Abstract
Podocin is a key protein of the kidney podocyte slit diaphragm protein complex, an important part of the glomerular filtration barrier. Mutations in the human podocin gene NPHS2 cause familial or sporadic forms of renal disease owing to the disruption of filtration barrier integrity. The exclusive expression of NPHS2 in podocytes reflects its unique function and raises interesting questions about its transcriptional regulation. Here, we further define a 2.5-kb zebrafish nphs2 promoter fragment previously described and identify a 49-bp podocyte-specific transcriptional enhancer using Tol2-mediated G0 transgenesis in zebrafish. Within this enhancer, we identified a cis-acting element composed of two adjacent DNA-binding sites (FLAT-E and forkhead) bound by transcription factors Lmx1b and FoxC. In zebrafish, double knockdown of Lmx1b and FoxC orthologs using morpholino doses that caused no or minimal phenotypic changes upon individual knockdown completely disrupted podocyte development in 40% of injected embryos. Co-overexpression of the two genes potently induced endogenous nphs2 expression in zebrafish podocytes. We found that the NPHS2 promoter also contains a cis-acting Lmx1b-FoxC motif that binds LMX1B and FoxC2. Furthermore, a genome-wide search identified several genes that carry the Lmx1b-FoxC motif in their promoter regions. Among these candidates, motif-driven podocyte enhancer activity of CCNC and MEIS2 was functionally analyzed in vivo. Our results show that podocyte expression of some genes is combinatorially regulated by two transcription factors interacting synergistically with a common enhancer. This finding provides insights into transcriptional mechanisms required for normal and pathologic podocyte functions.
Keywords: Combinatorial regulation, NPHS2 expression, Lmx1b and FoxC, transgenic zebrafish, podocyte
Normal glomerular filtration function depends on structural integrity of the filtration barrier. Glomerular podocytes play a key role in establishing and maintaining this unique filtration barrier structure. Mature podocytes are characterized by cell cycle arrest, foot process formation, and the presence of the slit diaphragm,1 which bridges the gaps between the interdigitating foot processes of neighboring podocytes and functions as a size-selective filtration barrier.2,3 For their differentiation, as well as for the maintenance of their complex architecture, podocytes require the expression of several specific genes in a correct spatial and temporal fashion. This notion is supported by the identification of many mutations in podocyte-expressed genes as the underlying cause of inherited renal diseases.4 Moreover, recent studies from genetically modified mice and the identification of genes responsible for human podocyte diseases have revealed a complex transcriptional network in podocytes critical for podocyte specification, differentiation, and contributing to renal disease pathogenesis.5,6 However, transcriptional regulatory mechanisms by which the transcription factors govern expression of their target genes in podocytes remain incompletely understood.
NPHS2 was identified by positional cloning because its mutations cause familial or sporadic forms of steroid-resistant nephrotic syndrome.7–9 Podocin is a key component of the slit diaphragm, where it interacts with nephrin, NEPH1, and CD2AP.10,11 In contrast to many other podocyte genes, NPHS2 is exclusively and constitutively expressed in podocytes.7 This likely reflects its unique function, and in particular implies the presence of a podocyte-specific enhancer. A putative enhancer element in NPHS2 has been localized within a 2.5-kb DNA fragment upstream of its transcriptional start site, and it drives reporter gene expression in transgenic mouse podocytes.12 We recently identified a zebrafish podocyte-specific enhancer element, which also lies within the 2.5-kb 5′ flanking region.13 However, the precise DNA-binding motifs in these regions and their potential interaction with specific transcription factors remain unknown.
Previous studies have shown that Lmx1b is essential for mouse Nphs2 expression.14,15 Lmx1b is a LIM-homeobox transcription factor that controls dorsal-ventral limb patterning during vertebrae development.16 Mutations in human LMX1B cause nail-patella syndrome, which is characterized by skeletal abnormality, nail hypoplasia, and nephropathy.17,18 In mice, genetic ablation of Lmx1b leads to loss of Nphs2 expression14,19 as well as loss of expression of the glomerular basement membrane (GBM) collagens Col4a3 and Col4a4,20 suggesting that Lmx1b potentially acts as a common upstream regulator of these genes through binding to the FLAT elements.21 Although this hypothesis is supported by an electrophoresis mobility shift assay (EMSA), conflicting results have been reported regarding the ability of a putative Lmx1b-binding enhancer to activate reporter gene expression.14,15 Moreover, Lmx1b is exclusively expressed in the glomerulus of the kidney,22 as well as in other organs, including limb, eye, and brain, during development.19,23 Thus, although Lmx1b is necessary for podocyte-specific expression of certain genes, it is not specific to podocytes, nor is it alone sufficient to direct podocyte-specific gene expression. Authors have argued that Lmx1b may interact with coactivators through its LIM domains,19 and Ldb1 has been shown as a candidate coactivator in podocytes.22 However, its role in regulating Nphs2 expression remains to be determined.
In a previous study, we demonstrated that Foxc2−/− mice, similar to Lmx1b−/− mice, lose the expression of Nphs2, Col4a3, and Col4a4.24 FoxC2 belongs to a subgroup of the forkhead transcription factor family that is involved in a broad range of developmental processes.25 The mammalian FoxC subfamily has two highly related members, FoxC1 and FoxC2, where mouse Foxc2 is expressed in multiple tissues26 in addition to podocytes.24,27 Mutations in FOXC2 cause lymphedema-distichiasis syndrome, which is characterized by limb lymphedema and double rows of eyelashes. Lymphedema-distichiasis syndrome with proteinuria has been reported in a family of German-Irish descent,28 suggesting that certain FOXC2 mutations may cause renal disease. Thus, both Lmx1b and FoxC2 have been implicated in the transcriptional regulation of Nphs2. However, the molecular mechanism and potential interaction between these two transcription factors have not been addressed previously.
In this study, we identified a 49-bp podocyte-specific enhancer in the zebrafish nphs2 promoter. This enhancer contains a conserved cis-acting motif composed of two adjacent DNA-binding sites, combinatorially bound and activated by two transcription factors Lmx1b and FoxC. We show that the human NPHS2 promoter also contains the cis-acting motif regulated by the mammalian orthologs LMX1B and FoxC2. Further, we genome-wide detected 26 genes carrying the Lmx1b-FoxC motifs in their promoter regions. Among them, motif-driven podocyte enhancer activity of CCNC and MEIS2 was functionally analyzed in vivo. The findings provide insights into the transcriptional regulatory mechanisms required for normal podocyte functions, and for the development of certain kidney diseases.
Results
Identification of a Podocyte-Specific Enhancer by Analysis of Reporter Gene Expression in Zebrafish
Using a 2.5-kb zebrafish nphs2 promoter fragment (Supplemental Figure 1A), we previously generated a Tg(podocin:GFP) zebrafish line, in which green fluorescence protein (GFP) is exclusively expressed in podocytes.13 To fine-map this 5′ sequence by in vivo experiments, we first compared the GFP expression patterns driven by this promoter between injected G0 and Tg(podocin:GFP) G1 embryos. The two types of embryos exhibited similar podocyte-specific GFP expression pattern at 4 days postfertilization (dpf), despite the expected mosaic expression in G0 embryos (Supplemental Figure 1B). The robust expression was observed in 18%±4% of G0 embryos, verified by three independent injections. Thus, we found it plausible to use G0 transgenic zebrafish for a rapid fine-mapping of the promoter fragment.
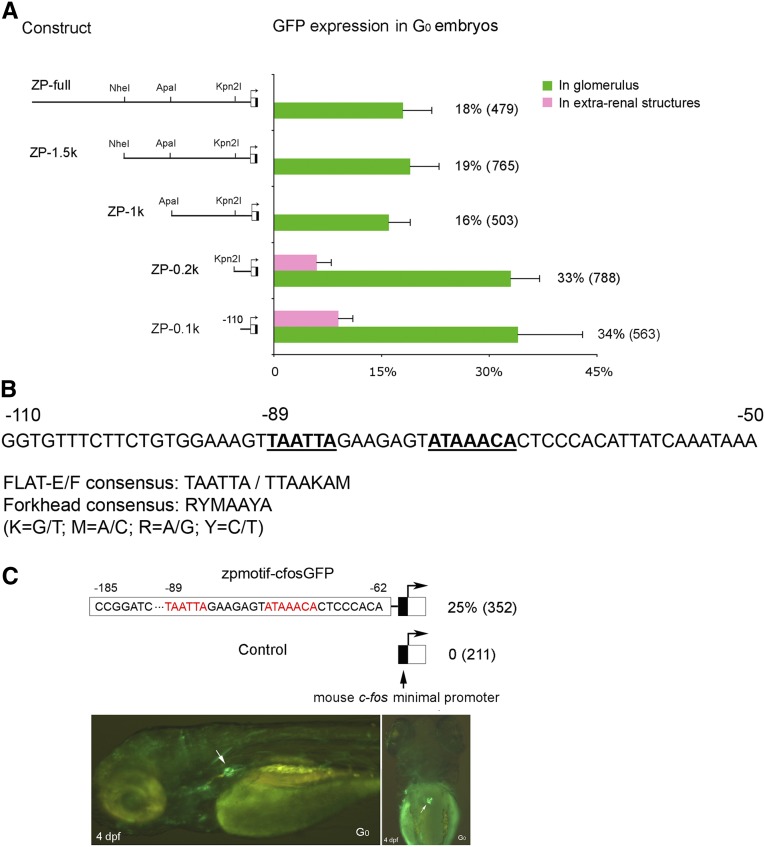
Further analysis of the 2.5-kb promoter fragment revealed that deletions from −2.5 kb to an ApaI site situated at −1.0 kb preserved the podocyte specificity and frequency of GFP expression (Figure 1A). However, further deletion to a Kpn2I site situated at −185 bp increased the expression rate from 18% to 33% and also induced a low but significant frequency (5%–10%) of ectopic GFP expression in extrarenal tissues (Figure 1A). This expression pattern was preserved upon further deletion to −110 bp (Figure 1A), suggesting that a podocyte-specific enhancer element resides within 110 bp upstream of the transcription start site. Within the 110-bp sequence, we observed two adjacent DNA-binding sites: a 5′ located FLAT-E element (TAATTA) and a 3′ located forkhead-binding site (ATAAACA) separated by seven nucleotides (Figure 1B). To test the cis-acting potential, we coupled the zebrafish motif element between −185 and −62 bp to a mouse c-fos minimal promoter and analyzed GFP expression in G0 embryos (Figure 1C). This resulted in glomerular GFP expression in 25% of embryos, whereas no glomerular GFP expression was observed in 211 control embryos (Figure 1C), suggesting that the 49-bp element containing a FLAT-E/forkhead motif constitutes a podocyte-specific enhancer sufficient to direct GFP expression in podocytes independent of the native nphs2 minimal promoter.
