Abstract
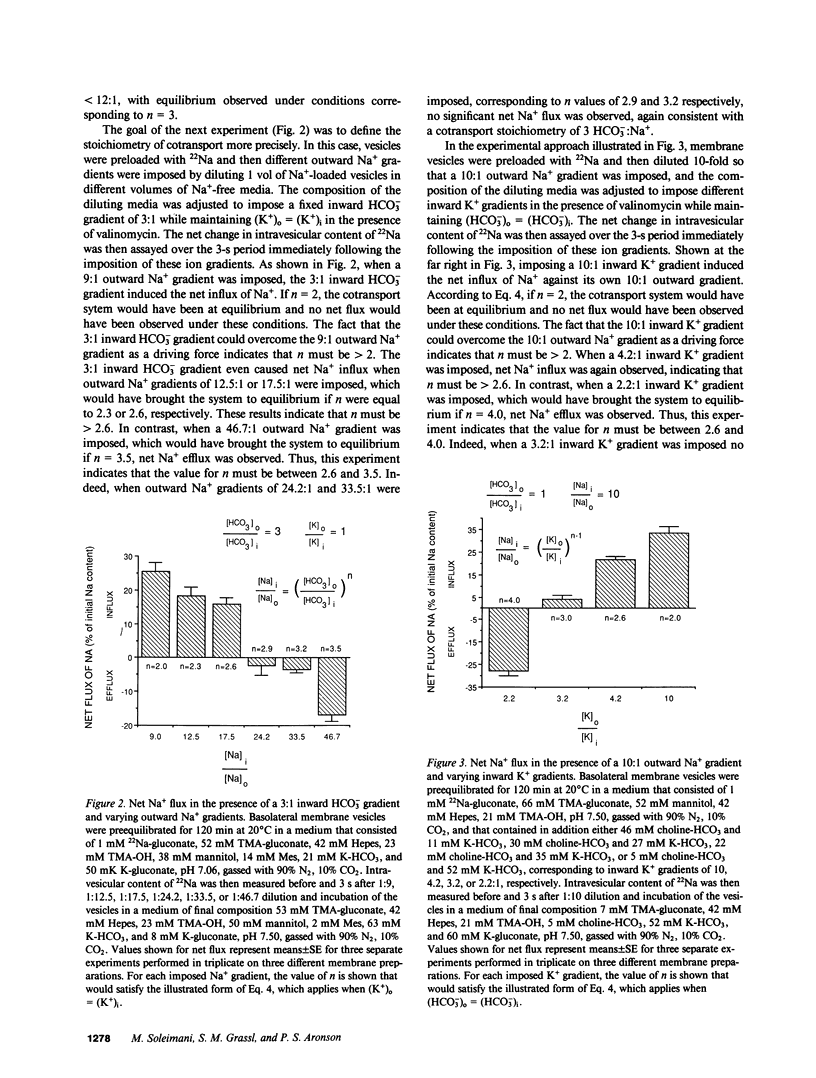
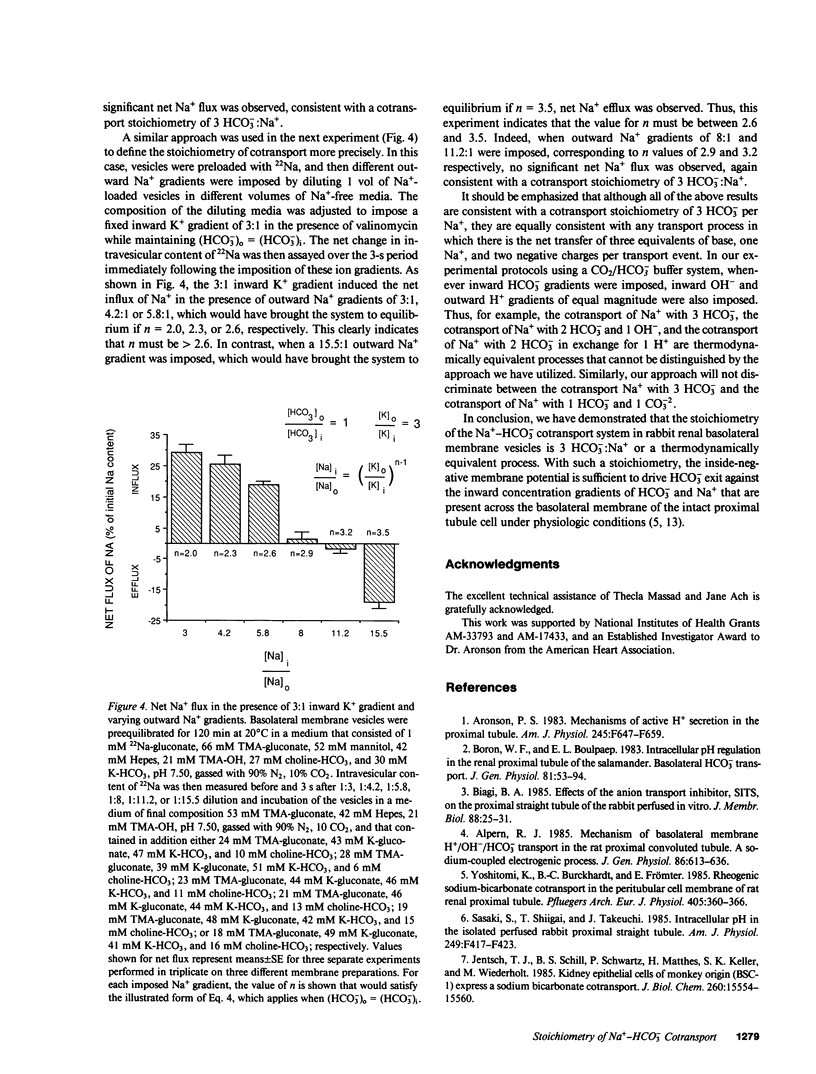
The major pathway for HCO3- transport across the basolateral membrane of the proximal tubule cell is electrogenic Na+-HCO3- cotransport. In this study, we have determined the stoichiometry of the Na+-HCO3- cotransport system in basolateral membrane vesicles that were isolated from rabbit renal cortex by Percoll gradient centrifugation. When the membrane potential is approximated by the Nernst potential for K+, as in the presence of the K+ ionophore valinomycin, equilibrium thermodynamics predicts that the Na+-HCO3- cotransport system should come to equilibrium and mediate no net flux when (Na)i/(Na)o = [(HCO3)o/(HCO3)i]n[(K)o/(K)i]n-1, where n is the HCO3-:Na+ stoichiometry. Our experimental approach was to impose transmembrane Na+, HCO3-, and K+ gradients of varying magnitude and direction, and then to measure the net flux of Na+ over the subsequent 3-s period. In this way, we could determine the conditions for equilibrium of the transport system and thereby calculate n. The results of these experiments indicate that the value of n is greater than 2.6 and less than 3.5, consistent with a stoichiometry of 3 HCO3-:1 Na+, or a thermodynamically equivalent process. Based on reported intracellular potentials and ion activities, this value for the stoichiometry indicates that the inside-negative membrane potential is sufficient to drive HCO3- exit against the inward concentration gradients of HCO3- and Na+ that are present across the basolateral membrane of the intact proximal tubule cell under physiologic conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Chambers M. Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest. 1986 Aug;78(2):502–510. doi: 10.1172/JCI112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Biagi B. A. Effects of the anion transport inhibitor, SITS, on the proximal straight tubule of the rabbit perfused in vitro. J Membr Biol. 1985;88(1):25–31. doi: 10.1007/BF01871210. [DOI] [PubMed] [Google Scholar]
- Biagi B. A., Sohtell M. Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule. Am J Physiol. 1986 Feb;250(2 Pt 2):F267–F272. doi: 10.1152/ajprenal.1986.250.2.F267. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Hamilton K. L., Johnson K. E. Intracellular acidosis blocks the basolateral Na-K pump in rabbit urinary bladder. Am J Physiol. 1984 Dec;247(6 Pt 2):F946–F954. doi: 10.1152/ajprenal.1984.247.6.F946. [DOI] [PubMed] [Google Scholar]
