Abstract
In a recent randomized trial, weekly recombinant tissue plasminogen activator (rt-PA), 1 mg per lumen, once per week, and twice-weekly heparin as a locking solution (rt-PA/heparin) resulted in lower risks of hemodialysis catheter malfunction and catheter-related bacteremia compared with thrice-weekly heparin (heparin alone). We collected detailed costs within this trial to determine how choice of locking solution would affect overall health care costs, including the cost of locking solutions and all other relevant medical costs over the course of the 6-month trial. Nonparametric bootstrap estimates were used to derive 95% confidence intervals (CIs) and mean cost differences between the treatment groups. The cost of the locking solution was higher in patients receiving rt-PA/heparin, but this was partially offset by lower costs for managing complications. Overall, the difference in unadjusted mean cost for managing patients with rt-PA/heparin versus heparin alone was Can$323 (95% CI, −$935 to $1581; P=0.62). When the costs were extrapolated over a 1-year time horizon using decision analysis, assuming ongoing rt-PA effectiveness, the overall costs of the strategies were similar. This finding was sensitive to plausible variation in the frequency and cost of managing patients with catheter-related bacteremia, and whether the benefit of rt-PA on catheter-related bacteremia was maintained in the long term. In summary, we noted no significant difference in the mean overall cost of an rt-PA/heparin strategy as a locking solution for catheters compared with thrice-weekly heparin. Cost savings due to a lower risk of hospitalization for catheter-related bacteremia partially offset the increased cost of rt-PA.
An increasing number of patients undergoing hemodialysis are managed with permanent hemodialysis catheters.1,2 In North America, approximately 80% of patients initiate hemodialysis with a catheter, and 20%–60% of prevalent hemodialysis patients receive dialysis with a catheter.3,4 Most hemodialysis catheters fail within 1 year of insertion; up to two thirds of these failures are due to thrombosis, and most of the remainder are removed because of catheter-related bacteremia.5,6 The need for catheter thrombolysis, catheter replacement, and management of catheter-related bacteremia contributes to significant costs.7 As such, hemodialysis patients managed with a catheter have significantly higher costs than those who have a fistula.8,9
Given these adverse outcomes and high costs, further research on strategies to optimize the use of hemodialysis catheters is required, particularly with regard to preventing the most common adverse catheter outcomes of malfunction (i.e., inability to initiate or continue dialysis because of to inadequate bloodflow, often the result of catheter thrombosis) and bacteremia.5,6 Heparin has traditionally been used as an intradialytic catheter locking solution, although other agents are now used, including citrate10,11 and recombinant tissue plasminogen activator (rt-PA).12 Intradialytic locking with a thrombolytic agent has many appealing features. For example, it may reduce intraluminal thrombosis and improve catheter function while also eliminating the nidus for biofilm formation and infection.
We recently completed a randomized clinical trial (the Prevention of Dialysis Catheter Malfunction with Recombinant Tissue Plasminogen Activator [PreCLOT]) in hemodialysis patients with a newly inserted tunnelled central venous catheter. The study compared rt-PA once per week and twice-weekly heparin as a locking solution (rt-PA/heparin) with thrice-weekly heparin (heparin alone) as the catheter-locking solution.13 During the 6-month study period, the catheter malfunctioned in 40 (34.8%) patients assigned to heparin alone and 22 (20.0%) patients assigned to rt-PA/heparin (hazard ratio, 1.91; 95% confidence interval [95% CI], 1.13 to 3.22; P=0.02), and catheter-related bacteremia occurred in 16 (13.9%) patients assigned to heparin alone and 5 (4.6%) assigned to rt-PA/heparin (hazard ratio, 3.30; 95% CI, 1.18 to 9.22; P=0.02).
Although rt-PA is significantly more expensive than heparin as a locking solution, the consequences of catheter malfunction and catheter-related bacteremia must be considered because they may contribute to substantial costs. We a priori identified the need to collect costs throughout the trial,12 enabling a detailed comparison of the overall health care costs of rt-PA/heparin versus those of heparin alone. Because the treatment period within the clinical trial was only 6 months in duration, we also created a decision analytic model to estimate the costs and clinical effectiveness of using rt-PA/heparin versus heparin alone as the locking solution over a 1-year period.