Figure 1.
Identification of a 49-bp enhancer sufficient to direct GFP expression in zebrafish podocytes. (A) Fine-mapping of the zebrafish nphs2 promoter fragment by deletion analysis. The constructs carrying different sizes of inserts are schematically shown in left panel. GFP expression rate, defined as percentage of positive G0 embryos expressing GFP in glomeruli or extrarenal structures out of total embryos, is illustrated with bar graphs. Total number of G0 embryos is indicated in parentheses. SD in the bar graph shows a variation in three independent injections. (B) DNA sequence analysis. The zebrafish nphs2 promoter element from −110 to −50 bp is shown. Consensus of the FLAT-E or -F element and the forkhead-binding site are denoted.21,25 The putative DNA-binding sites in the sequence are marked in bold and underline. (C) Heterologous promoter analysis. The zebrafish promoter element with two putative binding sites marked in red between −185 and −62 bp was subcloned in the Tol2-cfos-GFP plasmid (zpmotif-cfos-GFP) and is schematically illustrated. The mouse c-fos minimal promoter (arrow) in the plasmid is indicated. The empty plasmid was used as a control. GFP expression rate described above is shown. Microscopic imaging laterally and dorsally shows GFP expression in G0 glomerulus (arrow). GFP is also visible in other tissues.
FLAT-E/forkhead Motif Defines the Enhancer Required for Podocyte-Specific Expression
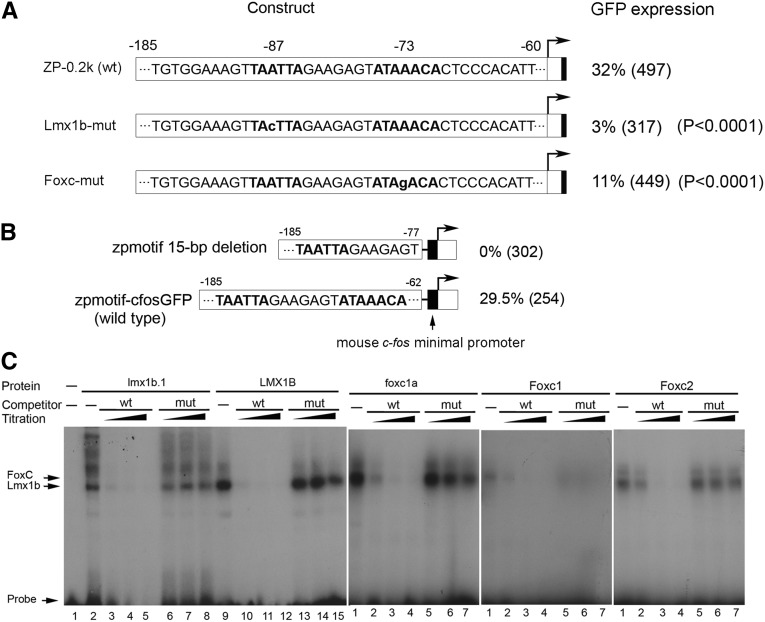
By analyzing the 5-kb Nphs2 promoter with a complete sequence in all vertebrates available, the motif with two adjacent binding sites is present in 90% of analyzed species (28 of 31 species) and the motif with 5-bp or 7-bp spacing between two sites accounts for 75% of 28 species (Supplemental Table 1), suggesting that the binding motif is highly conserved. To characterize the motif, we generated mutations targeting the FLAT-E and forkhead sites, respectively. A single mutation (A→C) in the FLAT-E element almost completely abolished expression (reduced to 3%; P<0.001) in comparison with the wild-type (32%). A point mutation at the forkhead site (A→G) likewise resulted in a significantly decreased expression frequency (11%; P<0.001) (Figure 2A). Further deletion of the entire forkhead site led to a complete loss of glomerular expression (Figure 2B), suggesting that the two sites are both required for podocyte expression.
Figure 2.
Characterization of two putative DNA-binding sites bound by Lmx1b and FoxC proteins. (A) Mutagenesis analysis of two putative DNA-binding sites. Two point-mutation carrying plasmids and their wild-type (ZP-0.2k) are illustrated. The putative binding sites are marked in bold, and the mutation at the −87 or −73 position is indicated in lowercase letters. GFP expression rate and number of G0 embryos in parentheses are shown. (B) Deletion of the forkhead-binding site. The sequence between −185 and −77 bp, in which the forkhead-binding site was completely deleted, was subcloned in the Tol2-based cfos-GFP plasmid (see Figure 1C). GFP expression rate controlled by the 15-bp deletion plasmid and its wild-type (zpmotif-cfos-GFP) are displayed. (C) EMSA. Radiolabeled oligonucleotide probes (wt) encompassing the zebrafish FLAT-E and the forkhead-binding sites and recombinant Lmx1b and FoxC proteins were used in EMSA. The mutations of the mutant probe (mu) are identical to that used in mutagenesis assay, shown in part A. An efficient binding of zebrafish lmx1b.1 and human LMX1B to the FLAT-E site was observed (lanes 2, 9 in Lmx1b panel). Zebrafish foxc1a strongly bound to the forkhead-binding site (lane 1 in foxc1a panel). The binding of Foxc1 and Foxc2 was notably weaker than zebrafish foxc1a (lanes 1 in panels for Foxc1 and Foxc2). To test binding specificity, 12.5-fold, 25-fold, and 37.5-fold excess unlabeled wild-type probes or mutant probes, indicated with gradients from low to high, were used for competition assays. Labeled probes were efficiently competed by unlabeled wild-type probes with elevated amounts for five tested proteins (lanes 3–5, lanes 10–12 in Lmx1b panel; lanes 2–4 in panels for foxc1a, Foxc1, and Foxc2), but not by unlabeled mutant probes (lanes 6–8, lanes 13–15 in Lmx1b panel; lanes 5–7 in panels for foxc1a, Foxc1, and Foxc2).
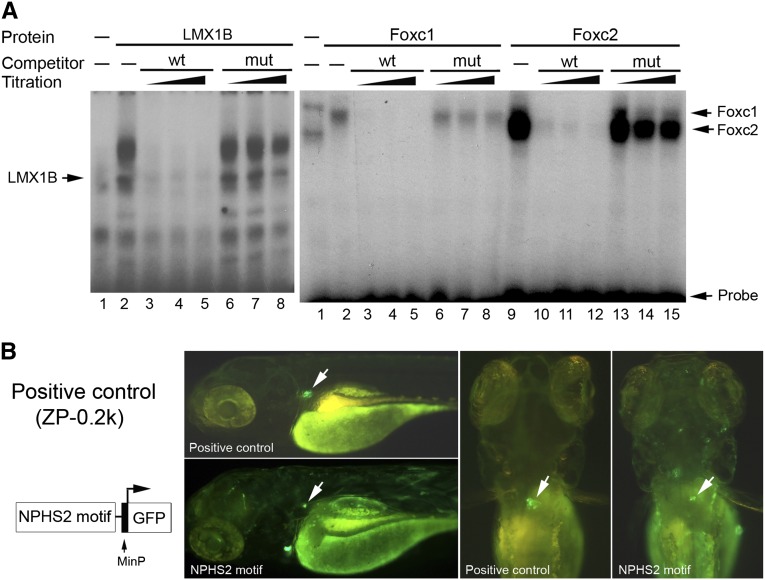
It is known that Lmx1b binds to the FLAT elements.21 Moreover, Lmx1b and Foxc2 are critical for the Nphs2 expression.14,19,24 Therefore, we hypothesized that the two proteins bind to the FLAT-E/forkhead motif with a synergistic effect on Nphs2 transcription. In zebrafish, no FoxC2 homolog exists. Instead, two foxc1 paralogs (foxc1a, b) have been identified, of which only foxc1a is expressed in the pronephros.29 We next performed EMSA to test binding potentials of the two proteins to the zebrafish motif. Lmx1b (lmx1b.1 and LMX1B) and foxc1a robustly and specifically bound to the FLAT-E element and the forkhead site, respectively (Figure 2C). Mouse Foxc1 and Foxc2 could also bind to the zebrafish forkhead-binding site, but this binding was significantly weaker than that observed for zebrafish foxc1a (Figure 2C). Competition assay supported the binding specificity, since the bindings were efficiently competed by the same unlabeled wild-type probes with different amounts, but not by the unlabeled mutant probes with different amounts (Figure 2C). High homology (84% identity and 89% similarity) of LMX1B with zebrafish lmx1b.130 is in agreement with sufficient binding of LMX1B to the zebrafish enhancer. Although repeated attempts with different methods and conditions were made, we could not detect a tertiary band that indicates co-binding of two proteins to the probe at the same time by a conventional EMSA in vitro.
