Abstract
Epidemiologic and preclinical data support the oral-cancer prevention potential of green tea extract (GTE). We randomly assigned patients with high-risk oral premalignant lesions (OPLs) to receive GTE at 500 mg/m2, 750 mg/m2, or 1000 mg/m2 or placebo TID for 12 weeks, evaluating biomarkers in baseline and 12-week biopsies. The OPL clinical response rate was higher in all GTE arms (n=28; 50%) versus placebo (n=11; 18.2%; p=0.09) but did not reach statistical significance. However, the 2 higher-dose GTE arms (58.8% [750, 1000 mg/m2], 36.4% [500 mg/m2], and 18.2%, [placebo], p=0.03) had higher responses, suggesting a dose-response effect. GTE treatment also improved histology (21.4% versus 9.1%, p=0.65), though not statistically significant. GTE was well-tolerated although higher doses increased insomnia/nervousness but produced no grade-4 toxicity. Higher mean baseline stromal VEGF correlated with a clinical (p=0.04) but not histologic response. Baseline scores of other biomarkers (epithelial VEGF, p53, Ki-67, cyclin D1, and p16 promoter methylation) were not associated with a response or survival. Baseline p16 promoter methylation (n=5) was associated with a shorter cancer-free survival. Stromal VEGF and cyclin D1 expression were downregulated in clinically responsive GTE patients and upregulated in non-responsive patients at 12 weeks (versus at baseline). An extended (median 27.5 months) follow-up showed a median time to oral cancer of 46.4 months. GTE may suppress OPLs, in part through reducing angiogenic stimulus (stromal VEGF). Higher doses of GTE may improve short-term (12 week) OPL outcome. The present results support longer-term clinical testing of GTE for oral cancer prevention.
Keywords: green tea extract, oral premalignant lesions, chemoprevention
Over 170,000 people develop oral cancer annually worldwide (1), and oral cancer is associated with severe morbidity and a 5-year overall survival rate of less than 50% (2). Oral carcinogenesis is a multi-step process involving the accumulation of genetic changes leading to progressive dysplasia, unregulated cell growth, and cancer. Oral cancer frequently arises from oral premalignant lesions (OPLs), which have a 2%–3% overall risk of developing into carcinoma (3). This risk increases to 17.5% within 8 years for dysplastic or high-risk OPLs (4). The reported cancer risks of OPLs also increase in association with erythroplasia (erythroleukoplakia) and proliferative verrucous hyperplasia, a history of never smoking, polysomy (5), early chromosomal alterations (9p, 3p, and 17p; ref (6–8)), and inactivation of p16INK4a (9).
Green tea extract (GTE) contains high amounts of polyphenols including epigallocatechin 3-gallate (EGCG), which inhibits carcinogenesis in preclinical models and potentially prevents oral cancer (10, 11). Although EGCG is the most-abundant and best-studied of the tea polyphenols (12), it appears that preventive effects are stronger with a mixture of tea catechins, such as polyphenon E (a decaffeinated green tea catechin mixture) or GTE, than with EGCG alone (13, 14). In preclinical models, EGCG treatment arrested cells in the G0/G1 phase; downregulated cyclin D1 (15); increased p14ARF and/or p16 protein levels and thus stabilized p53 and regulated apoptosis (16); and blocked angiogenesis by decreasing phosphorylation of vascular endothelial growth factor receptor (VEGFR; ref. (17)) and inhibiting VEGF secretion by tumor cells (18).
Preliminary evidence from numerous studies supports the preventive potential of tea and tea polyphenols (10, 19, 20). Asian and other epidemiologic studies have suggested a potential protective effect of green tea against epithelial malignancies (21). A prospective cohort survey of over 8,552 Japanese individuals showed that drinking over 10 daily cups (120 mL each) of green tea reduced malignancy (22). Oral GTE (23) had a reasonable safety profile in a phase I trial by members of our group involving patients with advanced pretreated cancer, causing mainly caffeine-related gastrointestinal (abdominal bloating, sore throat, nausea), cardiovascular (palpitations), and neurologic (insomnia, paresthesia, restlessness) side effects. The phase I investigators recommended a maximum dose of 1000 mg/m2 (equivalent to 7 to 8 cups of green tea given 3 times daily (TID; ref.(23)). Although this phase I trial (23) did not show a significant anti-cancer benefit, GTE is nevertheless an ideal agent for chemoprevention study because it has a low cost and toxicity and is readily available.
Successful chemopreventive agents must be effective and safe enough for extended use since short-term interventions are not expected to substantially reduce cancer risk over the longer term. Most active chemopreventive agents studied to date are only likely to delay malignant transformation (24). For example, although high-dose 13-cis-retinoic acid reversed OPLs (25), its chemopreventive usefulness was limited by toxicity that precluded long-term use. GTE toxicity in the initial clinical reports (23) and anti-tumor effects in preclinical studies support its safe use for an extended period of time and provided a reasonable scientific rationale for the randomized, placebo-controlled phase II chemoprevention trial of GTE in the setting of OPLs that we report here.
Patients and Methods
Patient selection
The major inclusion criterion was the presence of one or more histologically confirmed, bidimensionally measurable OPLs that could be sampled by biopsy and had at least one of the following high-risk features of malignant transformation: harboring at least mild dysplasia, located in a high-risk area (i.e., floor of mouth, ventrolateral tongue, and soft palate), significant extent of OPL tissue involvement, and presence of symptoms (pain or substantial discomfort). Additional inclusion criteria included age between ≥ 18 and ≤ 75; Zubrod performance status < 2; adequate hematologic, liver and renal function; adequate cardiac function (defined as no clinically significant electrocardiogram abnormality, unstable atrial or ventricular arrhythmias requiring medical control, or ischemic event experienced within the prior 6 months); negative pregnancy test in females of childbearing potential within 7 days prior to first dose of study medication; use of effective contraceptive method while on the trial; and written informed consent for participation. The main exclusion criteria were as follows: known hypersensitivity to oral GTE or its analogs; use of prior investigational agents within 30 days; any serious intercurrent illness; history of prior malignancy with less than a 1-year disease-free interval prior to study entry; lactating females; patients who were not able to abstain from the consumption of methylxanthine-containing products (including coffee, tea, chocolate, caffeinated soft drinks, and theophylline) and decaffeinated tea.
This phase II placebo-controlled, dose-finding trial was conducted exclusively at The University of Texas M. D. Anderson Cancer Center, approved by the M. D. Anderson Institutional Review Board, and conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Treatment plan
Upon enrollment, patients provided a medical history (including complete alcohol, tobacco, and caffeine consumption history, use of concomitant medications and adverse symptoms) and underwent a physical examination, clinical laboratory tests, color photography, bi-dimensional measurements, and a biopsy of the OPL. Smoking and alcohol cessation counseling was provided. All biopsy slides were reviewed for confirmation of the diagnosis and suitability for biomarker analysis. During active treatment, both safety and clinical efficacy evaluations occurred every 4 weeks. Histologic and biomarker evaluations were added to the other evaluations at baseline and after 3 months (12 weeks) of therapy. Plasma concentrations of EGCG and caffeine were measured at baseline, before each TID dose on the last day of intervention, and 3 hours after the last dose on the last day of intervention (week 12) to identify any correlations between efficacy and/or toxicity and EGCG or caffeine plasma concentrations.
Eligible patients were equally randomized to one of the four following treatment arms in a double-blinded manner: GTE (500 mg/m2), GTE (750 mg/m2), GTE (1000 mg/m2), or placebo. Capsules of a GTE formulation called THEA-FLAN 30 ARG were supplied by ITO EN, LTD (Tokyo, Japan); each capsule contained 350 mg of GTE. The first batch of study GTE capsules (lot number 00-09-26A) was manufactured on September 26, 2000, stable for at least 45 months, and replaced with a new batch (lot number 04-03-30A) in June 2004. The composition of GTE has been reported previously (23). GTE and placebo capsules were supplied to the pharmacy in blister packs containing 10 capsules each that were sealed in aluminum foil bags containing 10 blister packs each. After removal from the foil bag, capsules were stored in a closed container at 4° C.
In each arm, the dose of GTE or placebo was given orally TID after meals for 12 weeks. No intra-patient dose-escalations were allowed. The randomization was performed with the Pocock-Simon dynamic allocation scheme (26) to balance three prognostic factors in each of the four arms. The prognostic factors were tobacco use (current, stopped, never), alcohol use (current, stopped, never), and tea use (0 to 4 eight-ounce cups daily or 5 or more eight-ounce cups daily). GTE dosage calculations were based on the participant’s body surface area (BSA) according to the following formula: BSA (m2) = weight (kg)0.425 × height (cm)0.725 × 0.007184. The calculated dose was adjusted downward to the closest dose that could be administered using one or more 350 mg capsules. Dosing was based on a prior M. D. Anderson phase I trial (23) of dose levels from 500 mg/m2 to 5050 mg/m2, which identified a maximum tolerated dose of 4200 mg/m2 once daily and an optimal dose of 1000 mg/m2 given TID for phase II testing. Patients received a 1-month supply of GTE every 4 weeks and were instructed to record compliance in a diary card and to return any unused medication. Study monitors conducted diary checks and pill counts every 4 weeks during active therapy. A patient was considered compliant if the 4-week pill counts determined that 90% of the scheduled doses of drug or placebo were taken during the trial.
