularly prone to develop serious complications, including toxic megacolon, colonic perforation, sepsis, and death (36, 37). Further studies are warranted to investigate the utility of HLA-DR expression measurements as diagnostic markers of C. difficile infections.
In contrast to a study by Gardiner et al. (10) that demonstrated a downregulation of HLA-DR expression in patients with active UC, but not in patients with quiescent UC or CD, we found downregulated HLA-DR expression in both conditions, irrespective of disease activity. The reasons for these differences may be several-fold. First, the sample size in the study by Gardiner et al. (10) was very small (11 patients with UC and seven patients with CD), and particularly patients with CD were underrepresented. Second, patients aged up to 73 years were included in the study by Gardiner et al., but the age distribution within both patient groups was not stated. Because HLA-DR expression is diminished in elderly subjects (38), this could be another reason for the differences between our results. Third, HLA-DR expression was not quantitatively analyzed in terms of molecules per cell in the study of Gardiner et al, but by percentage of HLA-DR–positive monocytes and MCF. These variables, however, are influenced by the instrument settings of the flow cytometer. We believe that the quantitative approach we chose, using beads with defined amounts of fluorochrome molecules to calibrate fluorescence reading, may be more accurate to avoid possible interassay variances.
According to previous experimental studies with different cell lines or blood samples from patients with malignancies, HLA-DR expression was not altered by steroids (29), azathioprine, or TNF-α (39). Notably, regarding the effects of steroids on HLA-DR expression, divergent results have been obtained by others (40, 41). In our study, HLA-DR expression levels in the subgroups of patients who received immunosuppressive or steroid treatments were not significantly different, as compared with patients who did not receive such treatments. We therefore conclude that HLA-DR expression levels in IBD patients are influenced primarily by inflammatory mechanisms rather than by the effects of anti-inflammatory medications. This finding is important because it supports our conclusion that HLA-DR expression can serve as a robust diagnostic clinical marker. To further validate this concept, longitudinal studies in individual patients receiving different treatments will be necessary.
A potential disadvantage of HLA-DR measurements as a diagnostic tool lies in the instability of HLA-DR. After pre-analytical storage of blood for 2 h, HLA-DR expression increases presumably because of endogenous IFN-γ production. Döcke et al. (42) demonstrated that the stability of HLA-DR expression is preserved up to 24 h by storage at 4°C. However, the lowest interassay variance was obtained when processing of blood samples was performed within 4 h after blood samples were drawn (42). Our experiments showed that sample storage at 4°C provides sufficient stability of HLA-DR to allow storage for up to 72 h, if necessary.
The kinetics of HLA-DR expression in the course of an acute inflammatory response needs to be considered for data interpretation. In the present study, blood samples were collected within 48 h after the onset of symptoms of acute diarrhea. We observed that HLA-DR upregulation rapidly decreases after this time interval (data not shown). Therefore, the time frame when HLA-DR expression can serve as a robust diagnostic marker for intestinal bacterial infections may be limited to the first 48 h after the onset of symptoms.
In conclusion, we propose that the quantitative measurement of HLA-DR offers a novel diagnostic tool in patients with diarrheal diseases, which is, however, restricted to larger hospitals by the availability of flow cytometry. The prognostic impact of this biomarker needs to be addressed in further studies.
Footnotes
This work was performed at Hietzing Hospital, Vienna, Austria
The authors have no conflicts of interest to declare.