Lmx1b and Foxc1a Are Both Required for Nphs2 Expression and Slit Diaphragm Formation in Zebrafish
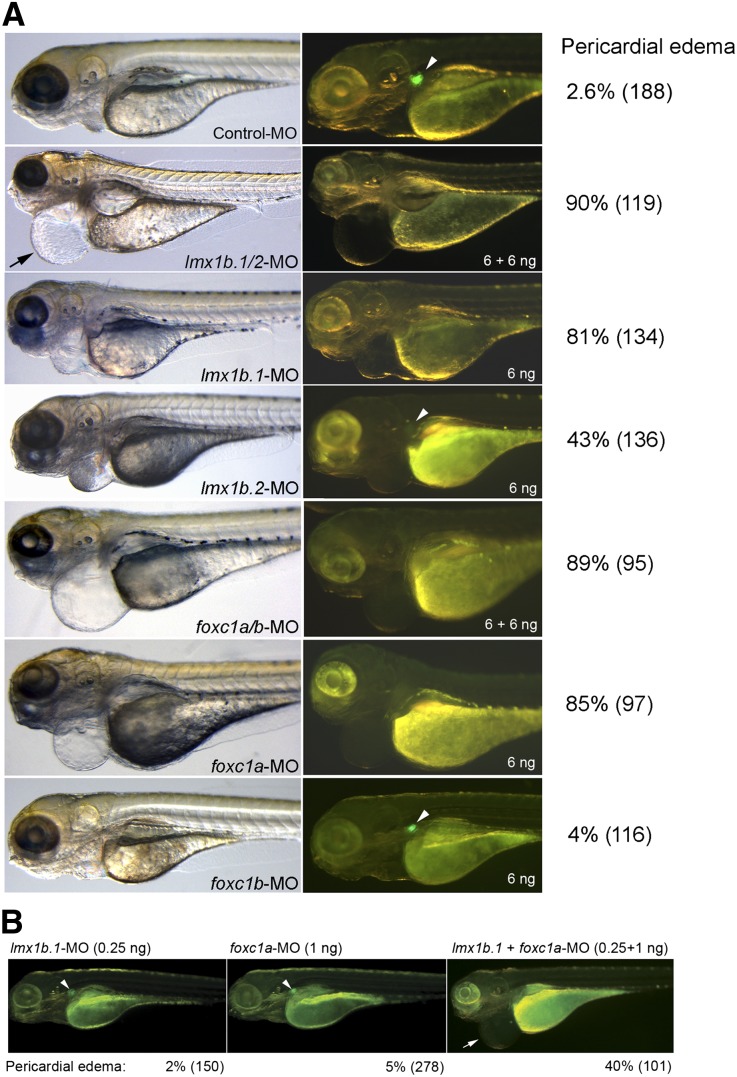
To determine whether two proteins are required for nphs2 expression in vivo, we knocked down lmx1b.1, lmx1b.2, foxc1a and foxc1b by injection of morpholinos (MOs) in Tg(podocin:GFP) embryos. Pericardial edema, associated with pronephric kidney dysfunctions,31,32 and glomerular GFP loss in 4-dpf embryos were used to evaluate the kidney phenotypic consequences of the knockdowns (Figure 3A). Double knockdown of lmx1b.1 and lmx1b.2 led to kidney phenotype in 90% of morphants. Single knockdown of lmx1b.1 resulted in a phenotype that was almost as severe as the double knockdown, whereas single knockdown of lmx1b.2 resulted in a less penetrant, but qualitatively similar, phenotype. These results suggest nonredundant but overlapping roles of lmx1b.1 and lmx1b.2. Double knockdown of foxc1a and foxc1b also led to severe phenotype, but so did single foxc1a knockdown (89% and 85%, respectively). Foxc1b knockdown lacked these effects. To test a combinatorial requirement of the two proteins for nphs2 expression in vivo, we first determined subphenotypic doses of MOs for individual knockdown. Individual injection of 0.25 ng lmx1b.1-MO or 1 ng foxc1a-MO led to no or little phenotype (Figure 3B). Combinatorial knockdown of lmx1b.1 and foxc1a with the same doses of MOs resulted in severe kidney phenotype in 40% of morphants (Figure 3B).
Figure 3.
Lmx1b and FoxC are both required for nphs2 expression in zebrafish. (A) MO-mediated knockdown of lmx1b and foxc1 genes. Microscopic images of 4-dpf morphants of individual or double knockdown as well as controls are displayed. Pericardial edema is indicated (arrow) in bright-field imaging, and glomerular GFP expression is indicated (arrowhead) in dark-field imaging. The percentage of pericardial edema is shown. Number of morphants is indicated in parentheses. (B) Combinatorial knockdown of lmx1b.1 and foxc1a genes. A low dose of lmx1b.1-MO (0.25 ng) or foxc1a-MO (1 ng) was individually injected in Tg(podocin:GFP) embryos. Glomerular GFP expression is indicated (arrowhead) in dark-field imaging. For double knockdown, 0.25 ng lmx1b.1-MO and 1 ng foxc1a-MO were coinjected in embryos. The percentage of pericardial edema in 4-dpf morphants is shown. Number of morphants is indicated in parentheses.
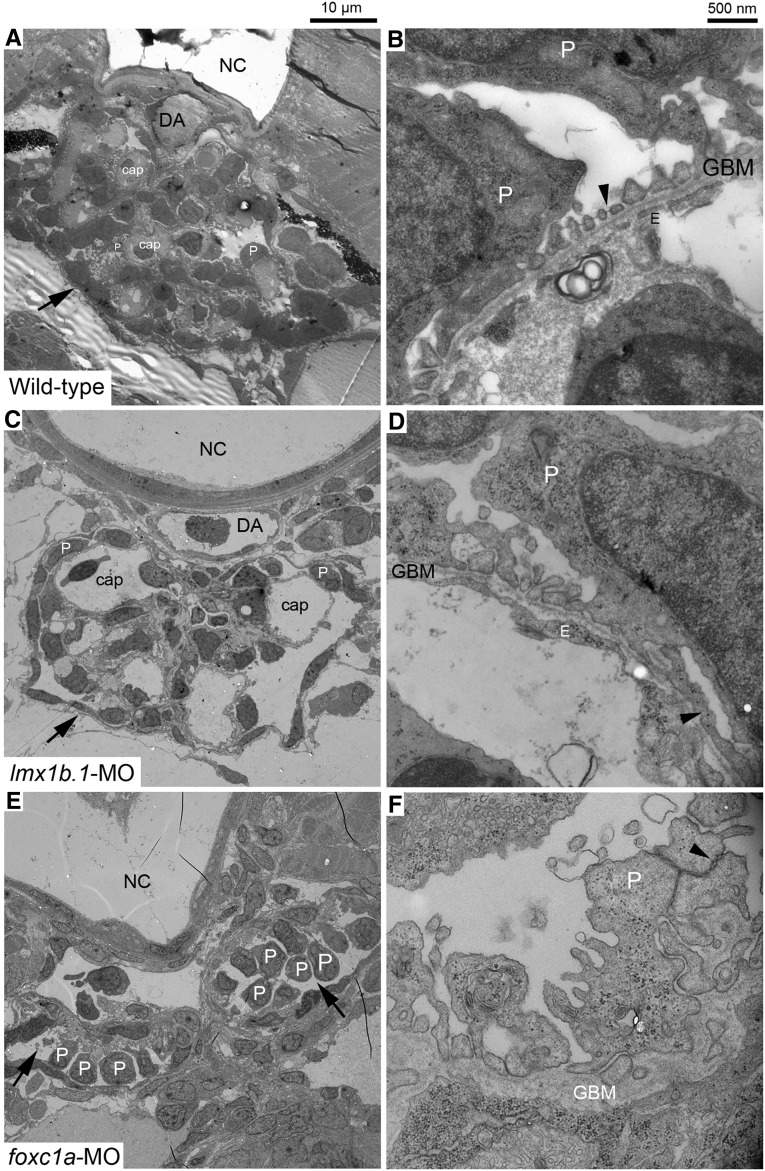
Glomerular ultrastructure of lmx1b.1 and foxc1a morphants exhibit significant developmental defects in podocytes as well as capillary endothelium, which disrupt the glomerular filtration barrier integrity, resulting in the phenotype observed (Figure 4). At low magnification, defects in the lmx1b.1 morphant appear to be podocyte-restricted. However, the foxc1a morphant shows severe defects in developing podocytes and migration of glomerular capillary endothelial cells, although the podocyte specification already occurs (Figure 4, A, C, and E). At higher magnification, two morphants showed a common defect in glomeruli: aberrant or flattened podocyte foot processes and a complete loss of the slit diaphragm in comparison to wild-type (Figure 4, B, D, and F). This is compatible with the known role of podocin in podocyte foot process formation.31,33,34 Taken together, Lmx1b and FoxC are both required for nphs2 expression through a combinatorial action on the enhancer and the formation of a functional pronephric kidney.