Results
Costing Analysis Alongside the Clinical Trial
Baseline patient characteristics are reported in Table 1. For most patients in both the rt-PA/heparin and heparin-only groups, this was their first hemodialysis catheter (67% and 70%, respectively). The mean age in the rt-PA group was slightly lower (61.6 years versus 64.8 for heparin; P=0.13). The total mean duration of patient follow-up for assessment of costs (including ongoing follow-up after clinical outcomes) was 133.0 and 126.2 days in the rt-PA/heparin and heparin groups, respectively.
Table 1.
Baseline characteristics of the PreCLOT study patients
| Characteristic | rt-PA (n=110) | Heparin Only (n=115) |
|---|---|---|
| Age (yr) | 61.6±16.6 | 64.8±15.2 |
| Women, n (%) | 39 (35.5) | 49 (42.6) |
| First catheter ever, n (%) | 67 (60.9) | 70 (60.9) |
| Indication for current catheter, n (%) | ||
| Catheter-related bacteremia | 4 (3.6) | 10 (8.7) |
| Failed access, or awaiting arteriovenous access | 21 (19.1) | 27 (23.5) |
| Starting dialysis without arteriovenous access | 60 (54.5) | 58 (50.4) |
| Transfer from peritoneal dialysis or transplant | 13 (11.8) | 8 (7.0) |
| Other | 12 (11.0) | 12 (10.4) |
| Duration of hemodialysis (yr) | ||
| Median | 0.5 | 1.0 |
| Interquartile range | 0–1.0 | 0–6.0 |
| Comorbid illnesses, n (%) | ||
| Diabetes mellitus | 60 (54.5) | 64 (55.7) |
| Ischemic heart disease | 26 (23.6) | 21 (18.3) |
| Congestive heart failure | 28 (25.5) | 22 (19.1) |
| Cerebral vascular disease | 12 (10.9) | 17 (14.8) |
| Hypertension | 104 (94.5) | 102 (88.7) |
| Prior pulmonary embolism or DVT | 5 (4.5) | 6 (5.2) |
| Prior gastrointestinal bleed | 12 (10.9) | 9 (7.8) |
Values expressed with a plus/minus sign are the mean±SD. DVT, deep venous thrombosis.
The unadjusted costs per patient, by treatment groups, over the 6-month study are displayed in the Supplemental Material. The mean total cost for managing patients with rt-PA/heparin and heparin were Can$1872 (95% CI, Can$1142 to Can$2601) and Can$1538 (95% CI, Can$503 to Can$2573), with no difference in the mean costs for the rt-PA/heparin and heparin groups (Can$323; 95% CI, Can$−935 to Can$1581; P=0.62) (Table 2). In a secondary analysis including the cost of bleeding complications, the mean total cost for managing patients with rt-PA/heparin and heparin were Can$2015 (95% CI, Can$1268 to Can$2763) and Can$1872 (95% CI, Can$803 to Can$2941), with the mean difference in cost similar for the rt-PA/heparin and heparin groups ($134 ;95% CI, Can$−2346 to Can$2286; P=0.66). To assess the robustness of the bootstrapping method for comparing costs, we also used generalized linear regression and a Gaussian linear model to compare costs, noting a similar difference in costs between rt-PA/heparin and heparin (Can$334; 95% CI, Can$−929 to Can$1597; P=0.60). Acknowledging that the price of rt-PA is higher in the United States ($107 per 2 mg compared with Can$64 in Canada, and that hospitalizations and procedures are typically 50% higher in the United States than in Canada,14 we recalculated the mean total costs for the rt-PA/heparin and heparin strategies, noting no difference in the mean costs for the rt-PA/heparin and heparin groups ($689; 95% CI, $−1187 to $2566; P=0.47).
Table 2.
Unadjusted mean cost of managing patients in the PreCLOT study with rt-PA or heparin over the 6-month study period
| Variable | rt-PA (n=110) | Heparin Only (n=115) |
|---|---|---|
| Drug (rt-PA/ heparin) costs | 1206±598 | 130±85 |
| Cost of managing patients experiencing catheter malfunction | 47±197 | 113±446 |
| Cost of managing patients with catheter-related bacteremia | 619±3957 | 1295±5629 |
| Total cost | 1872±3861 | 1538± 5605 |
| Difference in total costsa (rt-PA minus heparin) (95% CI) | 323 (−935 to 1581) | |
| P value | 0.616 | |
Results reported as mean±SD unless otherwise indicated. Costs are expressed in 2011 Can$.