References
- 1.DuPont HL. Review article: infectious diarrhoea. Aliment Pharmacol Ther. 1994;8:3–13. doi: 10.1111/j.1365-2036.1994.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 2.Herrlinger KR, Dittmann R, Weitz G, Wehkamp J, Ludwig D, Schwab M, Stange EF, Fellermann K. Serum procalcitonin differentiates inflammatory bowel disease and self-limited colitis. Inflamm Bowel Dis. 2004;10:229–233. doi: 10.1097/00054725-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lin RY, Astiz ME, Saxon JC, Rackow EC. Altered leukocyte immunophenotypes in septic shock. Studies of HLA-DR, CD11b, CD14, and IL-2R expression. Chest. 1993;104:847–853. doi: 10.1378/chest.104.3.847. [DOI] [PubMed] [Google Scholar]
- 4.Ditschkowski M, Kreuzfelder E, Rebmann V, Ferencik S, Majetschak M, Schmid EN, Obertacke U, Hirche H, Schade UF, Grosse-Wilde H. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229:246–254. doi: 10.1097/00000658-199902000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimmel H, Luschin U, Kohrer K, Anzur C, Vevera D, Spittler A. Clinical outcome of critically ill patients cannot be defined by cutoff values of monocyte human leukocyte antigen-DR expression. Shock. 2012;37:140–144. doi: 10.1097/SHK.0b013e31823f1866. [DOI] [PubMed] [Google Scholar]
- 6.Shirakawa F, Yamashita U, Suzuki H. Decrease in HLA-DR-positive monocytes in patients with systemic lupus erythematosus (SLE) J Immunol. 1985;134:3560–3562. [PubMed] [Google Scholar]
- 7.Steinbach F, Henke F, Krause B, Thiele B, Burmester GR, Hiepe F. Monocytes from systemic lupus erythematosus patients are severely altered in phenotype and lineage flexibility. Ann Rheum Dis. 2000;59:283–288. doi: 10.1136/ard.59.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Highton J, Carlisle B, Palmer DG. Changes in the phenotype of monocytes/macrophages and expression of cytokine mRNA in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 1995;102:541–546. doi: 10.1111/j.1365-2249.1995.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller RB, Skapenko A, Wendler J, Schuch F, Kalden JR, Schulze-Koops H. MHC class II expression on myeloid cells inversely correlates with disease progression in early rheumatoid arthritis. Rheumatology. 2007;46:931–933. doi: 10.1093/rheumatology/kem025. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner KR, Crockard AD, Halliday MI, Rowlands BJ. Class II major histocompatibility complex antigen expression on peripheral blood monocytes in patients with inflammatory bowel disease. Gut. 1994;35:511–516. doi: 10.1136/gut.35.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkstra G, Zandvoort AJH, Kobold ACM, de Jager-Krikken A, Heeringa P, van Goor H, van Dullemen HM, Tervaert JWC, van de Loosdrecht A, Moshage H, et al. Increased expression of inducible nitric oxide synthase in circulating monocytes from patients with active inflammatory bowel disease. Scand J Gastroenterol. 2002;37:546–554. doi: 10.1080/00365520252903099. [DOI] [PubMed] [Google Scholar]
- 12.Liu ZX, Hiwatashi N, Noguchi M, Toyota T. Increased expression of costimulatory molecules on peripheral blood monocytes in patients with Crohn’s disease. Scand J Gastroenterol. 1997;32:1241–1246. doi: 10.3109/00365529709028154. [DOI] [PubMed] [Google Scholar]
- 13.Mangan DF, Wahl SM, Sultzer BM, Mergenhagen SE. Stimulation of human monocytes by endotoxin-associated protein: inhibition of programmed cell death (apoptosis) and potential significance in adjuvanticity. Infect Immun. 1992;60:1684–1686. doi: 10.1128/iai.60.4.1684-1686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diament D, Brunialti MKC, Romero EC, Kallas EG, Salomao R. Peripheral blood mononuclear cell activation induced by Leptospira interrogans glycolipoprotein. Infect Immun. 2002;70:1677–1683. doi: 10.1128/IAI.70.4.1677-1683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahl SM, McCartney-Francis N, Hunt DA, Smith PD, Wahl LM, Katona IM. Monocyte interleukin 2 receptor gene expression and interleukin 2 augmentation of microbicidal activity. J Immunol. 1987;139:1342–1347. [PubMed] [Google Scholar]
- 16.Jørgensen PF, Wang JE, Almlöf M, Thiemermann C, Foster SJ, Solberg R, Aasen AO. Peptidoglycan and lipoteichoic acid modify monocyte phenotype in human whole blood. Clin Diagn Lab Immunol. 2001;8:515–521. doi: 10.1128/CDLI.8.3.515-521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saikh KU, Kissner TL, Sultana A, Ruthel G, Ulrich RG. Human monocytes infected with Yersinia pestis express cell surface TLR9 and differentiate into dendritic cells. J Immunol. 2004;173:7426–7434. doi: 10.4049/jimmunol.173.12.7426. [DOI] [PubMed] [Google Scholar]
- 18.Basham TY, Merigan TC. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983;130:1492–1494. [PubMed] [Google Scholar]
- 19.Livingston DH, Loder PA, Kramer SM, Gibson UE, Polk HC. Interferon gamma administration increases monocyte HLA-DR antigen expression but not endogenous interferon production. Arch Surg (Chicago, Ill: 1960) 1994;129:172–178. doi: 10.1001/archsurg.1994.01420260068009. [DOI] [PubMed] [Google Scholar]