Figure 4.
Glomerular ultrastructures in lmx1b.1 and foxc1a morphants at 4 dpf. At low magnification, whole glomeruli (arrow) are exhibited (A, C, and E). Compared with wild-type (A), capillary lumens in the lmx1b.1 morphant are abnormally enlarged (C), and the foxc1a morphant shows that two glomeruli fail to merge at the midline and glomerular capillaries are missing (E). Detailed morphology of the glomerular filtration barrier is displayed at higher magnification (B, D, and F). In wild-type, fine foot processes, slit diaphragm (arrowhead) and fenestrated endothelium together with GBM are clearly visible (B). The lmx1b.1 morphant displays typical effacement (arrowhead), lack of endothelial fenestration, and a complete loss of the slit diaphragm (D). The foxc1a morphant shows aberrant podocyte foot processes on disorganized GBM and absence of endothelial cells. Normal cell-cell junctions by the slit diaphragm disappear and instead typical adherens junctions (arrowhead) occur between adjacent podocytes (F). cap, capillary; DA, dorsal aorta; E, endothelium; NC, notochord; P, podocyte. Magnification is shown by scale bars 10 µm and 500 nm.
Lmx1b.1 and Foxc1a Synergistically Induce nphs2 Expression in Zebrafish
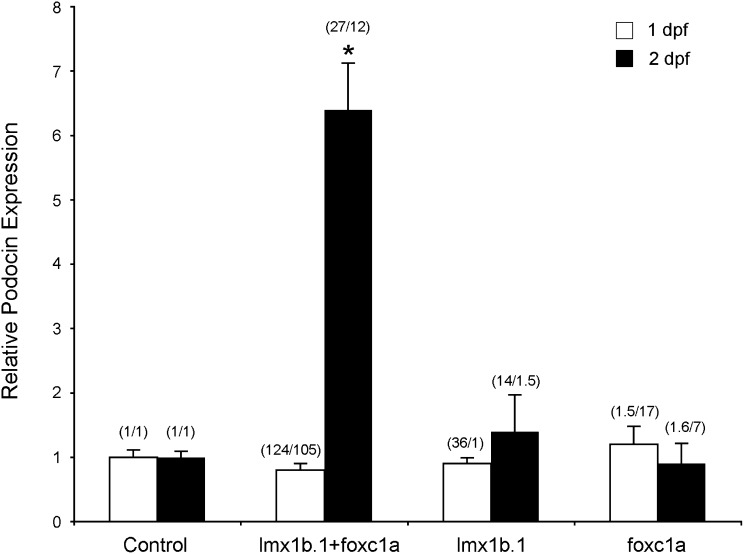
To test whether coexpression of lmx1b and foxc1a could induce endogenous and/or ectopic nphs2 expression in vivo, we generated the transgene of lmx1b.1 and foxc1a in zebrafish G0 embryos (see Concise Methods). To visualize potential ectopic GFP expression, the Tg(podocin:GFP) embryos were used for injection. At 1 dpf, quantitative PCR showed no significant difference of nphs2 expression between any of the experimental situations, although lmx1b.1 and foxc1a were strongly overexpressed (17- to 124-fold) individually or in combination (Figure 5). At 2 dpf, however, co-overexpression (12- to 27-fold) of the combined lmx1b.1 and foxc1a potently induced nphs2 expression, whereas overexpression (7- to 36-fold) of the single transcription factor lacked effect (Figure 5). The results provide additional evidence for that lmx1b and foxc1a combinatorially regulate nphs2 expression in zebrafish podocytes. In addition, we did not observe notable ectopic expression, implying that a unique cellular environment in the podocytes also plays a role in nphs2 expression.
Figure 5.
Lmx1b.1 and foxc1a potently induces endogenous nphs2 expression in zebrafish. Transient overexpression of lmx1b.1 and foxc1a was made by the Tol2 transposon-mediated transgenesis (see Concise Methods). The Tol2-based plasmids coexpressing lmx1b.1 and foxc1a at the same time, and expressing the two genes individually, under the control of the universal EF1α promoter, were injected in Tg(podocin:GFP) embryos. An empty plasmid was injected as a control. Gene expression (ΔCt) was evaluated by quantitative PCR and presented as fold changes, defined as mean 2−ΔCt in tested divided by that in control. Error bars indicate the SEM. Expression levels of lmx1b.1 and foxc1a in fold changes are indicated in parentheses above corresponding bars, respectively. *P<0.001.
Lmx1b-FoxC Motif Is Present in Human NPHS2 and Many Other Genes
By conservation analysis (Supplemental Table 1), we found that the binding motif is also present in the human NPHS2 proximate promoter, suggesting a similar regulatory mechanism controlling its expression by mammalian orthologs Lmx1b and FoxC2. Thus, we evaluated its binding potentials by EMSA. LMX1B and Foxc2 potently and specifically bound to the FLAT-F site and the forkhead site, respectively (Figure 6A). The bindings were specific because the binding signals were completely competed out by unlabeled wild-type probes with three different titrations, but not by unlabeled mutant probes. We observed that the binding by Foxc1 was significantly weaker than that by Foxc2 (Figure 6A).
Figure 6.
Characterization of the Lmx1b-FoxC motif in the human NPHS2 promoter. (A) EMSA. A putative Lmx1b-FoxC motif was identified at −743 bp in the NPHS2 promoter. Radiolabeled wild-type probes (wt) with the FLAT-F (TTAATAA) and the forkhead-binding site (CTAAATA) and recombinant LMX1B, Foxc1, and Foxc2 proteins were used in EMSA. Gel shift assay shows that LMX1B (lane 2 in LMX1B panel) binds to the FLAT-F element. Both Foxc1 and Foxc2 (lanes 5, 8 in FoxC panel) also bind to the forkhead site, but this binding of Foxc2 is much stronger than of Foxc1. For competition assays, 12.5-, 25-, and 37.5-fold excess unlabeled wild-type (wt) or mutant probes (mu), indicated with gradients from low to high, were used. Labeled probes were efficiently competed by unlabeled wild-type probes with elevated amounts, but not by unlabeled mutant probes (lanes 3–8 in LMX1B panel; lanes 3–8 for Foxc1, lanes 10–15 for Foxc2 in FoxC panel). This supports specificity of the bindings. (B) In vivo evaluation of the human cis-acting motif. A 178-bp motif-containing element was subcloned in the Tol2-based cfos-GFP plasmid illustrated in left panel. The plasmid ZP0.2k (Figure 1A) was used as a positive control. GFP expression (arrow) in zebrafish glomerulus is displayed laterally and dorsally.
We then tested cis-acting potentials of the NPHS2 motif using the transgenic zebrafish in vivo. For this experiment, the NPHS2 element containing the putative motif was subcloned in the Tol2-based cfos-GFP plasmid (Figure 6B) and was injected in wild-type embryos. The NPHS2 motif drove glomerular GFP expression in 5.5% (11 of 200) of embryos, compared with no glomerular expression in controls injected with an empty cfos-GFP plasmid (0 of 211). A low homology (48% identity) between FoxC2 and zebrafish foxc1a may lead to this low expression rate. As shown in Figure 6B, glomerular expression driven by the NPHS2 motif element was validated through their location and appearance. Together, the motif is likely to be a cis-acting element controlling podocyte expression of NPHS2.