Toxicity prior to starting each 4-week cycle was evaluated according to the National Cancer Institute Revised Common Toxicity Criteria (CTC) version 2.0. For non-hepatic grade-2 or −3 toxicity, study medication was withheld until the toxicity resolved to ≤ grade 1, when GTE was restarted at a 25% reduced dose; for grade-1 to −3 increased serum ALT, GTE was withheld until normalization, when the full dose was resumed. Any grade-4 or life-threatening toxicity required study drug discontinuation. Subjects were discontinued from study if they withdrew consent; requested discontinuation; had evidence of histologic or clinical progression of disease, persistent grade-2 or greater elevation in ALT, non-hepatic unacceptable toxicity, or an intercurrent illness necessitating premature termination; were pregnant or failed to use adequate birth control; were unwilling to comply with protocol requirements and follow up; or died. At the time of discontinuation or as soon thereafter as possible, patients who prematurely discontinued treatment underwent all evaluations and procedures (if possible) that otherwise would have been conducted at the scheduled end of treatment (week 12).
Study endpoints and statistical analysis
The primary endpoint was clinical and histologic response of high-risk OPLs at 12 weeks to three different doses of GTE compared with placebo. Secondary endpoints were the qualitative and quantitative toxicities of GTE, effects of treatment on the expression of biomarkers, and any potential correlation between treatment efficacy and/or toxicity with plasma concentrations of EGCG or caffeine. Efficacy was analyzed on an intent-to-treat basis. Patients with evaluable disease were defined as having bidimensionally measurable lesions. Lesions with clearly defined margins were measured in two dimensions and then the surface area was calculated by multiplying the longest diameter measurement by the greatest perpendicular diameter measurement. Standard punch biopsies were performed by the same dental oncologist (J. Martin) for all patients on this study. The clinical responses were assessed according to previously described criteria (27) for bidimensional measurements: disappearance of all lesions was considered a complete response (CR); 50% or greater decrease in the sum of products of diameters of all measured lesions was considered a partial response (PR); and increase by 25% or greater in size of lesions or appearance of new lesions or progression to invasive cancer was considered progression of disease (PD). Any response that did not meet criteria for CR, PR, or PD was considered stable disease (SD). The histologic response was assessed according to the following categories: CR was defined as a complete reversal of premalignancy to normal epithelium with no new lesions, PR was defined as an improvement in degree of maturation of epithelium with no new lesions and no progression of any lesion, SD was defined as no change in histology and no appearance of new lesions or progression of any lesion. Histologic PD was defined as progression from hyperplasia and/or hyperkeratosis to dysplasia, or from a lower to a higher degree of dysplasia or invasive carcinoma, or appearance of any new lesions. The sample size comprised 9 patients per treatment arm, for a total of 36 evaluable patients. We based this sample size on historical estimates of response to chemoprevention agents such as retinoids aiming at a conservative estimate of 20% response rate (25, 27). The minimum sample size was calculated in order to provide a preliminary assessment of the dose effect. Using nine patients per treatment arm would rule out a 20% or better response rate with 86% power if no response (or zero response) was observed for a specified treatment arm. Although this trial was not designed or powered to detect statistically significant differences in response between treatment groups, comparison of the incidence of complete or partial response versus stable or progressive disease was made between the treatment groups using Fisher’s exact test. Concordance between histologic and clinical response was assessed within each treatment (pooled GTE or placebo) using Bowker’s test. Response rates were summarized and compared by smoking, alcohol consumption status, and baseline histology. Baseline biomarker data were summarized and compared and correlated with smoking status, alcohol use, histology groups, and clinical and histologic response by Wilcoxon rank sum test or Kruskal-Wallis test. The difference in biomarker modulation was compared between responders versus non-responder groups and among treatment arms using Wilcoxon rank sum test or Kruskal-Wallis test. Logistic regression was used to assess the effects of dose, histology and baseline biomarkers on response. Kaplan-Meier curves were used to demonstrate the oral cancer free survival in the different treatment arms and a log-rank test was used to test the difference between the groups. Both univariate and multivariate Cox models were used to assess the effect of covariates, such as histology and biomarkers, on oral cancer free survival. All tests were two-sided and were considered statistically significant if p≤ 0.05. All computations were carried out in SAS 9.1 (Cary, NC) and S-plus 7.0 for Windows (Insightful Corporation).
Biomarker assays
Immunohistochemistry
Tissue biopsies (at baseline and 12 weeks) were assessed by immunohistochemistry for the following biomarkers: cytoplasmic expression of vascular endothelial growth factor (VEGF) in the epithelium and also in the stroma, nuclear staining for p53, Ki-67, and cyclin D1 (CD1) in normal or premalignant epithelium (in basal, suprabasal or superficial layers using the most histologically advanced area). Biopsies were processed and IHC was carried out per standard protocol and validated antibodies. Unstained 4-µM thin slides were prepared from formalin-fixed and paraffin-embedded tissue sections for immunohistochemical analyses. The slides were deparaffinized, hydrated, and heated in a steamer for 10 minutes with 10 mMol/L of sodium citrate (pH 6.0) for antigen retrieval, and washed in Tris buffer. Slides were treated with 3% H2O2 in methanol and non-specific-protein blocking and were incubated with the primary and then second antibodies. All antibody information and incubation conditions are shown in Table 1. Diaminobenzidine chromogen was subsequently applied for 5 minutes and the slides were then counterstained with hematoxylin. The immunohistochemistry staining was evaluated independently by two pathologists (X. Tang and I.I. Wistuba, who were both blinded to the clinical and molecular variables) using light microscopy (200× magnification). The staining intensity (range 0–3) and percentage of positive cells (range 1–100) have been previously documented and described in prior publications (28). A cytoplasmic immunohistochemical stain was scored by multiplying the intensity and extension for VEGF (scores ranged from 0 to 300) or the nuclear stain was scored as percentage of positive cells (0–100%) for CD1, Ki-67 and p53 in the basal and suprabasal epithelium. The stain intensity was defined as follows: 0, no appreciable staining; 1, barely detectable staining compared with the stromal elements; 2, readily appreciable staining; 3, dark brown staining. In addition, the VEGF expression intensity of stromal cells was recorded as 0–3 based on stain intensity and CD1 expression in epidermal superficial layer was recorded as “present” or “absent.”
Table 1.
List of antibodies utilized and tissue biopsy treatment conditions for the immunohistochemical biomarker evaluation conducted at baseline and at week 12
| Manufacture | Clone name |
H202 block |
Non-specific protein block |
1st antibody concentration and incubation time |
2nd antibody polymer and incubation time |
|
|---|---|---|---|---|---|---|
| VEGF | Santa Cruz Bio Tech (Santa Cruz, CA, USA) |
A-20, RXH |
22’ | Dako Serum Free Protein Block for 7’ |
1:200,65’ | DAKO LSAB+Streptavidin for 15’, HPR for 15’ |
| Cyclin D1 | Lab Vision (Fremont, CA, USA) |
SP-4, RXH |
15’ | 10% FBS for 35’ |
1:100,65’ | DAKO Envision Link+ for 30’ |
| Ki-67 | DAKO (Carpinteria, CA, USA) |
MIB- 1, MXH |
15’ | 10% FBS for 35’ |
1:200,65’ | DAKO Envision Link+ for 30’ |
| P53 | DAKO (Carpinteria, CA, USA) |
Do-7, MXH |
15’ | 10% FBS for 35’ |
1:400,65’ | DAKO Envision Link+ for 30’ |
Methylation analysis - bisulfite pyrosequencing
Sections of the lesion biopsies were microdissected to obtain epithelial cells for genomic DNA extraction. Extracted genomic DNA was used for bisulfite treatment to modify unmethylated cytosine residues. Pyrosequencing was used to quantitatively measure the levels of cytosine methylation of CpG sites of promoters, as described previously (29). The primers used for the bisulfite pyrosequencing of the p16 promoter region (CDKN2A) are listed here: PCR step 1: p16-270F1 (GGGGTAGGTGGGGAGGAGTT), p16-270R1 (CCCCTCCTCTTTCTTCCTCC); PCR step 2: p16-169F2 (GGTTGTTTTAGGTTGGTGTTTT), p16-R-Biotin (Biotin-accctatccctcaaatcctctaaaa); Sequencing: p16-Pyro1F (TTTTTGTTTGGAAAGAT), p16-S3F (GATTTTAGGGGTGTTATATT). The quantification of cytosine methylation was performed using Pryo-Q-CpG software (Biotage, Uppsala, Sweden).
Pharmacokinetic sampling
Pharmacokinetic plasma samples were collected at baseline (prior to treatment) and on the last day of active intervention at Week 12, Day 84 (30 minutes prior to each of three daily doses and 3 hours after the last dose). The average time elapsed between administration of the medication and blood collection was 3.3 hours. Whole blood was collected and each sample was placed on ice immediately following collection and centrifuged within 30 minutes at 4° C for 10 minutes at 3000 rpm. Two 1 mL aliquots of plasma were transferred to 5 mL polypropylene cryogenic vials (Corning, Corning, NY) and stored at −20° C or colder until analysis.