Mean difference, 95% CI, and P value were calculated with the bootstrap method.
Decision Analytic Modeling
Model Validity
We established internal and predictive validity of our model by comparing the proportion of patients who developed catheter malfunction and catheter-related bacteremia at 6 months, noting similar results between the model and the randomized trial. Moreover, at 6 months, we noted similar relative cost differences compared with the trial (Table 3). Using our model, we noted that 50.0%, 28.8%, 8.0%, and 13.2% of patients were using a catheter, had a fistula, had received a transplant, or had died at 1 year, respectively.
Table 3.
Clinical inputs and cost of treatments and managing complications in the base-case decision analysis
| Outcome Variables | Values | Range | Source |
|---|---|---|---|
| Clinical variables | |||
| Risk of catheter malfunction rt-PA over 3 mo | 0.145 | 0.093–0.211 | PreCLOT |
| Risk of catheter-related bacteremia rt-PA over 3 mo | 0.040 | 0.013–0.092 | PreCLOT |
| Hazard ratio heparin | PreCLOT | ||
| Catheter malfunction | 1.910 | 1.130–3.220 | PreCLOT |
| Catheter-related bacteremia | 3.300 | 1.180–9.220 | PreCLOT |
| Probability of needing a new catheter | PreCLOT | ||
| After catheter malfunction | 0.034 | 0.009–0.085 | PreCLOT |
| After catheter-related bacteremia | 0.854 | NA | PreCLOT |
| Probability of patients with catheter malfunction returning to a functional catheter, without need for new catheter | PreCLOT | ||
| First 3 mo | 0.677 | NA | PreCLOT |
| After 3 mo | 1.000a | NA | PreCLOT |
| Risk of hospitalization for catheter-related bacteremia | 0.550 | NA | PreCLOT |
| Probability of a establishing a functioning fistula enabling catheter removal every 3 mo | 0.115 | 0.080–0.158 | PreCLOT |
| Risk of fistula failure | |||
| First 6 mo | 0.0086 | NA | Ravani et al.15 |
| After 6 mo | 0.0005 | NA | Ravani et al.15 |
| Risk of transplant for age <65 y every 3 mo | 0.047 | NA | CORR35 |
| Risk of mortality over 3 mo | |||
| Patient on dialysis age <65 yr | 0.019 | 0.018–0.021 | CORR35 |
| Patient on dialysis age >65 yr | 0.052 | 0.049–0.054 | CORR35 |
| Cost variables (in 2011 Can$) | |||
| Cost of treatment over 3 mo (Can$) | |||
| rt-PA | 897 | 449–1346 | PreCLOT |
| Heparin | 98 | 73–122 | PreCLOT |
| Mean cost of hospitalization for catheter-related bacteremia (Can$) | 16,440 | 7297–25,585 | PreCLOT |
| Mean cost of outpatient care for managing catheter-related bacteremia (Can$) | 1731 | 865–2596 | PreCLOT |
| Cost of catheter replacement (Can$) | 1409 | 704–2123 | PreCLOT |
| Cost of rescue rt-PA for patients who develop catheter malfunction (Can$) | 533 | NA | PreCLOT |
| Mean cost of managing patients who had a bleeding complication (Can$) | 6775 | NA | PreCLOT |
| Cost of maintaining a functioning fistula (per 3 mo) (Can$) | 928 | NA | Manns et al.9 |
| Discount rate | 0.05 | NA |
NA, not applicable; CORR, Canadian Organ Replacement Registry.
Of those who continued to have a poorly functioning catheter at 3 months, all patients who did not require a new line returned to a functional catheter state by the end of the second 3-month period.
Base-Case Analysis
Over a 1-year time horizon, the cost of rt-PA/heparin and heparin prophylaxis that would be required to manage 100 hemodialysis patients with a recently inserted catheter was $173,700 and $19,000, respectively. The overall cost, excluding dialysis costs but including the cost of managing complications (catheter malfunction and catheter-related bacteremia), to manage 100 hemodialysis patients with a recently inserted catheter was $316,300 with rt-PA/heparin and $328,300 with heparin alone. On the basis of the results of the clinical trial, and assuming ongoing efficacy, the use of rt-PA/heparin reduced episodes of catheter-related bacteremia by 62% and catheter replacements by 68% (Table 4). Additional analyses suggested that the discrepancy between the results of the decision analysis (slightly lower costs for rt-PA/heparin compared with heparin alone) and the costing analysis over the trial period (slightly higher costs for rt-PA/heparin compared with heparin alone) was due to the longer observation period for the rt-PA/heparin patients in the trial,13 as well as the fact that patients with catheter malfunction (more than one third of patients in the heparin group) were censored after 30 days and therefore were not “eligible” during the trial observation period to develop bacteremia (the more expensive of the adverse outcomes).