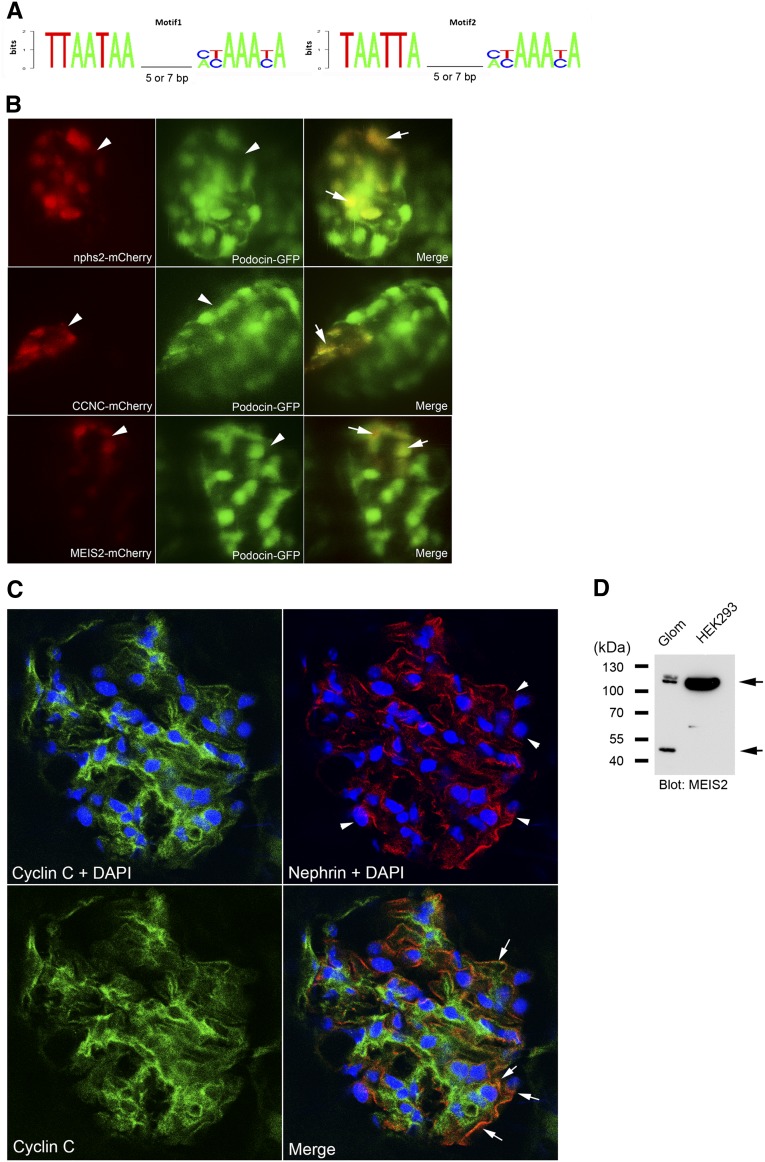
It is interesting to know whether the identified motifs could predict novel genes coexpressed with NPHS2 in podocytes. On the basis of the motif consensus sequences (Figure 7A), we detected 26 candidates genome-wide that carry the motifs in their promoter regions in addition to NPHS2 (Table 1). Among them, glomerular expression levels of CCNC, which encodes the cyclin C protein, and MEIS2, which encodes the Meis homeobox 2 protein, were downregulated almost 3-fold in Foxc2−/− mice,24 suggesting that their expression is regulated by Foxc2. Thus, we tested cis-acting potentials of these two genes. To verify podocyte expression for the two predicted podocyte genes, we reconstructed Tol2-based plasmids, where the CCNC and MEIS2 motifs were coupled with the mCherry cDNA, and then injected them in the Tg(podocin:GFP) embryos. Colocalization of mCherry (red) with glomerular GFP (green) analyzed using confocal microscopy could provide direct evidence for podocyte expression. In addition, we also examined whether the proteins are present in mouse glomeruli. The CCNC element drove glomerular mCherry expression in 7% (7 of 100) of embryos. Similar to the positive control for glomerular colocalization (Figure 7B, upper panel), mosaic mCherry expression driven by the CCNC motif was co-localized with glomerular GFP expression (Figure 7B, middle panel). The MEIS2 motif also drove mCherry expression in 5.4% (6 of 110) of injected embryos, which was colocalized with glomerular GFP (Figure 7B, lower panel). Furthermore, immunostaining of mouse kidney sections showed that cyclin C was present in mouse glomeruli, and was mainly localized in cytoplasm (Figure 7C). Confocal images showed partial overlap (arrow) between cyclin C and nephrin, suggesting its expression in podocytes as well as other cells such as mesangial cells and endothelial cells (Figure 7C). MEIS2 shows strong nuclear expression in human glomeruli (http://www.proteinatlas.org). This antibody did not work in our immunofluorescence staining on mouse kidney sections. Glomerular expression of mouse Meis2 was, however, clearly detected by Western blotting (Figure 7D).
Figure 7.
Podocyte enhancer activity analyses of the predicted Lmx1b-FoxC motifs in CCNC and MEIS2 promoter. (A) Two sequence logos representing the position weight matrix of the Lmx1b-FoxC motif were used for the genome-wide search. Spacing sizes of 5 or 7 nucleotides between two sites were included. (B) In vivo assay for podocyte enhancer activity. Colocalization between transient mCherry expression and stable glomerular GFP expression in living zebrafish was analyzed using confocal microscopy. The zebrafish nphs2 enhancer was used as a positive control (upper panel). Mosaic mCherry expression (red, arrowhead) driven by the motifs of CCNC (middle panel) and MEIS2 (lower panel) shows colocalization (arrow) with glomerular GFP (green, arrowhead). (C) Confocal images of the mouse kidney section stained for cyclin C (green), nephrin (red), and nucleus with DAPI (blue). An image of cyclin C staining without DAPI (lower left panel) is included for assessment of nuclear staining in comparison to cyclin C staining with DAPI. Arrowhead indicates podocytes, and arrow indicates the overlap between cyclin C and nephrin. (D) In Western blotting, Meis2 was detected as two distinct bands (arrows), one with approximately 52-kD (predicted molecular mass, 52 kD) and one with about 110 kD, in mouse whole glomerular lysates (Glom). In nuclear extract of HEK293 cells, only a single strong band with about 110 kD was detected.
Table 1.
Candidate podocyte genes identified by a genome-wide search of the Lmx1b-FoxC motif in human and mouse genomes
| Gene | Human | Mouse | Description | ||
|---|---|---|---|---|---|
| Motif Sequence | Position | Motif Sequence | Position | ||
| ABHD1 | TAATTAccctaATAAACA | −6418 | TATTTATttaatTAATTA | −5998 | Abhydrolase domain containing 1 |
| ANKRD6 | TATTTGTgataacaTAATTA | −2998 | TAATTAcatgcagATAAACA | −7198 | Ankyrin repeat domain 6 |
| ATP5H | TAATTAcagtgACAAACA | −1978 | TGTTTGTtttgtttTTATTAA | −3178 | ATP synthase, H+ transporting, mitochondrial Fo complex, subunit d |
| CASQ2 | TAATTAtttagaaATAAACA | −9778 | TAATTAttttaATAAATA | −8518 | Calsequestrin 2 (cardiac muscle) |
| CCNC | TATTTAGaaaaaTAATTA | −1438 | TGTTTATgtaagTAATTA | −4258 | Cyclin C |
| COL6A3 | TAATTAtaaaaCCAAACA | −7018 | TAATTAaaaaaCCAAATA | −7798 | Collagen, type VI, alpha 3 |
| CYB5R4 | TATTTGTatataaaTTATTAA | −2218 | TATTTGTatatacaTTATTAA | −2158 | Cytochrome b5 reductase 4 |
| DCAF15 | TATTTATtaaatTAATTA | −8398 | TATTTATtaccaTTATTAA | −838 | DDB1 and CUL4 associated factor 15 |
| DUT | TGTTTGGtttaataTAATTA | −6238 | TAATTAtctggATAAACA | −5398 | Deoxyuridine triphosphatase |
| EREG | TTAATAAtttacACAAATA | −6418 | TGTTTAGtatgaaaTAATTA | −4798 | Epiregulin |
| FOXA2 | TATTTAGctcatcaTTATTAA | −8098 | TAATTAttataATAAATA | −8158 | Forkhead box A2 |
| GABRP | TATTTATaatcaTTATTAA | −9418 | TTAATAAtaataATAAATA | −2398 | γ-aminobutyric acid (GABA) A receptor, pi |
| GATA6 | TTAATAAtgataATAAATA | −4798 | TTAATAAtgactCTAAATA | −5038 | GATA binding protein 6 |
| KIF2B | TATTTAGgagatagTTATTAA | −5218 | TATTTAGaagacaaTTATTAA | −6298 | Kinesin family member 2B |
| LMBRD2 | TATTTATtttctTAATTA | −3298 | TAATTAgaatttgATAAATA | −8758 | LMBR1 domain containing 2 |
| MEIS2 | TATTTATccctcTAATTA | −8938 | TATTTATccctcTAATTA | −9598 | Meis homeobox 2 |
| MIR128-1 | TAATTAttcaaCCAAATA | −1018 | TAATTAttcagCCAAATA | −1318 | MicroRNA 128-1 |
| NPHS2 | TTAATAAagaccCTAAATA | −778 | TATTTGTtgtctggTAATTA | −4138 | Nephrosis 2, idiopathic, steroid-resistant (podocin) |
| POGZ | TGTTTAGctgagccTAATTA | −2338 | TGTTTGTgaagcTAATTA | −358 | Pogo transposable element with ZNF domain |
| RAB14 | TAATTAcaaagATAAATA | −6778 | TGTTTAGttttactTAATTA | −5638 | Rab14, member RAS oncogene family |
| SLC16A9 | TGTTTAGtatttTAATTA | −7138 | TTAATAAacaggggATAAACA | −2998 | Solute carrier family 16, member 9 |
| SOHLH2 | TGTTTAGtgaaattTAATTA | −9598 | TATTTATaaatataTAATTA | −2038 | Spermatogenesis and oogenesis specific basic helix-loop-helix 2 |
| SRPX2 | TATTTGTtgaatTAATTA | −1318 | TATTTGTtgaatTAATTA | −2278 | Sushi-repeat containing protein, X-linked 2 |
| STMN4 | TGTTTATtttgaaaTAATTA | −6958 | TATTTATttattgtTAATTA | −4078 | Stathmin-like 4 |
| TSPAN6 | TAATTAattcaACAAATA | −2938 | TAATTAattcaACAAATA | −7798 | Tetraspanin 6 |
| VPS37B | TATTTGTgatcgccTAATTA | −298 | TAATTAaataacaATAAATA | −6718 | Vacuolar protein sorting 37 homolog B (S. cerevisiae) |
| ZSCAN30 | TGTTTAGagcagctTTATTAA | −8458 | TAATTAagaaaATAAATA | −1738 | Zinc finger and SCAN domain containing 30 |
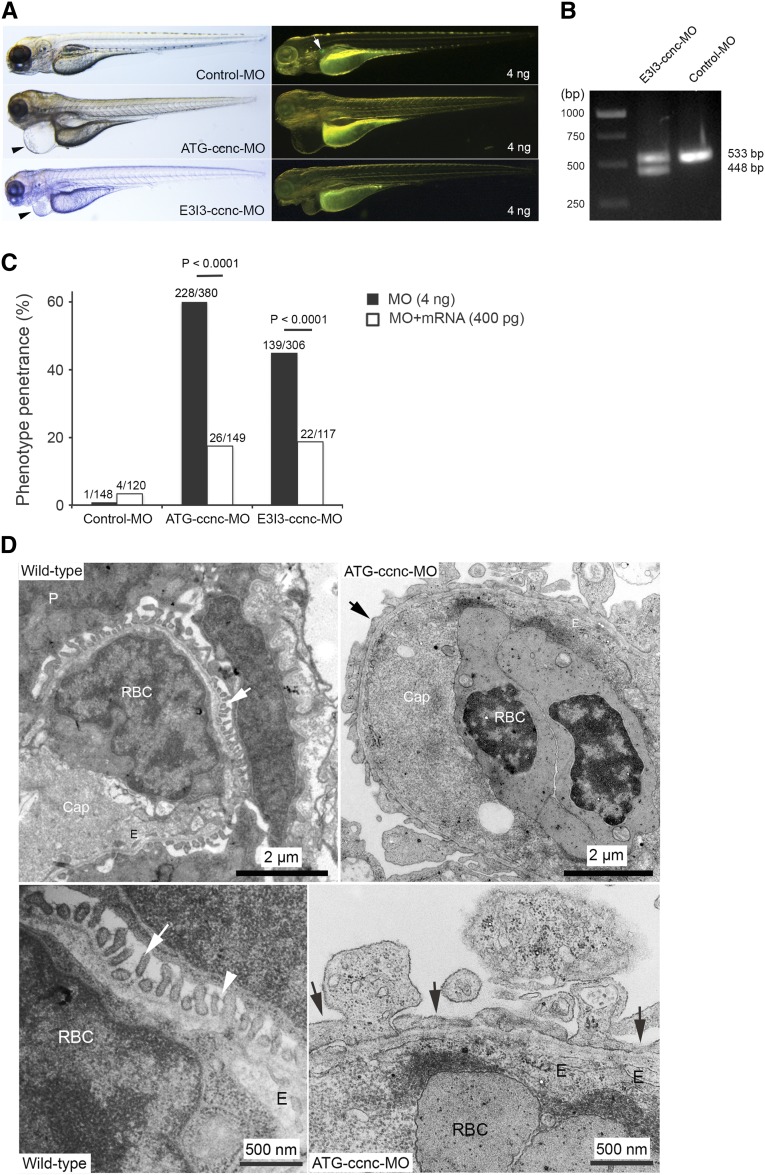