Pharmacokinetic Analysis Methods
Both caffeine and EGCG concentrations were determined using a LC/MS/MS assay. An Agilent 1100 series LC pump (Agilent, Santa Clara, CA) was tandemed with a Micromass Quattro Micro mass spectrometer (Waters, Milford, MA) using MassLynx/QuanLynx software for data acquisition and processing.
Caffeine
Caffeine was extracted from 100 µL plasma in duplicate using MTBE (Sigma-Aldrich, St. Louis, MO) and dried at 40° C under gentle nitrogen gas stream then reconstituted with 200 µL 20:80, methanol:0.2% formic acid (v:v). Chromatographic separation was carried out using a Phenomenex Luna C8, 50 × 2.1 mm, 3.5 µm analytical column over 7 minutes at 0.35 mL/min using 15:85, methanol:0.2% formic acid, (v:v) mobile phase with a linear gradient to 95% methanol over the first 3 minutes. The analyte retention time was 2.8 ± 0.1 minutes. The mass spectrometer monitored the analyte transition of 195.4 > 138.1 (m/z) in electrospray ionization positive (ESI+) mode. The calibration curve (quadratic) ranged from 0.1 – 20 µg/mL. In order to avoid interference due to the high background signal of caffeine in donor human plasma, we purchased frozen fresh rhesus plasma and used it as our biomatrix for calibration and quality control samples. Caffeine reference standard, USP was purchased from Sigma-Aldrich.
Unconjugated (free) EGCG
EGCG was extracted from 1 mL plasma in duplicate using MTBE and dried at 40° C under gentle nitrogen gas stream then reconstituted with 100 µL 10:90, methanol:0.2% formic acid (v:v). Chromatographic separation was carried out using a Ascentis Express, 50 × 2.1 mm, 2.7 µm analytical column over 12 minutes at 0.3 mL/min using 20:80; 1:1, methanol:acetonitrile:0.2% formic acid, (v:v) mobile phase with a linear gradient to 100% organic over the first 4.5 minutes. The analytical retention time was 6.1 ± 0.1 minutes. The monitored transition was 456.8 > 168.9 (m/z) in ESI (−) mode. The calibration curve ranged from 1 – 1000 ng/mL.
Results
Patient and treatment characteristics
We enrolled 41 patients between August 2002 and March 2008 in order to achieve 36 evaluable patients (9 per arm). Of the 41 patients, 11 were randomized to placebo, 11 to GTE at 500 mg/m2, 9 to GTE at 750 mg/m2, and 10 to GTE at 1 g/m2 given orally three times a day. Our analysis of efficacy included 39 of these patients, excluding 2 who did not have a 3-month efficacy assessment. The baseline demographics of the patients are seen in Table 2. There were fourteen patients (34%) with a prior head and neck cancer history, eleven of whom had a prior oral tongue cancer. The remaining three patients had a prior history of a larynx cancer, base of tongue, and a tonsil cancer. The arms were similar with respect to distribution of patients with a prior history of head and neck squamous cell cancer. The distribution of tobacco use across the treatment groups was similar with approximately 37% never-smokers, 54% former smokers, and 10% actively smokers at the time of trial enrollment. The number of former and current alcohol users was similar between the treatment arms, with the exception of fewer never-drinkers in the GTE (500 mg/m2) arm. One patient had prior betel nut use and was enrolled in the GTE 750 mg/m2 arm. Patients were also assessed for caffeine use and 39 (95.1%) had used prior caffeine. The distribution of caffeine use across treatment groups was similar. Current caffeine use at baseline occurred in 25 (61%) patients with soft drinks, 21 (51.2%) with coffee, 26 (63.4%) with tea, and 27 (65.9%) with chocolate. None of the patients had used or were currently using theophylline medication for a medical indication.
Table 2.
Patient demographics
| GTE |
|||||
|---|---|---|---|---|---|
| Placebo (n=11) |
500 mg/m2 (n=11) |
750 mg/m2 (n=9) |
1000 mg/m2 (n=10) |
Total (n=41) |
|
| Median age (range) | 57(33–76) | 57(35–71) | 49(41–71) | 59(36–70) | 57(33–76) |
| Gender, n (%) | |||||
| Male | 6 (54.5%) | 5 (45.5%) | 4 (44.4%) | 4 (40%) | 19 (46.3%) |
| Female | 5 (45.5%) | 6 (45.5%) | 5 (55.6%) | 6 (60%) | 22 (53.7%) |
| Ethnicity, n (%) | |||||
| Caucasian | 11(100%) | 10 (90.9%) | 8 (88.9%) | 8 (80%) | 37 (90.2%) |
| Asian | 0 | 1(9.1%) | 1(11.1%) | 0 | 2 (4.9%) |
| Hispanic | 0 | 0 | 0 | 2 (20%) | 2 (4.9%) |
| Smoking Status, n (%) | |||||
| Never smoked | 4 (36.4%) | 4 (36.4%) | 3 (33.3%) | 4 (40%) | 15 (36.6%) |
| Former smoker | 6 (54.5%) | 6 (54.5%) | 5 (55.6%) | 5 (50%) | 22 (53.7%) |
| Current smoker | 1 (9.1%) | 1(9.1%) | 1(11.1%) | 1 (10%) | 4 (9.8%) |
| Cigar use | |||||
| Former | 1(9.1%) | 0 | 0 | 0 | 1 (2.4%) |
| Current | 0 | 2 (18.2%) | 0 | 1 (10%) | 3 (7.3%) |
| Smokeless Tobacco | |||||
| Former | 1(9.1%) | 0 | 0 | 0 | 1 (2.4%) |
| Current | 0 | 0 | 0 | 0 | 0 |
| Alcohol Status, n (%) | |||||
| Never | 2 (18.2%) | 1(9.1%) | 2 (22.2%) | 3 (30%) | 8 (19.5%) |
| Former | 2 (18.2%) | 3 (27.3%) | 2 (22.2%) | 2 (20%) | 9 (22%) |
| Current | 7 (63.6%) | 7 (63.6%) | 5 (55.6%) | 5 (50%) | 24 (58.5%) |
| Prior cancer history, n (%) | |||||
| HNSCC | 3 (27.3%) | 4 (36.4%) | 3 (33.3%) | 4 (40%) | 14(34.1%) |
| Other | 1 (9.1%) | 1(9.1%) | 1(11.1%) | 3 (30%) | 6 (14.6%) |
| Prior treatment, n (%) | |||||
| No prior radiotherapy | 10 (90.9%) | 10 (90.9%) | 9 (100%) | 7 (70%) | 36 (87.8%) |
| No prior surgery | 3 (27.3%) | 0 | 1(11.1%) | 0 | 4 (9.8%) |
| Prior radiotherapy | 1 (9.1%) | 1 (9.1%) | 0 | 3 (30%) | 5 (12.2%) |
| Prior surgery | 8 (72.7%) | 11(100%) | 8 (88.9%) | 10 (100%) | 37 (90.2%) |
| Baseline histology, n (%) | |||||
| Hyperplasia | 5 (45.5%) | 2 (18.2%) | 2 (22.2%) | 2 (20%) | 11(26.8%) |
| Mild dysplasia | 5 (45.5%) | 5 (45.5%) | 7 (77.8%) | 6 (60%) | 23 (56.1%) |
| Moderate dysplasia/-carcinoma in situ | 1 (9.1%) | 4 (36.4%) | 0 | 2 (20%) | 7(17.1%) |
Efficacy
Response rates are reported in Table 3. Thirty-nine patients were evaluable for the 12-week clinical and histologic response assessments. The clinical response rate was higher in the 3 combined GTE arms (50%) versus placebo (18.2%, p=0.09), as was the histologic response rate 21.4% (GTE arms) versus 9.1% (placebo, p=0.65). The clinical response rate was dose-dependent—58% in the combined higher-dose GTE arms (750 mg/m2 and 1000 mg/m2) versus 36.4% (GTE at 500 mg/m2) and 18.2% (placebo; p=0.03, Cochran-Armtage trend test). Dose dependency was not seen in histologic response (p=0.68). In the fifteen patients with a clinical response, 3 had hyperkeratosis/hyperplasia, 9 had mild dysplasia, and 3 had moderate dysplasia in their baseline tumor biopsy. In the seven histologic responders, 3 had mild dysplasia, 3 had moderate dysplasia, and 1 had severe dysplasia. There were no correlations between the 12-week clinical or histologic response rate and baseline patient demographics, with the one exception of a higher 12-week histologic response rate in never drinkers (p=0.01). Neither caffeine consumption nor prior cancer history impacted the clinical or histologic response rate. Fig. 1 illustrates examples of a clinical and histologic response to GTE treatment.
Table 3.