- 20.Schiller JH, Hank J, Storer B, Borchert AA, Moore KH, Albertini M, Bechhofer R, Wesley O, Brown RR, Bastin AM. A direct comparison of immunological and clinical effects of interleukin 2 with and without interferon-alpha in humans. Cancer Res. 1993;53:1286–1292. [PubMed] [Google Scholar]
- 21.Gerrard TL, Dyer DR, Mostowski HS. IL-4 and granulocyte-macrophage colony-stimulating factor selectively increase HLA-DR and HLA-DP antigens but not HLA-DQ antigens on human monocytes. J Immunol. 1990;144:4670–4674. [PubMed] [Google Scholar]
- 22.Al-Banna N, Raghupathy R, Albert MJ. Correlation of proinflammatory and anti-inflammatory cytokine levels with histopathological changes in an adult mouse lung model of Campylobacter jejuni infection. Clin Vaccine Immunol. 2008;15:1780–1787. doi: 10.1128/CVI.00193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam D, Bardhan PK, Lindberg AA, Christensson B. Shigella infection induces cellular activation of T and B cells and distinct species-related changes in peripheral blood lymphocyte subsets during the course of the disease. Infect Immun. 1995;63:2941–2949. doi: 10.1128/iai.63.8.2941-2949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida Y, Maegawa T, Kondo T, Kimura A, Iwakura Y, Nakamura S, Mukaida N. Essential involvement of IFN-gamma in Clostridium difficile toxin A–induced enteritis. J Immunol. 2004;172:3018–3025. doi: 10.4049/jimmunol.172.5.3018. [DOI] [PubMed] [Google Scholar]
- 25.van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Stange E, Travis S, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Flejou J, Herfarth H, Hommes D, et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. YGAST. 2011;140:1756.e1–1767.e1. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Autschbach F, Giese T, Gassler N, Sido B, Heuschen G, Heuschen U, Zuna I, Schulz P, Weckauf H, Berger I, et al. Cytokine/chemokine messenger-RNA expression profiles in ulcerative colitis and Crohn’s disease. Virchows Arch. 2002;441:500–513. doi: 10.1007/s00428-002-0684-z. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe Y, Lee S, Allison AC. Control of the expression of a class II major histocompatibility gene (HLA-DR) in various human cell types: down-regulation by IL-1 but not by IL-6, prostaglandin E2, or glucocorticoids. Scand J Immunol. 1990;32:601–609. doi: 10.1111/j.1365-3083.1990.tb03202.x. [DOI] [PubMed] [Google Scholar]
- 30.de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, Velde te A, Figdor C, Johnson K, Kastelein H, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolk K, Kunz S, Crompton NEA, Volk H-D, Sabat R. Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. J Biol Chem. 2003;278:18030–18036. doi: 10.1074/jbc.M207714200. [DOI] [PubMed] [Google Scholar]
- 32.Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, Maxwell RJ, Rowlands BJ. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastor Rojo Ó, López San Román A, Albéniz Arbizu E, la Hera Martínez de A, Ripoll Sevillano E, Albillos Martínez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:269–277. doi: 10.1002/ibd.20019. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 35.Blumenstein M, Boekstegers P, Fraunberger P, Andreesen R, Ziegler-Heitbrock HL, Fingerle-Rowson G. Cytokine production precedes the expansion of CD14+ CD16+ monocytes in human sepsis: a case report of a patient with self-induced septicemia. Shock. 1997;8:73–75. doi: 10.1097/00024382-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Ananthakrishnan AN, Issa M, Binion DG. Clostridium difficile and inflammatory bowel disease. Med Clin North Am. 2010;94:135–153. doi: 10.1016/j.mcna.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–1450. doi: 10.1111/j.1572-0241.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 38.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiller JH, Storer BE, Witt PL, Alberti D, Tombes MB, Arzoomanian R, Proctor RA, McCarthy D, Brown RR, Voss SD. Biological and clinical effects of intravenous tumor necrosis factor-alpha administered three times weekly. Cancer Res. 1991;51:1651–1658. [PubMed] [Google Scholar]
- 40.Fischer A, König W. Influence of cytokines and cellular interactions on the glucocorticoid-induced Ig (E, G, A, M) synthesis of peripheral blood mononuclear cells. Immunology. 1991;74:228–233. [PMC free article] [PubMed] [Google Scholar]
- 41.Kim O, Monsel A, Bertrand M, Coriat P, Cavaillon J-M, Adib-Conquy M. Differential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammation. Crit Care. 2010;14:R61. doi: 10.1186/cc8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Döcke W-D, Höflich C, Davis KA, Röttgers K, Meisel C, Kiefer P, Weber SU, Hedwig-Geissing M, Kreuzfelder E, Tschentscher SD, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem. 2005;51:2341–2347. doi: 10.1373/clinchem.2005.052639. [DOI] [PubMed] [Google Scholar]