We further asked whether a low rate of glomerular reporter expression driven by the human motifs in zebrafish could reflect real podocyte enhancer activity. To answer this question, we knocked down zebrafish ccnc and tested whether the phenotype generated by MOs was related to podocyte defects. As shown in Figure 8A, injection of MOs, targeting two different regions of ccnc, in Tg(podocin:GFP) embryos resulted in severe pericardial edema and glomerular GFP loss (Figure 8, A and C). The phenotype was significantly rescued by co-injection of heterologous mouse mRNA (P<0.001) (Figure 8C). Glomerular ultrastructure of ccnc morphants displayed widespread effacement of podocyte foot processes and lack of the slit diaphragm (Figure 8D). Together, the observed phenotype caused by ccnc MOs suggests that the defect is podocyte-restricted, although ccnc expression occurs also in other cell types.
Figure 8.
Morpholino-mediated knockdown of zebrafish ccnc gene. (A) Individual injection of two ccnc MOs, ATG-ccnc-MO and E3I3-ccnc-MO, in zebrafish embryos resulted in similar kidney phenotype (pericardial edema and glomerular GFP loss) at 4 dpf, compared with the control-MO. Pericardial edema (arrowhead) in bright-field imaging and glomerular GFP signal (arrow) in dark-field imaging are indicated, respectively. (B) RT-PCR demonstrated efficacy of ccnc knockdown generated by E3I3-ccnc-MO. At 4 dpf, compared with control mRNA showing a single 533-bp band, ccnc morphants appear as two bands (533 bp and 448 bp), indicating deletion of the 85-bp exon 3 generated by this MO. (C) The phenotype caused by ccnc MOs was significantly rescued by coinjection of mouse Ccnc mRNA (400 pg) (P<0.001). The phenotype penetrance generated by MOs and mRNA rescue is illustrated by bar graphs. Phenotypic number of morphants and total number of morphants are indicated. (D) Glomerular ultrastructural analysis of ccnc morphants at 4 dpf. In the wild-type glomerulus, fine foot processes of podocytes (arrowhead), fenestrated endothelial cells and GBM are clearly visible (upper panel). At higher magnification (lower panel), the slit diaphragm (arrowhead) is visible. In contrast, podocytes in the morphants generated by injection of the ATG-ccnc-MO exhibit irregular, abnormally flattened podocyte foot processes (arrow in upper panel) and lack of the slit diaphragm (lower panel), suggesting diffuse podocyte effacement. Normal glomerular endothelium in ccnc morphants becomes invisible at higher magnification (lower panel). Compared with wild-type, GBM shows no notable difference (upper panel). cap, capillary lumen; E, endothelium; P, podocyte; RBC, red blood cells. Magnification is shown by scale bars 2 µm and 500 nm.
Discussion
Expression of NPHS2 is highly restricted to podocytes and is constantly active once it is activated during the podocyte development.7 Here, we show that podocyte-specific expression of NPHS2 or zebrafish nphs2 is combinatorially regulated by Lmx1b and FoxC through binding to two adjacent DNA-binding sites. We designate this cis-acting motif as the Lmx1b-FoxC enhancer, in which the forkhead site is bound by FoxC2 in mammals and foxc1a in zebrafish. This enhancer is also present in many other genes.
Combinatorial control represents an important gene regulatory mechanism in which multiple transcription factors come together to exert specific transcriptional control.35 This regulatory manner has been hypothesized for some tissue-restricted expression in multicellular organisms. A recent study revealed that transcriptional regulation of endothelium-specific gene expression follows this principle, and specifically that FoxC and Ets transcription factors cooperate by interacting with neighboring binding sites in a FOX:ETS enhancer present in several endothelial-specific genes.36 Accordingly, this principle is likely to apply to a portion of podocyte-specific gene expression. Thus, NPHS2 may represent an ideal gene for test because of its unique expression pattern. Using in vivo zebrafish models, we identified a 49-bp podocyte-specific enhancer that contains two adjacent DNA-binding sites, potentially bound by Lmx1b and FoxC. To claim combinatorial control, several basic points should be addressed: the DNA binding sites of the enhancers, coexpression of transcription factors required in a target cell, and cooperativity of protein-protein as well as protein-DNA. For the binding site, we found that the putative binding motif within the zebrafish nphs2 proximal promoter is highly conserved across species, implying a functional potential. Interestingly, 5- or 7-bp spacing between two sites represents a major form. The spacing in the motif may be critical in configuration of the DNA-protein complex. The DNA double-helical periodicity is 10.4 bp per turn,37 and therefore the interaction between two transcription factors may or may not be possible depending on what side of the double helix they bind. By in vitro EMSA and in vivo mutagenesis analyses, we demonstrate that the two adjacent DNA-binding sites are specifically bound by Lmx1b and FoxC and are both required for the activation of transcription, providing molecular basis for the combinatorial binding by two proteins. At the structure level, DNA binding of multiple proteins may lead to various alterations in protein structure, including the formation of additional secondary structural elements, reorientation of loops, rearrangements of hydrophobic cores, and changes of their quaternary structure.35 These alterations in the stereo-specific complex may highly depend on the cellular microenvironment, and detecting the cobinding of two proteins to DNA as a complex by a conventional EMSA in vitro is likely to be difficult.
Lmx1b and FoxC2 are both expressed in mammalian podocytes.14,15,24 In frog glomerulus, foxc2 is one of the earliest expressed transcription factors at stage 20, and lmx1b and nphs2 simultaneously appear at stage 27.38 In zebrafish, foxc1a expression appears in podocyte progenitors at 8-somite stage, while nphs2 expresses in mature podocytes at 1.5 dpf.39 In agreement with data from mouse knockout studies,14,15 our zebrafish data support that lmx1b.1 is essential for nphs2 expression because the ultrastructural alteration by lmx1b.1 knockdown is compatible with the observed pronephros dysfunction and with the known role of podocin in podocyte foot process formation.31,33,34 The role of foxc1a in developing glomeruli seems to be more complex. Our data show that foxc1a knockdown is unlikely to affect the podocyte specification; instead it significantly disrupts podocyte differentiation and recruitment of endothelial cells, suggesting that foxc1a plays a role in cross-talking between two types of cells for functional glomerulus formation in addition to regulating the nphs2 expression. FoxC2/foxc1a is probably involved in regulating VEGF-A gene expression in podocytes and contributes to the VEGF signaling cascade.