Efficacy outcomes (n=39)
| GTE |
||||||
|---|---|---|---|---|---|---|
| Efficacy Parameter | Placebo (n=11) |
500 mg/m2 (n=11) |
750 mg/m2 (n=8) |
1000 mg/m2 (n=9) |
All GTE doses (n=28) |
P-value |
| Clinical Response | 18.2% | 50% | 0.09 | |||
| CR | 0 | 1 | 0 | 0 | 1 | |
| PR | 2 | 3 | 5 | 5 | 13 | |
| SD | 4 | 4 | 1 | 1 | 6 | |
| PD | 5 | 3 | 2 | 3 | 8 | |
| Histologic Response | 9.1% | 21.4% | 0.65 | |||
| PR | 1 | 3 | 0 | 3 | 6 | |
| SD | 7 | 5 | 5 | 3 | 13 | |
| PD | 3 | 3 | 3 | 3 | 9 | |
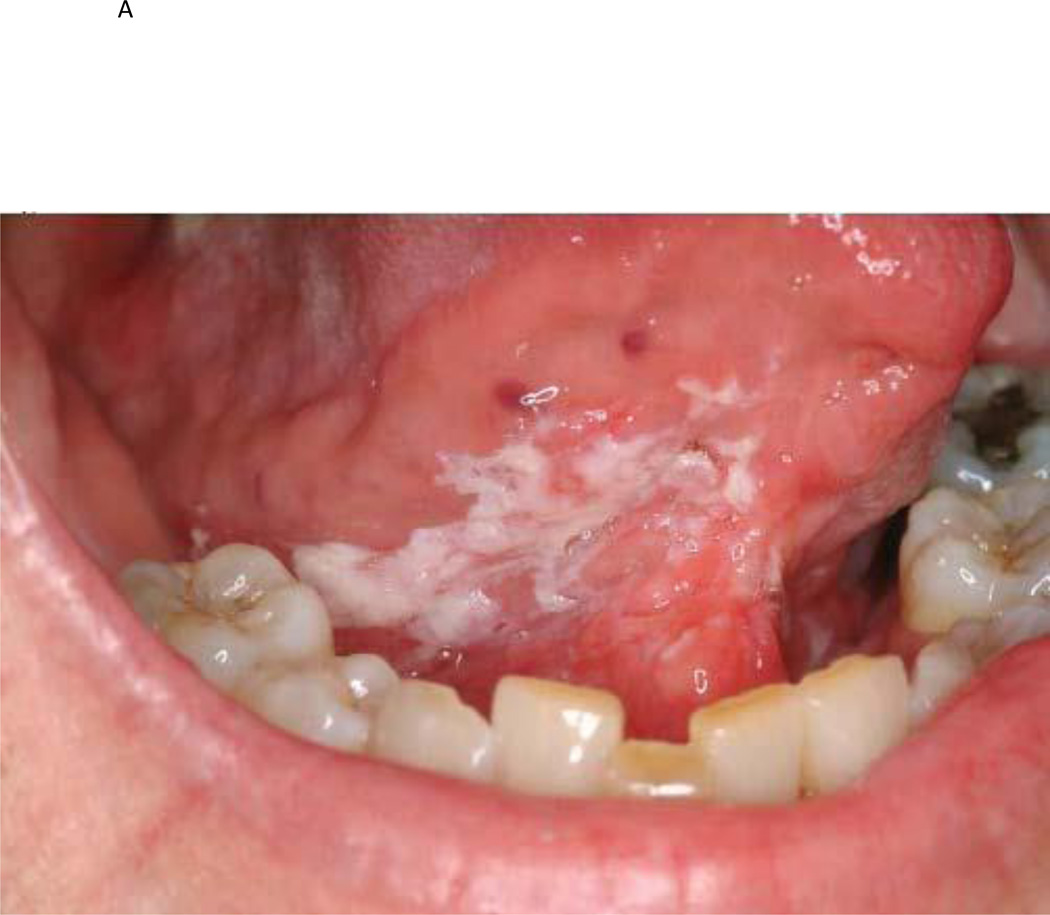
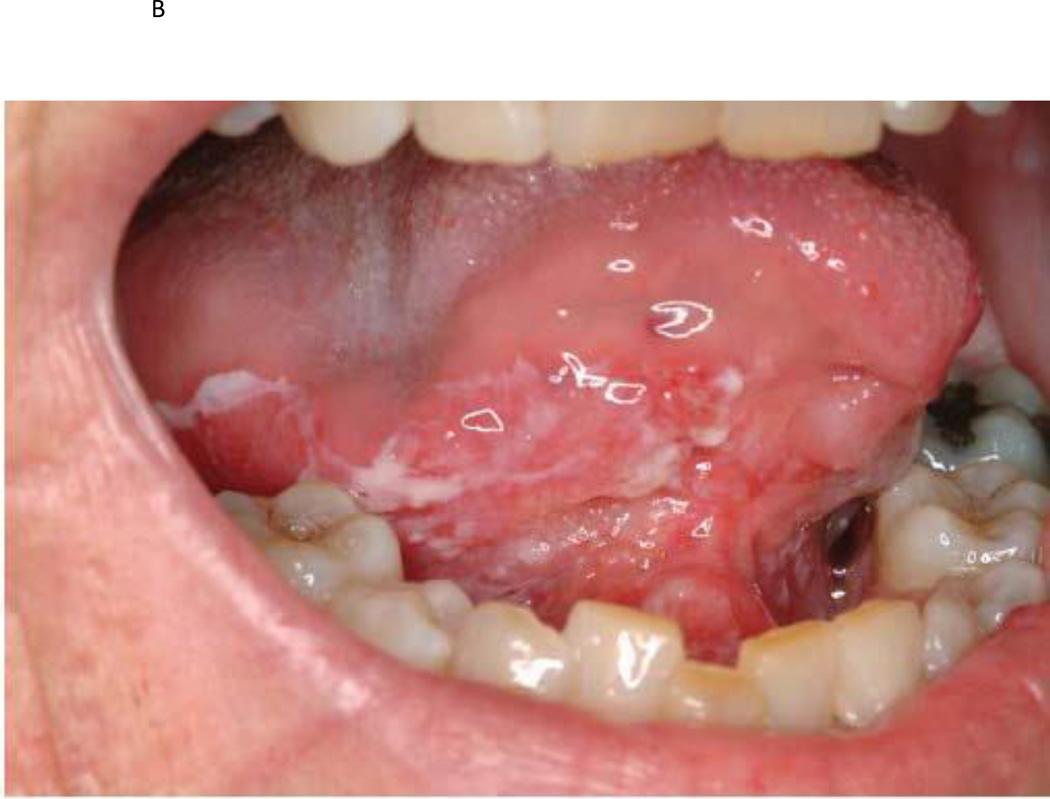
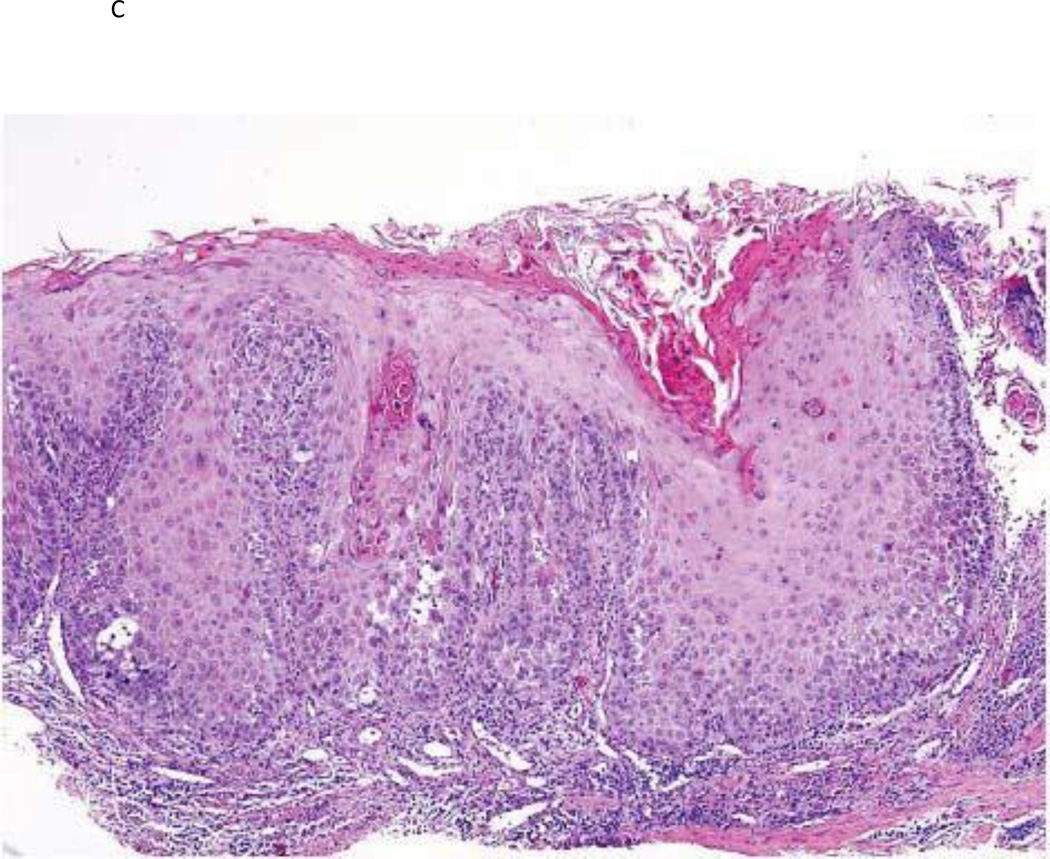
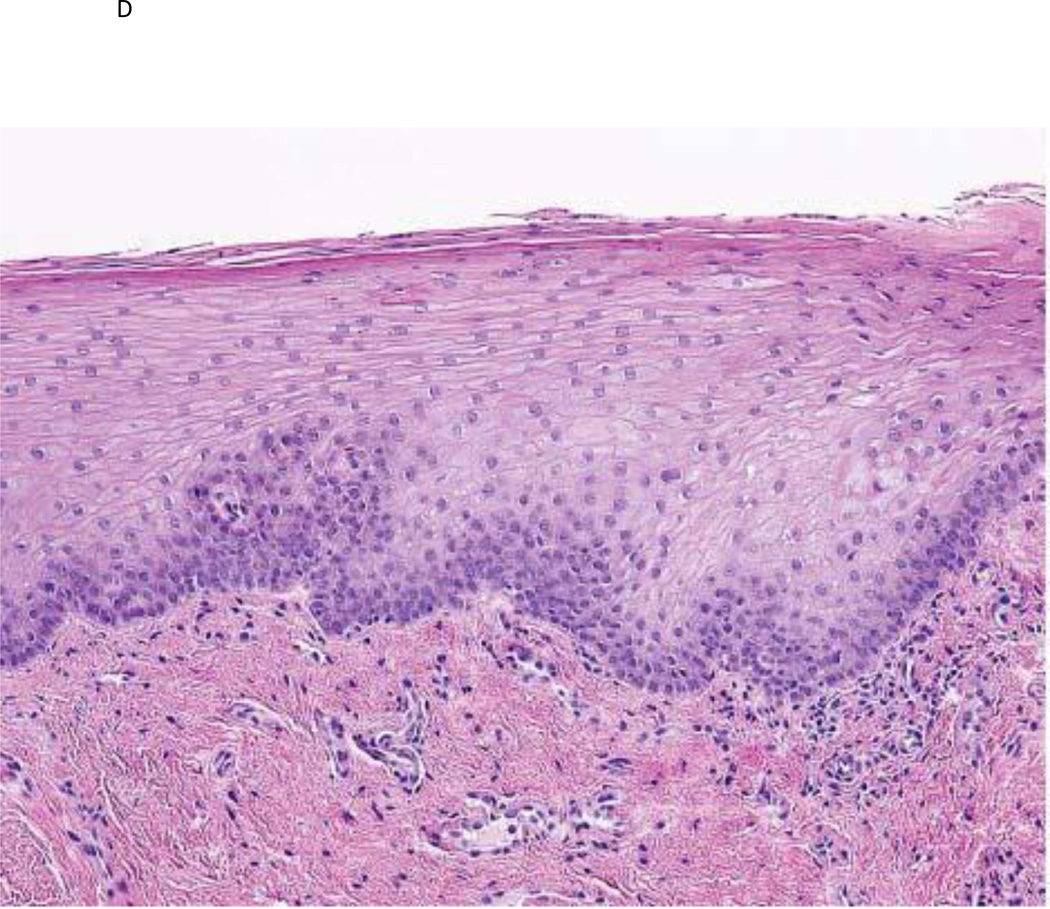
Figure 1.
a) Photographic example of a high risk OPL patient at baseline; b) same patient after GTE treatment demonstrating a clinical response; c) baseline histologic example of a high-risk OPL patient at baseline demonstrating dysplasia; d) same patient demonstrating a histologic response after GTE treatment.
With a median follow-up time of 27.5 months, 15 patients subsequently developed oral cancer with a median time to oral cancer development of 46.4 months. There was no difference in oral cancer-free survival between the GTE and placebo arms. None of the baseline patient demographics of age, gender, smoking status, nor alcohol history correlated with development of oral cancer. However, in patients with mild to moderate dysplasia, there was a shorter median time to oral cancer development of 35.9 months when compared to patients with hyperplasia, where the median time to oral cancer development has not been reached (p=0.005). Neither a clinical or histologic response to GTE intervention was associated with oral cancer development.
Toxicity
Overall, the therapy was well-tolerated and safe with treatment-related adverse events reported by 28 of the 30 (93.3%) patients who received GTE and 9 of the 11 (81.8%) patients who received placebo. Table 4 summarizes the adverse event information. The most frequently reported grade 1–2 adverse events were insomnia, headache, nausea, and nervousness; the incidence of insomnia and nervousness was higher in patients receiving GTE at 750 mg/m2 and 1 gm/m2. Insomnia was reported in 11 (36.7%) patients who had received GTE and 2 (18.2%) that received placebo. There were only three grade 3 adverse events reported and no grade 4 or 5 adverse events. The grade 3 toxicity was reported by 2 patients that received GTE 750 mg/m2 and included insomnia, diarrhea, and oral/neck pain. Two patients, one in each arm GTE 750 mg/m2 and GTE 1 gm/m2) discontinued therapy due to side effects of insomnia, gastrointestinal symptoms (diarrhea, flatulence), and nervousness. There were no significant differences in hematologic or blood chemistry results between the GTE and placebo arms at baseline and week 12 of therapy.