Table 4.
Base-case and sensitivity analysis results for a cohort of 100 hemodialysis patients with a recently inserted catheter, using decision analysis over a 1-year period
| Variable | Drugs Costs (Can$) | Overall Costs (Can$) | Patients with Bacteremia (n) | New Catheters (n) | ||||
|---|---|---|---|---|---|---|---|---|
| rt-PA | Heparin Only | rt-PA | Heparin Only | rt-PA | Heparin Only | rt-PA | Heparin Only | |
| Base case analysis | 173,700 | 19,000 | 316,300 | 328,300 | 13 | 34 | 6 | 19 |
| Variation in rt-PA hazard ratio for catheter malfunction | ||||||||
| Lower 95% CI | 173,700 | 19,000 | 310,200 | 328,300 | 13 | 34 | 6 | 19 |
| Upper 95% CI | 173,700 | 19,000 | 328,200 | 328,300 | 14 | 34 | 6 | 19 |
| Variation in rt-PA hazard ratio for catheter-related bacteremia | ||||||||
| Lower 95% CI | 173,700 | 19,000 | 269,800 | 328,300 | 4 | 34 | 4 | 19 |
| Upper 95% CI | 173,700 | 19,000 | 440,400 | 328,300 | 31 | 34 | 23 | 19 |
| Ongoing effectiveness of rt-PA over time: varying rt-PA hazard ratio for catheter malfunction and catheter-related bacteremia after 6 mo | ||||||||
| HRs after 6 mo based on upper 95% CIs | 173,700 | 19,000 | 357,100 | 328,300 | 15 | 34 | 10 | 23 |
| HRs after 6 mo set at 1.0 (i.e., assumes rt-PA not effective after 6 mo) | 173,700 | 19,000 | 367,600 | 328,300 | 17 | 34 | 10 | 23 |
| Variation in risk of catheter-related bacteremia (base case, 0.04 episode of bacteremia per 1000 patient-days) | ||||||||
| 0.013 episode of bacteremia per 1000 patients-days (lower 95% CI) | 173,700 | 19,000 | 182,700 | 266,400 | 3 | 13 | 3 | 10 |
| 0.092 episode of bacteremia per 1000 patients-days (upper 95% CI) | 173,700 | 19,000 | 407,900 | 557,700 | 26 | 53 | 19 | 33 |
| Variation in cost of rt-PA | ||||||||
| Decreased by 50% | 93,500 | 19,000 | 226,100 | 311,800 | 13 | 34 | 6 | 19 |
| Increased by 50% | 255,100 | 19,000 | 406,600 | 344,800 | 13 | 34 | 6 | 19 |
| Scenario analysis | ||||||||
| Using United States costs for rt-PA and hospitalization (in $) | 280,400 | 16,700 | 469,000 | 450,500 | 13 | 34 | 6 | 19 |
| Risk of fistula reduced by 50% | 194,800 | 21,200 | 328,000 | 342,200 | 14 | 36 | 9 | 25 |
Costs in 2011 Can$, unless otherwise noted. HR, hazard ratio.