Whether Lmx1b and FoxC cooperatively act on nphs2 enhancer represents a key aspect for a combinatorial control, although they are both required for nphs2 expression. To address their cooperativity, we carried out assays for loss-of-function and gain-of-function strategies. First, combinatorial knockdown of the two genes completely disrupts the podocyte development in 40% of morphants, in comparison with no or little phenotype by individual knockdown of lmx1b.1 or foxc1a using the same doses of MOs. Thus, if lmx1b.1 or foxc1a acts independently on the targets, the phenotypic penetrance by double knockdown is likely to be similar to that by individual knockdowns. Furthermore, in vivo overexpression experiments showed that endogenous expression of nphs2 in zebrafish was potently induced only by co-overexpression of lmx1b.1 and foxc1a, but not by individual overexpression. Taken together, these two experiments strongly support a combinatorial action of the two proteins on the nphs2 enhancer. The LIM domains of Lmx1b mediate protein-protein interaction and have been shown to interact with LDB1, E47/shPan1,40 and PAX2.41 LIM-homeodomain proteins might interact combinatorially with other transcription factors in a homomeric or heteromeric fashion, and the formation of higher-order transcriptional-regulator complex may regulate expression in a tissue-specific manner.42 We thus speculate that the Nphs2 enhancer motif is bound specifically by the homeodomain of Lmx1b and the helix-turn-helix structure of FoxC. The specific spacing between two sites allows the interaction of two DNA-bound proteins through the LIM domain of Lmx1b, leading to the formation of a regulator complex for activating Nphs2 transcription. In vivo chromatin-immunoprecipitation or in vitro co-immunoprecipitation in transfected podocytes is of great help in providing evidence for the interaction. The work deserves further exploration.
We reasoned that the evolutionarily conserved Lmx1b-FoxC enhancer might regulate many other genes important for the podocyte development and function. Through a genome-wide search for the motifs in the promoter regions throughout all human genes, we identified 26 novel podocyte-expressed genes, but, to our knowledge, none of them have been previously demonstrated to play a functional role in podocytes. Therefore, we are cautious in characterizing the predicted data. To validate podocyte expression of these predicted genes, we used two evaluating assays: (1) in vivo reporter expression in zebrafish and (2) endogenous expression in mouse glomeruli, preferably using double immunofluorescence staining. Two selected genes—CCNC and MEIS2—for follow-up analysis met the criteria. Podocyte expression of CCNC was further supported by the zebrafish ccnc knockdown, demonstrating that CCNC is required for podocyte development and normal podocyte functions. Together, the Lmx1b-FoxC motif may be an efficient predictor to identify novel podocyte-expressed genes, although they are not necessarily podocyte-specific. It is interesting how a member of the cyclin protein family that is involved in regulating cell cycle progression plays an important role in terminally differentiated podocytes. Cyclins function through activating their partners, cyclin-dependent kinases. The role of cyclin C in the cell cycle is unclear.43 Thus far, Cdk8 and Cdk3 are the only known kinases associated with cyclin C.44,45
Podocytes rapidly lose their characteristic and specific protein expression pattern when cultured in vitro.1 To evaluate cis-regulatory elements controlling podocyte gene expression, we therefore reasoned that in vivo models are likely required. In this study, we validate that G0 embryos are suitable for rapid evaluation of potential cis-acting elements in vivo. Interestingly, the human Lmx1b-FoxC motif can be activated in zebrafish podocytes, albeit at a relatively low expression rate. Thus, this system may be useful in identifying other cell type–specific enhancers.
In summary, the complex architecture and unique function of podocytes apparently require the cooperation of common transcription factors to achieve specific podocyte gene expression. We show that through binding to a unique DNA motif, Lmx1b and FoxC combinatorially regulate gene expression in podocytes. Our findings also indicate novel podocyte genes coexpressed with Nphs2. Our results provide insights into the transcriptional regulatory mechanisms required for normal podocyte functions and likely also important for renal diseases.
Concise Methods
Zebrafish
The wild-type AB zebrafish and the Tg(podocin:GFP) line13 were maintained at the Karolinska Institute zebrafish core facility.
Transgenic Zebrafish
To generate transgenic zebrafish, we used the Tol2 transposon-mediated transgenesis in zebrafish as described.46 The Tol2 transposon-based plasmids contain two Tol2 transposable sequences flanking the insertion fragment sites, and they were comicroinjected, together with the Tol2 transposase mRNA, in one- to two-cell zebrafish embryos. The translated transposase protein from injected mRNA in fertilized eggs catalyzes excision of the Tol2 transposable elements from the plasmid, leading to stable integration of the excised DNA fragments in the genome. Generally, the insertion fragment consists of a promoter and a downstream reporter gene such as GFP or any cDNA sequences to be expressed in zebrafish. In this study, we analyzed glomerular expression of GFP or mCherry in injected G0 embryos at 4 dpf as a rapid in vivo expression system. We showed that despite its mosaic pattern, this transient expression assay accurately reflects expression of germline zebrafish (see Results and Supplemental Figure 1). The Ethics Committee of Karolinska Institute has approved the experiments using the transgenic manipulation in zebrafish.
Morpholino-Mediated Knockdown
MOs were used to knock down zebrafish genes lmx1b.1, lmx1b.2, foxc1a, foxc1b, and ccnc. The sequences of these MOs used are shown in Supplemental Table 2. An unrelated standard control MO (control-MO), provided by the manufacturer, was used as a negative control. In addition, a mismatch lmx1b.1 MO was also used as a gene-specific control. About 4–6 ng of MOs were normally injected into the yolk of one- or two-cell Tg(podocin:GFP) embryos. For knockdown of zebrafish ccnc gene, a translation-blocking MO (ATG-ccnc-MO) and a splice-blocking MO (E3I3-ccnc-MO) were used. The splice-blocking efficacy by the E3I3-ccnc-MO was evaluated by using RT-PCR. As shown in Figure 8B, an in-frame deletion of exon 3 caused by this MO at 4 dpf was detected as a decrease in amplicon size (448 bp) compared with wild-type (533 bp). For the mRNA rescue, mouse ccnc mRNA (400 pg), in vitro transcribed from a mouse full-length ccnc cDNA plasmid (Origene) using the mMESSAGE mMACHINE kit (Ambion), was coinjected with MOs. Embryos injected with 4–6 ng control-MO and with 6 ng mismatch lmx1b.1-MO did not produce any discernable pericardial edema, similar to wild-type embryos.
Cloning, Plasmids and Mutagenesis
For fine-mapping the promoter, the Tol2-based plasmid carrying the 2.5-kb zebrafish nphs2 promoter fragment was subjected to a series of digestions at naturally occurring restriction sites (Supplemental Figure 1A), followed by religations. PCR was also used to generate the plasmid carrying the shortest element (ZP-0.1k) between −110 and 63 bp. For the heterologous promoter analysis and the forkhead site deletion analysis, the elements between −185 and −62 and between −185 and −77 were generated by PCR and then subcloned upstream of the mouse c-fos minimal promoter in the Tol2-based pGW-cfos-GFP plasmid, provided by Dr. Andrew S. McCallion (Johns Hopkins University School of Medicine, Baltimore, MD). This plasmid, with some modification, such as replacement of GFP by mCherry, was also used to evaluate cis-acting potentials of putative enhancer motifs in CCNC and MEIS2 in vivo. In addition, a mCherry plasmid coupled with zebrafish nphs2 promoter (nphs2-mCherry) was used as a positive control for glomerular colocalization. To induce zebrafish endogenous nphs2 expression, full-length cDNA of zebrafish foxc1a and lmx1b.1 was amplified from cDNA of 4-dpf wild-type embryos and was constructed in the pT2KXIG plasmid,46 in which GFP sequence was replaced by the lmx1b.1-IRES-foxc1a cDNA fragment. This construct contains a Xenopus EF1α enhancer/promoter that strongly drives ubiquitous expression in zebrafish. A pcDNA plasmid encoding human LMX1B was provided by Dr. Brendan Lee (Baylor College of Medicine, Houston, TX). In addition, two pcDNA plasmids expressing mouse Foxc1 and Foxc2 were obtained elsewhere. The point mutations were generated using QuickChange II Site-Direct Mutagenesis kit (Stratagen) according to the manufacturer’s instructions. The primer sequences used for cloning and mutagenesis are shown in Supplemental Table 3.
Quantitative PCR
Total RNA was isolated from a mixture of five whole embryos using the RNeasy Mini Kit (Qiagen). The first-strand cDNA synthesis was carried out using the iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed on the ABI PRISM 7300 Sequence Detection System using the TaqMan or SYBR Green method (Applied Biosystems). Triplicates for each sample were carried out. The relative quantification of gene expression was analyzed using the comparative threshold method. Data were presented as mean±SEM 2-ΔCt.
Immunofluorescence Staining and Western Blotting
Kidneys from C57BL/6 mice were snap-frozen and embedded in optimal cutting temperature media. Cryosections (8 µm) were postfixed with cold acetone for 10 minutes, followed by blocking in 5% normal goat or donkey serum. For immunofluorescence staining, the primary antibodies to cyclin C (1:100, ab2950; Abcam), MEIS2 (1:100, HPA003256; Sigma-Aldrich) and nephrin (1:200, guinea pig; Acris Antibodies GmbH) were incubated at 37°C for 1 hour, followed by 45-minute incubation with corresponding Alexa fluor (Invitrogen) secondary antibodies. Western blotting was performed with standard procedures. Whole glomerular lysates isolated from adult C57BL/6 mice using Dynabead perfusion24 and nuclear extract of HEK293 cells were used. The primary antibody to MEIS2 (1:800, HPA003256; Sigma-Aldrich) was used. The local ethical committee (the North Stockholm district court) approved studies in mice.