Table 4.
Most common adverse events Grades 1–2
| GTE |
|||||
|---|---|---|---|---|---|
| Adverse Events | Placebo (n=11) |
500 mg/m2 (n=11) |
750 mg/m2 (n=9) |
1 gm/m2 (n=10) |
All doses (n=30) |
| Insomnia | 18.2% | 18.2% | 44.4% | 50% | 36.7% |
| Nausea | 0% | 36.4% | 22.2% | 30% | 30% |
| Nervousness | 9.1% | 9.1% | 11.1% | 60% | 26.7% |
| Headache | 18.2% | 27.3% | 11.1% | 40% | 26.7% |
| Dyspepsia | 18.2% | 9.1% | 11.1% | 20% | 13.3% |
| Flatulence | 9.1% | 9.1% | 11.1% | 20% | 13.3% |
| Diarrhea | 18.2% | 9.1% | 11.1% | 10% | 10% |
| Abdominal pain | 18.2% | 9.1% | 11.1% | 0% | 6.7% |
| Back pain | 9.1% | 9.1% | 0% | 10% | 6.7% |
| Abnormal EKG | 0% | 9.1% | 0% | 10% | 6.7% |
| Gastric Reflux | 0% | 9.1% | 11.1% | 0% | 6.7% |
| Dizziness | 0% | 9.1% | 0% | 10% | 6.7% |
| Rhinitis | 0% | 0% | 22.2% | 0% | 6.7% |
| Sinusitis | 0% | 0% | 11.1% | 10% | 6.7% |
| Eyelid twitching | 0% | 18.2% | 0% | 0% | 6.7% |
| Urinary frequency | 0% | 9.1% | 0% | 10% | 6.7% |
| Constipation | 9.1% | 0% | 11.1% | 0% | 3.3% |
| Paresthesia | 9.1% | 0% | 0% | 10% | 3.3% |
Pharmacokinetic results
Caffeine
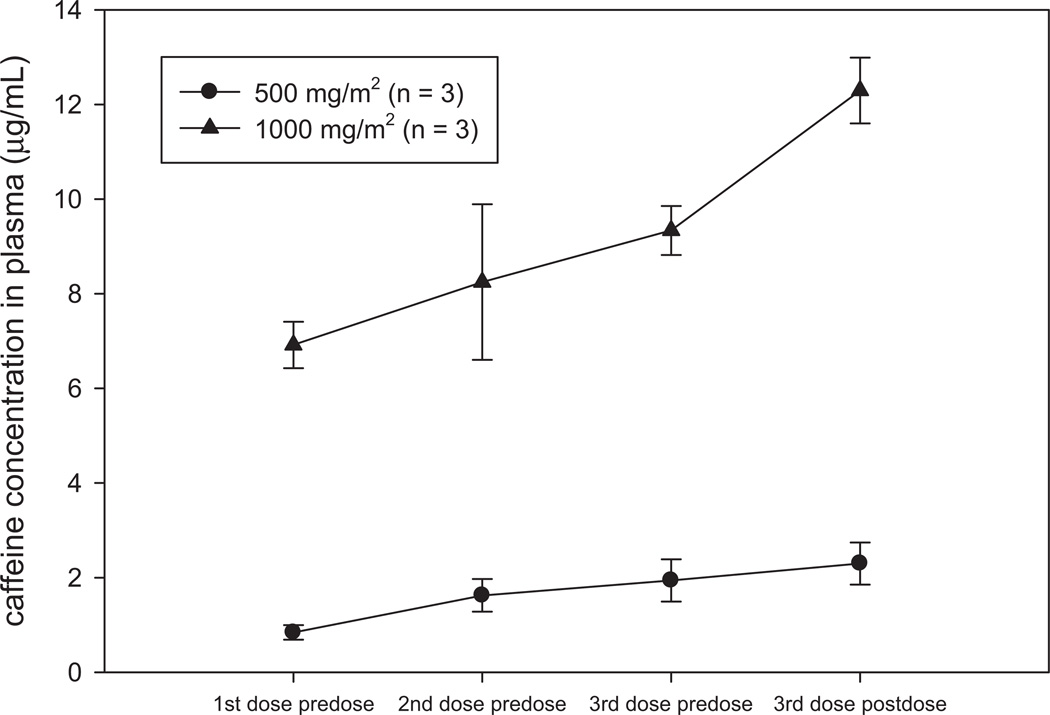
Caffeine concentrations in plasma on day 84 were dose- and schedule (TID)-dependent, ranging from 0.75–2.78 µg/mL in the 500 mg/m2 arm to 6.49–13.07 µg/mL in the 1000 mg/m2 arm (Fig. 2). The 5-fold greater mean peak concentration (12.29 versus 2.14) observed following 1000 mg/m2 dosing was most likely the reason for the increased insomnia and nervousness in this arm of the study. All concentrations in the placebo arm were < 0.1 µg/mL. Dose-dependent interday accumulation of caffeine was observed in the 1000 mg/m2 arm when comparing mean trough concentrations following the last dose from the previous day. Trough concentrations were 8-fold greater (6.92 vs. 0.84) in the 1000 mg/m2 arm when compared to the 500 mg/m2 arm yet the difference in dose was only 2-fold. Similar results were observed when comparing Cmax concentrations over an 8 week period which clearly showed a time-dependent accumulation of caffeine in the phase I trial (23). The expected fall in plasma concentration between the last dose from the previous day and the first dose of the next day in the 500 mg/m2 arm correlates well with the reported terminal half-life for caffeine of 5 – 7 hours but does not for the 1000 mg/m2 arm. This may be due to a drug-drug interaction between caffeine and the catechins in the GTE since both are metabolized in the liver via cytochrome P450 enzymes (30). From the limited PK sampling available, there was no correlation between clinical or histologic response and levels of caffeine.