Sensitivity Analysis
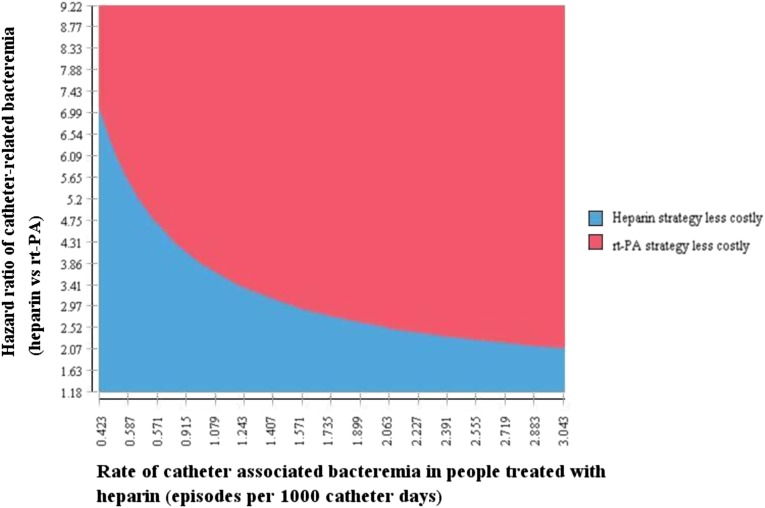
In general, the results of our analysis were robust to the uncertainty in most variables. For example, the overall costs were robust to plausible variation in the cost of rt-PA and heparin, cost of outpatient care, cost of catheter replacement, exclusion of rescue rt-PA cost, or the hazard ratio of catheter malfunction. However, the model was sensitive to plausible variation in the relative risk of developing catheter-related bacteremia, and whether the relative risk of catheter-related bacteremia was maintained after the 6-month period in which it was tested within the trial (Table 4). In addition, in scenarios where the rate of catheter-related bacteremia was assumed to be lower, or the relative risk of catheter-related bacteremia with heparin use was lower, the cost of the rt-PA/heparin strategy became less attractive (Figure 1). When we assumed that rt-PA was less effective after 6 months, the cost of the rt-PA strategy became higher than that of the heparin strategy (Table 4).
Figure 1.
Two-way sensitivity analysis assessing the impact of changing the baseline rate and hazard ratio of catheter-related bacteremia in the heparin group on overall costs over a one-year time horizon.
We also considered a scenario that might be more likely in the United States, where the cost of rt-PA and hospitalization is higher. In this scenario, we noted that the cost of rt-PA/heparin and heparin drug prophylaxis regimens that would be required to manage 100 hemodialysis patients with a recently inserted catheter was $280,400 and $16,700 over 1 year, respectively. Including the cost of managing complications (catheter malfunction and catheter-related bacteremia), the overall cost to manage these 100 hemodialysis patients with a recently inserted catheter was $469,000 with rt-PA/heparin and $450,500 with heparin alone over 1 year.
Discussion
In the original publication of the PreCLOT study,13 we estimated that use of rt-PA/heparin would be associated with much higher costs, although this prior “back of the envelope” analysis assumed that all patients would use their locking solution for 6 months, and we based the cost of managing patients hospitalized for catheter-related bacteremia on outdated costing estimates.8 In this updated analysis, using estimates of clinical effectiveness and health care cost data from trial patients exclusively, we noted similar costs for patients managed with rt-PA/heparin and heparin alone as a locking solution, regardless of the method used to compare costs. Specifically, we noted that the higher cost of rt-PA in the rt-PA locking strategy was partially offset by lower costs for managing catheter-related bacteremia and rescue rt-PA for managing catheter malfunction. Importantly, when we considered a scenario that might be more relevant in the United States, where the cost of rt-PA and hospitalization for catheter-related bacteremia is substantially higher,14 we noted similar results.
When we used decision analysis to extrapolate to a 1-year timeframe, we noted that the results of the decision analysis were sensitive to the frequency and cost of managing catheter-related bacteremia, and the relative risk of catheter-related bacteremia. Of note, the ongoing rate of bacteremia that we considered within our analysis (based on the PreCLOT trial data) was lower than that observed 6 months after catheter placement in a large analysis of the Dialysis Outcomes and Practice Patterns Study,15 and the costs of hospitalization we observed were lower than other published estimates14 suggesting that our results may be generalizable to other countries. However, as noted, the results of this analysis were largely driven by cost savings associated with lower rates of catheter-related bacteremia in the rt-PA/heparin group. Given the infrequent rate of catheter-related bacteremia in both groups, further evidence of long-term treatment efficacy is required to obtain a more precise estimate of the risk of catheter-related bacteremia associated with different locking strategies.