EMSA
EMSAs were performed as previously described.47 Recombinant proteins were in vitro synthesized using the TNT Quick Couple Transcription/Translation System (Promega) according to the manufacturer’s protocols. mRNA used for these protein synthesis was transcribed from plasmids expressing LMX1B, lmx1b.1, foxc1a, Foxc1, and Foxc2 using SP6 or T7 polymerase. Specificity of EMSA was evaluated by competition assay, where unlabeled wild-type or mutant probes in three titrations of 12.5-fold (0.25 µg), 25-fold (0.5 µg), and 37.5-fold (0.75 µg) excess were used as competitor to labeled wild-type probes. Oligonucleotide sequences used for EMSA are provided in Supplemental Table 4.
Confocal Imaging
For in vivo imaging of transgenic zebrafish glomerulus, confocal microscopy (Leica TCS SP8) was performed (magnification ×20). Larvae at 5 dpf were anesthetized by 0.1% tricaine and embedded in 1% low-melt agarose gel. Confocal imaging of mouse kidney immunostaining was performed using Zeiss LSM 700 (magnification ×63).
Transmission Electron Microscopy
Transmission electron microscopy was performed as previously described.48 Briefly, larvae were fixed in the fixation solution buffer (2% glutaraldehyde, 0.5% paraformaldehyde, 0.1 M cacodylate, 0.1 M sucrose, 3 mM CaCl2) and washed in 0.1 M cacodylate buffer (pH, 7.4) before staining in 2% OsO4 in cacodylate buffer for 1 hour at room temperature. Samples were dehydrated and en bloc staining was performed in 2% uranyl acetate in absolute ethanol for 1 hour at room temperature; samples were then taken through an Epon 812/acetone series and embedded at 60°C in pure Epon 812. Thin sections of 70 nm thickness were made on a Leica EM UC6 Ultratome and mounted on Formvar-coated copper slot grids. Poststaining was done with 2% aqueous acetate (pH, 3.5) and Venable and Coggleshall lead citrate. Grids were analyzed on an FEI TECNAI electron microscope.
Genome-Wide Identification of the Conserved Lmx1b-FoxC Motifs
The 5′ flanking DNA sequences within 10 kb in length upstream from the transcription start sites of all human and mouse genes were downloaded from the Ensembl databases (http://www.ensembl.org/downloads.html) and were scanned using the Lmx1b-FoxC motifs. The position weight matrix for the motif scanning, based on the two motif sequences from zebrafish and human with 5- or 7-bp spacing, was shown in Figure 7A. Resulting hits from the two genomes were further analyzed. Final candidates were determined if the hit was (1) present in two genomes and (2) protein-coding locus or noncoding locus with transcript evidence. Pseudogenes were excluded.
Disclosures
None.
Supplementary Material
Acknowledgments
We acknowledge Susan Warner and her colleagues at the Karolinska Institute zebrafish core facility for maintaining fish and providing embryos. Partial results of this study were presented in abstract form at the annual meeting of the American Society of Nephrology in 2012. This work was supported in part by grants from Foundations of the Knut and Alice Wallenberg, Söderberg and Hedlund Foundations, the Swedish Research Council, and the Swedish Foundation for Strategic Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012080823/-/DCSupplemental.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Somlo S, Mundel P: Getting a foothold in nephrotic syndrome. Nat Genet 24: 333–335, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Tryggvason K, Wartiovaara J: How does the kidney filter plasma? Physiology (Bethesda) 20: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Quaggin SE: Transcriptional regulation of podocyte specification and differentiation. Microsc Res Tech 57: 208–211, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: Good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Frishberg Y, Rinat C, Megged O, Shapira E, Feinstein S, Raas-Rothschild A: Mutations in NPHS2 encoding podocin are a prevalent cause of steroid-resistant nephrotic syndrome among Israeli-Arab children. J Am Soc Nephrol 13: 400–405, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Karle SM, Uetz B, Ronner V, Glaeser L, Hildebrandt F, Fuchshuber A: Novel mutations in NPHS2 detected in both familial and sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol 13: 388–393, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellin L, Huber TB, Gerke P, Quack I, Pavenstädt H, Walz G: NEPH1 defines a novel family of podocin interacting proteins. FASEB J 17: 115–117, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 13.He B, Ebarasi L, Hultenby K, Tryggvason K, Betsholtz C: Podocin-green fluorescence protein allows visualization and functional analysis of podocytes. J Am Soc Nephrol 22: 1019–1023, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miner JH, Morello R, Andrews KL, Li C, Antignac C, Shaw AS, Lee B: Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest 109: 1065–1072, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohr C, Prestel J, Heidet L, Hosser H, Kriz W, Johnson RL, Antignac C, Witzgall R: The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes. J Clin Invest 109: 1073–1082, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RL, Tabin CJ: Molecular models for vertebrate limb development. Cell 90: 979–990, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL: Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 19: 51–55, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, Cole W, Johnson RL, Lee B: Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet 19: 47–50, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Dreyer SD, Morello R, German MS, Zabel B, Winterpacht A, Lunstrum GP, Horton WA, Oberg KC, Lee B: LMX1B transactivation and expression in nail-patella syndrome. Hum Mol Genet 9: 1067–1074, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Morello R, Zhou G, Dreyer SD, Harvey SJ, Ninomiya Y, Thorner PS, Miner JH, Cole W, Winterpacht A, Zabel B, Oberg KC, Lee B: Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nat Genet 27: 205–208, 2001 [DOI] [PubMed] [Google Scholar]
- 21.German MS, Wang J, Chadwick RB, Rutter WJ: Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: Building a functional insulin minienhancer complex. Genes Dev 6: 2165–2176, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Suleiman H, Heudobler D, Raschta AS, Zhao Y, Zhao Q, Hertting I, Vitzthum H, Moeller MJ, Holzman LB, Rachel R, Johnson R, Westphal H, Rascle A, Witzgall R: The podocyte-specific inactivation of Lmx1b, Ldb1 and E2a yields new insight into a transcriptional network in podocytes. Dev Biol 304: 701–712, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Dunston JA, Reimschisel T, Ding YQ, Sweeney E, Johnson RL, Chen ZF, McIntosh I: A neurological phenotype in nail patella syndrome (NPS) patients illuminated by studies of murine Lmx1b expression. Eur J Hum Genet 13: 330–335, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsson P, Mahlapuu M: Forkhead transcription factors: Key players in development and metabolism. Dev Biol 250: 1–23, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Miura N, Iida K, Kakinuma H, Yang XL, Sugiyama T: Isolation of the mouse (MFH-1) and human (FKHL 14) mesenchyme fork head-1 genes reveals conservation of their gene and protein structures. Genomics 41: 489–492, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS: Defining the molecular character of the developing and adult kidney podocyte. PLoS ONE 6: e24640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yildirim-Toruner C, Subramanian K, El Manjra L, Chen E, Goldstein S, Vitale E: A novel frameshift mutation of FOXC2 gene in a family with hereditary lymphedema-distichiasis syndrome associated with renal disease and diabetes mellitus. Am J Med Genet A 131: 281–286, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Topczewska JM, Topczewski J, Shostak A, Kume T, Solnica-Krezel L, Hogan BL: The winged helix transcription factor Foxc1a is essential for somitogenesis in zebrafish. Genes Dev 15: 2483–2493, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Hara FP, Beck E, Barr LK, Wong LL, Kessler DS, Riddle RD: Zebrafish Lmx1b.1 and Lmx1b.2 are required for maintenance of the isthmic organizer. Development 132: 3163–3173, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA: Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol 285: 316–329, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumdar A, Drummond IA: The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev Biol 222: 147–157, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, Gubler MC, Antignac C, Esquivel EL: Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol 20: 2181–2189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C: Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol 24: 550–560, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reményi A, Schöler HR, Wilmanns M: Combinatorial control of gene expression. Nat Struct Mol Biol 11: 812–815, 2004 [DOI] [PubMed] [Google Scholar]
- 36.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL: Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 135: 1053–1064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prunell A, Kornberg RD, Lutter L, Klug A, Levitt M, Crick FH: Periodicity of deoxyribonuclease I digestion of chromatin. Science 204: 855–858, 1979 [DOI] [PubMed] [Google Scholar]
- 38.White JT, Zhang B, Cerqueira DM, Tran U, Wessely O: Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus. Development 137: 1863–1873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien LL, Grimaldi M, Kostun Z, Wingert RA, Selleck R, Davidson AJ: Wt1a, Foxc1a, and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev Biol 358: 318–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jurata LW, Gill GN: Functional analysis of the nuclear LIM domain interactor NLI. Mol Cell Biol 17: 5688–5698, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marini M, Giacopelli F, Seri M, Ravazzolo R: Interaction of the LMX1B and PAX2 gene products suggests possible molecular basis of differential phenotypes in Nail-Patella syndrome. Eur J Hum Genet 13: 789–792, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Hobert O, Westphal H: Functions of LIM-homeobox genes. Trends Genet 16: 75–83, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Sage J: Cyclin C makes an entry into the cell cycle. Dev Cell 6: 607–608, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Ren S, Rollins BJ: Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 117: 239–251, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Tassan JP, Jaquenoud M, Léopold P, Schultz SJ, Nigg EA: Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc Natl Acad Sci U S A 92: 8871–8875, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M: A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell 7: 133–144, 2004 [DOI] [PubMed] [Google Scholar]
- 47.De Val S, Anderson JP, Heidt AB, Khiem D, Xu SM, Black BL: Mef2c is activated directly by Ets transcription factors through an evolutionarily conserved endothelial cell-specific enhancer. Dev Biol 275: 424–434, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, Majumdar A: A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol 334: 1–9, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.