Figure 2.
Dose-dependent increases in caffeine concentrations during TID administration on Day 84 of GTE. Time-dependent accumulation of caffeine over 12 weeks of GTE is revealed by the 8-fold greater mean trough concentration observed at the 1000 mg/m2 dose. The average time elapsed between drug administration and predose or postdose blood collection was 3.3 hours. Each symbol represents arithmetic mean concentrations ± SD.
EGCG
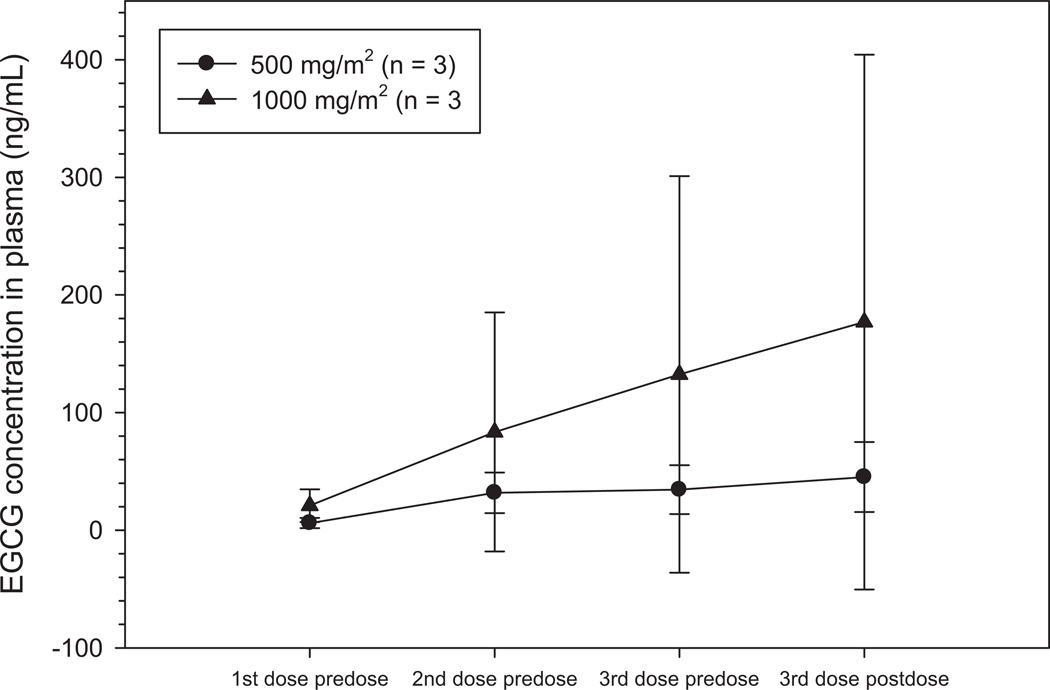
Concentrations of free EGCG reached almost 1 µM but were relatively more erratic than caffeine with a wide interpatient variability in the 1000 mg/m2 arm. Day 84 concentrations in plasma ranged from 1.7 – 75.3 ng/mL in the 500 mg/m2 arm and 5.7 – 435.2 ng/mL in the 1000 mg/m2 arm with peak concentrations from 22.1 – 75.3 ng/mL and 8.7 – 435.2 ng/mL, respectively (Fig. 3). There appears to be a trend toward a dose-dependent increase in concentration of EGCG but the distinction is not as evident as with caffeine due to the wide range of concentrations observed at the 1000 mg/m2 dose. The possible reasons for this are the very poor oral absorption of EGCG (< 1%) or the differences in dynamic metabolic capacity between these few patients at the time of dosing (30, 31). Free EGCG accounts for only 12–28% of total EGCG (conjugated and unconjugated) with the major conjugated forms being glucoronidated and sulfated products (32). No significant difference in trough concentrations can be observed at Day 84. All placebo concentrations were < 2.0 ng/mL. From the limited PK sampling available, there was no correlation between clinical or histologic response and levels of EGCG.
Figure 3.
A trend toward a dose-dependent increase in plasma EGCG concentrations during TID administration of green tea extract (GTE). Each symbol represents arithmetic mean concentrations ± SD. The data shows some dose-dependent accumulation of EGCG over the sampling period of approximately 9–12 hours although variability in plasma concentrations at 1000 mg/m2 is very high. Trough concentrations following the last dose from the previous day are similar for both arms and no significant accumulation of drug is observed.
Biomarker results
Baseline analyses were conducted in 35 tissue specimens for epithelial VEGF, p53 expression, and Ki-67 and in 34 tissue specimens for stromal VEGF and CD1 expression. Three-month (post-treatment) analyses were conduced in 34 tissue specimens for epithelial VEGF and p53, in 35 specimens for Ki-67, and in 33 specimens for stromal VEGF and CD1 expression. The only statistically significant difference in baseline biomarkers among study arms was a lower p53 expression in the 1000 mg/m2 arm (p=0.007). No baseline biomarker level correlated with patient demographics of age, gender, smoking status, or alcohol consumption. However, higher baseline mean and median values of stromal VEGF (p=0.24), p53 (p=0.34), Ki-67 (p=0.055), and CD1 (p=0.04) were found in the mild/moderate dysplasia versus the hyperplasia cohort (Wilcoxon rank sum test). Levels of epithelial VEGF were the same in the hyperplasia and mild/moderate dysplasia cohorts (p=1.0).
The baseline expression levels of the biomarkers were analyzed against both the clinical and histologic response rates; and none of the baseline biomarker expression scores, except that of stromal VEGF, correlated with response. A higher baseline stromal VEGF score was associated with a better clinical response to GTE treatment; with a median expression level of 3 in partial response group versus 1 in the stable disease/progressive disease group (p=0.02, Wilcoxon rank sum test). The multi-covariate analysis supported this finding by showing that a high baseline level of stromal VEGF is the single most significant predictor for clinical response. Interestingly, this effect was also seen in the 2 patients with spontaneous remissions in the placebo group where their baseline median stromal VEGF level was 2.5 compared to the median baseline expression level of 1 in the patients with stable or progressive disease. However, this association of stromal VEGF to response was not duplicated in the histologic response assessment.
To assess the treatment effect on modulation of the biomarkers, the expression level of the biomarkers at baseline were subtracted from those obtained at 12 weeks. Overall, there was no difference in biomarker modulation between the 3 different doses of GTE treatment. However, there was less up-regulation of stromal VEGF in the overall GTE group when compared to that seen in the placebo group (stromal VEGF mean levels 0.13 versus 0.67, median levels 0 versus 1, p=0.15). In the subgroup analysis of the clinical responders, GTE treatment led to lower levels of stromal VEGF and CD1 expression at 12 weeks, as opposed to the non-responders (stable and progressive disease) who had elevation of stromal VEGF and CD1 expression at 12 weeks. These differences in biomarker modulation did not reach statistical significance due to the small numbers of patients. In the placebo (n=10) group, a trend towards downregulation of p53, Ki-67, and CD1 were seen in the 2 patients with a clinical response and whereas the same biomarkers were upregulated in the non-responding group.