- Grassl S. M., Aronson P. S. Na+/HCO3-co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J Biol Chem. 1986 Jul 5;261(19):8778–8783. [PubMed] [Google Scholar]
- Jentsch T. J., Keller S. K., Koch M., Wiederholt M. Evidence for coupled transport of bicarbonate and sodium in cultured bovine corneal endothelial cells. J Membr Biol. 1984;81(3):189–204. doi: 10.1007/BF01868713. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Schill B. S., Schwartz P., Matthes H., Keller S. K., Wiederholt M. Kidney epithelial cells of monkey origin (BSC-1) express a sodium bicarbonate cotransport. Characterization by 22Na+ flux measurements. J Biol Chem. 1985 Dec 15;260(29):15554–15560. [PubMed] [Google Scholar]
- Jentsch T. J., Schwartz P., Schill B. S., Langner B., Lepple A. P., Keller S. K., Wiederholt M. Kinetic properties of the sodium bicarbonate (carbonate) symport in monkey kidney epithelial cells (BSC-1). Interactions between Na+, HCO-3, and pH. J Biol Chem. 1986 Aug 15;261(23):10673–10679. [PubMed] [Google Scholar]
- Jentsch T. J., Stahlknecht T. R., Hollwede H., Fischer D. G., Keller S. K., Wiederholt M. A bicarbonate-dependent process inhibitable by disulfonic stilbenes and a Na+/H+ exchange mediate 22Na+ uptake into cultured bovine corneal endothelium. J Biol Chem. 1985 Jan 25;260(2):795–801. [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Determination of the coupling ratio for Na+ -H+ exchange in renal microvillus membrane vesicles. Biochim Biophys Acta. 1982 Jul 14;689(1):161–164. doi: 10.1016/0005-2736(82)90200-0. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Shigai T., Takeuchi J. Intracellular pH in the isolated perfused rabbit proximal straight tubule. Am J Physiol. 1985 Sep;249(3 Pt 2):F417–F423. doi: 10.1152/ajprenal.1985.249.3.F417. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Stoichiometric studies of the renal outer cortical brush border membrane D-glucose transporter. J Membr Biol. 1982;67(1):73–80. doi: 10.1007/BF01868649. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Frömter E. Cell pH of rat renal proximal tubule in vivo and the conductive nature of peritubular HCO3- (OH-) exit. Pflugers Arch. 1984 Nov;402(3):300–305. doi: 10.1007/BF00585513. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Frömter E. How big is the electrochemical potential difference of Na+ across rat renal proximal tubular cell membranes in vivo? Pflugers Arch. 1985;405 (Suppl 1):S121–S126. doi: 10.1007/BF00581792. [DOI] [PubMed] [Google Scholar]