Univariate Cox model analysis was conducted using the biomarker expression levels as a continuous variable and demonstrated that higher levels of the following baseline biomarkers led to a higher risk of oral cancer development: stromal VEGF (HR 1.88, p=0.08), p53 (HR 18.2, p=0.001), Ki-67 (HR 20.8, p=0.04), and CD1 (HR 7.4, p=0.07). When using the median expression level of the biomarkers, the univariate analysis also noted that expression of p53 and Ki-67 above the median level were associated with a higher risk of development of oral cancer (HR 3.1, p=0.049; HR 3.1, p=0.06, respectively). In the multivariate Cox analysis when adjusting for histology, higher p53 levels predicted for a worse oral cancer free survival (HR 12.7, p=0.004).
The p16 promoter methylation analysis of the 19 tissue specimens with sufficient tissue for analysis, showed 5 patients to have p16 promoter methylation at baseline compared to 14 who did not. In these 5 patients, the median cancer-free survival was 31 months whereas the patients with no baseline p16 promoter methylation did not reach their median cancer-free survival yet (p=0.35). Only 7 patients in the study had paired tissue specimens from both pre- and post- treatment that yielded methylation results. In these seven patients, five showed no methylation of the p16 promoter at baseline and remained unmethylated after their respective treatments. Two patients had p16 promoter methylation at baseline and both were treated on the GTE 500 mg/m2 arm. Analysis of the post-GTE treatment tissue did not show modulation of the p16 promoter methylation status in either of the two patients.
Discussion
The two higher doses of GTE in this placebo-controlled trial produced a trend towards a greater clinical response in association with a high baseline level and downregulation of angiogenic stromal VEGF. These correlative findings suggest that potential GTE preventive activity may be linked mechanistically to the inhibition of angiogenesis, which is believed to promote carcinogenesis in premalignant conditions. The small trial population did not allow our analysis of downregulated stromal VEGF to reach statistical significance, and so we could not establish that modulation of this biomarker was directly linked to GTE activity; this result is consistent, however, with preclinical data supporting the anti-angiogenic properties of EGCG (18–20, 33, 34). EGCG decreases neovascularization via downregulation of VEGF and decreases endothelial cell growth by increasing fibroblast growth factor-2 (19). In head and neck, breast and other epithelial cancer cell lines, EGCG treatment led to reduced VEGF secretion (18) and binding to its receptor (35).
Biomarker evaluations and interpretations in a clinical trial are difficult and challenging. We evaluated 6 tissue-based biomarkers--Ki-67, p53, CD1, and p16 promoter methylation and angiogenesis-related epithelial and stromal VEGF. Ki-67 as a marker of the proliferative fraction of OPL cells appeared to increase as the tissue passed from normal histology to hyperplasia to dysplasia to cancer. Mutations of p53 occur in 40%–50% of head and neck squamous cell cancers and are associated with an increased immunohistochemical expression of p53 in malignant cells (36, 37). We did not find a correlation between levels of Ki-67 or p53 expression and either a clinical or histologic response. A higher baseline p53 level (above the median), however, was associated with a worse oral cancer-free survival in our multivariate analysis. This result is consistent with prior trials in which p53 protein overexpression frequently occurred in OPLs with a decreased response to retinoid chemoprevention and increased rates of progressive disease or cancer (38).
CD1 gene amplification frequently occurs and is associated with increased genomic instability in head and neck squamous cell tumors (39, 40). CD1 overexpression allows cells to continually enter the S phase independently of external stimulatory signals. Since dysregulation of the cell cycle, especially disruption of the G1-S checkpoint, is important to head and neck tumorigenesis, CD1 is an important potential marker of an OPL’s risk for oral cancer (39, 41). Prior studies have shown that high baseline CD1 expression has the highest correlation with increased cancer risk (41, 42). This finding specifically involves the truncated alternate CD1 protein, which is suspected of being a nuclear oncogene (43). In our trial, the median level of CD1 was higher in clinical responders than in non-responders (p=0.9287), and CD1 down-regulation was seen in clinical responders, whereas CD1 was up-regulated in patients with stable or progressive disease. Although higher levels of baseline CD1 were associated with a higher risk of oral cancer development (HR 7.4, p=0.07) in our univariate analysis, a result that is consistent with our prior observations (39, 41), this effect was weaker in our multivariate analysis. These data add to the growing body of evidence that CD1 may be an important regulatory molecule in OPLs.
Promoter methylation of p16 has been reported to predict the malignant transformation of oral epithelial lesions (44, 45). EGCG inhibited DNA methyltransferase activity and potentially reactivated methylation-silenced genes in preclinical studies (46). Conclusions about this marker were not possible, however, in our limited analysis. Only 2 patients, both of whom received 500 mg/m2 of GTE, had baseline p16 promoter methylation that could be evaluated following treatment, which did not reverse methylation status in either patient. Baseline p16 promoter methylation occurred in 5 patients overall, who had a shorter cancer-free survival than did the 14 patients without this methylation; this difference did not reach statistical significance, however, because of the limited patient numbers.
We also analyzed the pharmacokinetics of the GTE components caffeine and EGCG. A prior phase I analysis (23) found that the accumulation of caffeine was dose-dependent, but EGCG levels did not accumulate nor did they appear to be dose-related. Our present analysis confirms the dose-dependent elevation of intraday caffeine concentrations during TID administration of GTE, with the highest peak concentrations occurring after the last daily dose (in the evening). This finding most likely explains the greater incidence of insomnia and nervousness at higher GTE doses. EGCG levels were too erratic to establish dose-dependence, but the highest peak concentration of EGCG in the 1000 mg/m2 arm was 5-fold greater than the highest peak concentration in the 500 mg/m2 arm. A lack of EGCG accumulation was confirmed in this trial and most likely is due to poor bioavailability of oral GTE in the majority of our patients. Oral EGCG analogs with an improved bioavailability and consequently increased concentrations in plasma are being developed.
Promising green tea products in active cancer prevention research include GTE and polyphenon E (Poly E), which are prominent in clinical research, and EGCG alone. Both GTE and Poly E contain several catechins, including EGCG most abundantly, and perhaps the major distinction between them is caffeine, which is present in GTE but not in Poly E. The promise of catechin mixtures containing EGCG has been reinforced by mouse studies showing that Poly E has stronger effects against lung cancer than does EGCG alone or modified Poly E (stripped of EGCG; refs. (13, 14, 47). An important aspect of future research is to standardize as much as possible the formulation and contents of natural GTE or Poly E (not only the active but the believed-to-be inactive constituents as well) that are used in research so as to enhance the objective interpretation of different trials of the same-named agent (48). For example, the validity of follow-up testing of the clinical potential we report here will depend in large part on the formulation of GTE in the future being the same as or very similar to that of the present trial.
The present biomarker suggestion that a clinical response to GTE may involve the inhibition of angiogenesis is only preliminary, and the ability of GTE to prevent clinical OPL progression remains uncertain. Clinical response in our trial was not associated with a decreased risk of oral cancer. Therefore, the GTE intervention may not have been sufficient and/or clinical OPL response is not a valid intermediate surrogate marker for cancer development, leaving the need for true surrogate markers. Regarding the first possibility, our clinical findings may indicate that GTE was not given long enough and so was unable to completely reverse genetic OPL changes and ultimately prevent progression. We have shown this effect in retinoid-treated OPLs, where a transitory prevention benefit stopped once retinoid therapy stopped (24, 25, 27). Regarding the second possibility, other prevention trials have indicated that OPL response to treatment did not correlate with decreased cancer development (41, 49). Therefore, identifying molecular biomarkers that can serve as surrogate markers for preventive efficacy remains a research priority and should be incorporated within future clinical trials directed toward developing personalized chemoprevention strategies in the OPL setting. For example, our current results suggest that stromal VEGF levels deserve further prospective investigation for their ability to serve as a predictive biomarker of GTE treatment.
In conclusion, higher doses of GTE for 12 weeks in our trial led to a higher clinical response rate but did not generate an improvement in oral cancer-free survival. It remains unclear whether a longer duration of GTE would have led to a greater preventive benefit, but it appears that GTE administered at 750 mg/m2–1000 mg/m2 TID is feasible over a longer duration of time. Therefore, our present clinical results support future trials of GTE monotherapy given for a longer period of time or in combination with a targeted agent such as an epidermal growth factor receptor inhibitor. The potential synergism of EGCG and epidermal growth factor tyrosine kinase inhibitors in inhibiting aerodigestive tumor cell growth has been shown in preclinical studies (50–53).
Acknowledgements
We thank Dr. Robert Newman for his expertise and critical review of the pharmacokinetics in this manuscript and Nancy Shinn, RN, for her excellence in clinical research coordination.
Footnotes
Disclosure of Potential Conflicts of Interest
YM Sagesaka is an employee of Ito En, Ltd., which funded the trial reported here. WK Hong is an advisor to Ito En, Ltd.
References
- 1.Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol. 2003;39:770–780. doi: 10.1016/s1368-8375(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 2.Fuller CD, Wang SJ, Thomas CR, Jr., Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109:1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 3.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727–1730. [PubMed] [Google Scholar]
- 4.Silverman S, Jr., Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53:563–568. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Hittelman WN, Voravud N, Shin DM, Lee JS, Ro JY, Hong WK. Early genetic changes during upper aerodigestive tract tumorigenesis. J Cell Biochem Suppl. 1993;17F:233–236. doi: 10.1002/jcb.240531034. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Cheung KJ, Jr., Lam WL, Cheng X, Poh C, Priddy R, Epstein J, Le ND, Rosin MP. Increased genetic damage in oral leukoplakia from high risk sites: potential impact on staging and clinical management. Cancer. 2001;91:2148–2155. doi: 10.1002/1097-0142(20010601)91:11<2148::aid-cncr1243>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682–685. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 8.Rosin MP, Cheng X, Poh C, Lam WL, Huang Y, Lovas J, Berean K, Epstein JB, Priddy R, Le ND, Zhang L. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6:357–362. [PubMed] [Google Scholar]
- 9.Papadimitrakopoulou V, Izzo J, Lippman SM, Lee JS, Fan YH, Clayman G, Ro JY, Hittelman WN, Lotan R, Hong WK, Mao L. Frequent inactivation of p16INK4a in oral premalignant lesions. Oncogene. 1997;14:1799–1803. doi: 10.1038/sj.onc.1201010. [DOI] [PubMed] [Google Scholar]
- 10.Fujiki H, Yoshizawa S, Horiuchi T, Suganuma M, Yatsunami J, Nishiwaki S, Okabe S, Nishiwaki-Matsushima R, Okuda T, Sugimura T. Anticarcinogenic effects of (−)-epigallocatechin gallate. Prev Med. 1992;21:503–509. doi: 10.1016/0091-7435(92)90057-o. [DOI] [PubMed] [Google Scholar]
- 11.Fujiki H, Suganuma M, Okabe S, Sueoka E, Suga K, Imai K, Nakachi K, Kimura S. Mechanistic findings of green tea as cancer preventive for humans. Proc Soc Exp Biol Med. 1999;220:225–228. doi: 10.1046/j.1525-1373.1999.d01-38.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 13.Bode AM, Dong Z. Epigallocatechin 3-Gallate and Green Tea Catechins: United They Work, Divided They Fail. Cancer Prev Res (Phila Pa) 2009 doi: 10.1158/1940-6207.CAPR-09-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu H, He J, Mei F, Zhang Q, Hara Y, Ryota S, Lubet R, Chen R, Chen D, MY Lung Cancer Inhibitory Effect of Epigallocatechin-3-Gallate is dependenet on its presence in a complex mixture (Polyphenon E) Cancer Prev Res (Phila Pa) 2009;2:531–537. doi: 10.1158/1940-6207.CAPR-08-0185. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000;275:328–334. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- 16.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. Faseb J. 2005;19:789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 17.Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- 18.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 20.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 21.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- 23.Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, Glisson BS, Lee JS. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19:1830–1838. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 24.Papadimitrakopoulou VA, Hong WK, Lee JS, Martin JW, Lee JJ, Batsakis JG, Lippman SM. Low-dose isotretinoin versus beta-carotene to prevent oral carcinogenesis: long-term follow-up. J Natl Cancer Inst. 1997;89:257–258. doi: 10.1093/jnci/89.3.257. [DOI] [PubMed] [Google Scholar]
- 25.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, Fofonoff S, Byers R, Atkinson EN, Vaughan C, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 26.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 27.Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, Hays GL, Goepfert H, Hong WK. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 28.Tang X, Varella-Garcia M, Xavier AC, Massarelli E, Ozburn N, Moran C, Wistuba II. Epidermal growth factor receptor abnormalities in the pathogenesis and progression of lung adenocarcinomas. Cancer Prev Res (Phila Pa) 2008;1:192–200. doi: 10.1158/1940-6207.CAPR-08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S, Talpaz M, Garcia-Manero G, Kantarjian HM. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 30.Moridani MY, Scobie H, Salehi P, O’Brien PJ. Catechin metabolism: glutathione conjugate formation catalyzed by tyrosinase, peroxidase, and cytochrome p450. Chem Res Toxicol. 2001;14:841–848. doi: 10.1021/tx000235o. [DOI] [PubMed] [Google Scholar]
- 31.Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, Go VL, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 32.Lee MJ, Wang ZY, Li H, Chen L, Sun Y, Gobbo S, Balentine DA, Yang CS. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev. 1995;4:393–399. [PubMed] [Google Scholar]
- 33.Cao Y, Cao R, Brakenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13:380–390. doi: 10.1016/s0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 34.Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 35.Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180:139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 36.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, Couch MJ, Forastiere AA, Sidransky D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 37.Nees M, Homann N, Discher H, Andl T, Enders C, Herold-Mende C, Schuhmann A, Bosch FX. Expression of mutated p53 occurs in tumor-distant epithelia of head and neck cancer patients: a possible molecular basis for the development of multiple tumors. Cancer Res. 1993;53:4189–4196. [PubMed] [Google Scholar]
- 38.Shin DM, Xu XC, Lippman SM, Lee JJ, Lee JS, Batsakis JG, Ro JY, Martin JW, Hittelman WN, Lotan R, Hong WK. Accumulation of p53 protein and retinoic acid receptor beta in retinoid chemoprevention. Clin Cancer Res. 1997;3:875–880. [PubMed] [Google Scholar]
- 39.Izzo JG, Papadimitrakopoulou VA, Liu DD, den Hollander PL, Babenko IM, Keck J, El-Naggar AK, Shin DM, Lee JJ, Hong WK, Hittelman WN. Cyclin D1 genotype, response to biochemoprevention, and progression rate to upper aerodigestive tract cancer. J Natl Cancer Inst. 2003;95:198–205. doi: 10.1093/jnci/95.3.198. [DOI] [PubMed] [Google Scholar]
- 40.Izzo JG, Papadimitrakopoulou VA, Li XQ, Ibarguen H, Lee JS, Ro JY, El-Naggar A, Hong WK, Hittelman WN. Dysregulated cyclin D1 expression early in head and neck tumorigenesis: in vivo evidence for an association with subsequent gene amplification. Oncogene. 1998;17:2313–2322. doi: 10.1038/sj.onc.1202153. [DOI] [PubMed] [Google Scholar]
- 41.Papadimitrakopoulou V, Izzo JG, Liu DD, Myers J, Ceron TL, Lewin J, William WN, Jr., Atwell A, Lee JJ, Gillenwater A, El-Naggar A, Wu X, Lippman SM, Hittelman WN, Hong WK. Cyclin D1 and cancer development in laryngeal premalignancy patients. Cancer Prev Res (Phila Pa) 2009;2:14–21. doi: 10.1158/1940-6207.CAPR-08-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsit CJ, Black CC, Posner MR, Kelsey KT. A genotype-phenotype examination of cyclin D1 on risk and outcome of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:2371–2377. doi: 10.1158/1078-0432.CCR-07-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu F, Gladden AB, Diehl JA. An alternatively spliced cyclin D1 isoform, cyclin D1b, is a nuclear oncogene. Cancer Res. 2003;63:7056–7061. [PubMed] [Google Scholar]
- 44.Hall GL, Shaw RJ, Field EA, Rogers SN, Sutton DN, Woolgar JA, Lowe D, Liloglou T, Field JK, Risk JM. p16 Promoter methylation is a potential predictor of malignant transformation in oral epithelial dysplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2174–2179. doi: 10.1158/1055-9965.EPI-07-2867. [DOI] [PubMed] [Google Scholar]
- 45.Papadimitrakopoulou VA, Izzo J, Mao L, Keck J, Hamilton D, Shin DM, El-Naggar A, den Hollander P, Liu D, Hittelman WN, Hong WK. Cyclin D1 and p16 alterations in advanced premalignant lesions of the upper aerodigestive tract: role in response to chemoprevention and cancer development. Clin Cancer Res. 2001;7:3127–3134. [PubMed] [Google Scholar]
- 46.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 47.Yan Y, Cook J, McQuillan J, Zhang G, Hitzman CJ, Wang Y, Wiedmann TS, You M. Chemopreventive effect of aerosolized polyphenon E on lung tumorigenesis in A/J mice. Neoplasia. 2007;9:401–405. doi: 10.1593/neo.07160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila Pa) 2009;2:187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadimitrakopoulou VA, Lee JJ, William WN, Jr., Martin JW, Thomas M, Kim ES, Khuri FR, Shin DM, Feng L, Hong WK, Lippman SM. Randomized Trial of 13-cis Retinoic Acid Compared With Retinyl Palmitate With or Without Beta-Carotene in Oral Premalignancy. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, Yang CS, Chen ZG, Shin DM. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–1014. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for Cancer Prevention With Natural Compounds. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amin AR, Wang D, Khuri FR, Chen ZG, Shin DM. in vitro and in vivo synergistic anti-tumor efficacy of two naturally available dietary polyphenols EGCG and luteolin. In: Proceedings of the 100th Annual Meeting of the American Association for Cancer Research, Denver, CO. 2009 Abstract 33. [Google Scholar]
- 53.Amin AR, Khuri FR, Chen ZG, Shin DM. Synergistic growth inhibition of squamous cell carcinoma of the head and neck by erlotinib and epigallocatechin-3-gallate: the role of p53-dependent inhibition of nuclear factor-kappaB. Cancer Prev Res (Phila Pa) 2009;2:538–545. doi: 10.1158/1940-6207.CAPR-09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]