Some limitations of this analysis require consideration. Although this study was based largely on one randomized trial of moderate size,13 it is important to note that no other large randomized trials have compared rt-PA locking solutions in hemodialysis patients. However, a randomized trial of patients with indwelling central venous catheters and hematologic malignancies also observed a 39% lower risk of catheter-related coagulase-negative staphylococci infections.16 Among hemodialysis patients, a recent randomized trial comparing gentamicin/citrate and heparin locking solutions reported a significant decrease in the risk of catheter-related bacteremia for gentamicin/citrate locking solutions,17 consistent with earlier related studies.18 However, the potential side effects of antibiotic locking solutions have reduced their routine use in clinical practice.19 Further, studies comparing higher concentrations of trisodium citrate (46.7%) with heparin report no reduction in rates of catheter-related bacteremia.20,21 Another limitation is that our results in part relate to a small number of patients experiencing very high costs. Although it is not appropriate to exclude these patients (because the costs associated with their care were real), this does reduce the generalizability of our findings. While the cost of managing inpatients with catheter-related bacteremia (>Can$16,000) may seem high, our estimates are lower than those reported in other Canadian and United States studies.14,22 Finally, PreCLOT only compared rt-PA/heparin with heparin alone, although citrate is now used as the catheter-locking solution by many dialysis units in North America. The cost-effectiveness of rt-PA/heparin in comparison to other locking agents, such as citrate,10 and in comparison to other prophylactic strategies, such as the use of topical polysporin triple, or mupuricin19 is uncertain.
How might renal programs use the results of this study? From our costing analysis, we noted numerically higher costs associated with the use of rt-PA (Can$323; 95% CI, −Can$935 to Can$1581; P=0.62). In our decision analysis using a longer time horizon, we noted similar overall costs, although costs for the prophylaxis strategy itself were higher (Can$173,700 versus Can$19,000) if 100 hemodialysis patients with a newly inserted catheter received rt-PA/heparin versus heparin alone as a locking solution. Assuming that the effectiveness of rt-PA is maintained in the long term, our decision analysis suggests that dialysis units with a rate of catheter-related bacteremia >1 per 1000 catheter-days might not experience a significant increase in costs overall (Figure 1). However, although the additional costs associated with use of rt-PA as a locking solution are certain, potential downstream cost savings are less certain. Some additional considerations are needed in the United States, where the cost of rt-PA is borne by dialysis units, whereas any potential cost savings due to lower hospitalization rates would be a benefit to Medicare but not directly to the dialysis units.
In summary, we noted no significant difference in the mean overall cost of a strategy using once-weekly rt-PA, combined with twice-weekly heparin, as a locking solution for catheters compared with thrice-weekly heparin only. Cost savings due to lower risk of hospitalization for catheter-related bacteremia partially offset the increased cost of rt-PA. Further information on the long-term effectiveness of an rt-PA/heparin strategy as a locking solution for catheters is required to guide practice.
Concise Methods
Costing Analysis
We first conducted a costing analysis with the primary objective to determine the cost associated with using rt-PA (1 mg per lumen, administered once per week with heparin used for the remaining two sessions [rt-PA/heparin] or heparin [5000 U/ml after each dialysis session (heparin only)]), taking into account the cost of the locking solutions and all other relevant medical costs. This analysis was conducted alongside the clinical trial12 as detailed below.
Patient Population and Costing Time Period
All patients randomly assigned in the randomized controlled trial were included, with costs collected from study initiation until study completion (6 months for patients not censored for study outcomes or loss to follow-up). For patients experiencing catheter malfunction (the primary study outcome), the costing period was extended for at least an additional month, or until either six consecutive successful dialysis sessions were achieved (defined as a mean blood flow of ≥300 ml/min at each run) or the catheter was removed. Patients experiencing catheter-related bacteremia were followed until hospital discharge (if admitted) or completion of their outpatient antibiotics, ensuring complete capture of downstream health care costs related to all adverse events.
Costs Considered
In addition to drug costs for rt-PA and heparin (Can$299 and Can$33 per month, respectively), the resources to maintain a hemodialysis catheter were divided into relevant categories, including the cost of managing catheter malfunction (including catheter replacement when necessary) and the cost of managing catheter-related bacteremia (whether managed on an outpatient or inpatient basis). Following an episode of catheter malfunction, the cost of treating any repeat malfunction with rt-PA, or a diagnostic imaging procedure (catheter replacement, fibrin sheath stripping, or catheter re-wiring) was documented.
For patients with catheter-related bacteremia managed without hospital admission, management costs were estimated, including the cost of blood cultures, antibiotics used to treat the bacteremia (based on dose and duration of treatment), laboratory monitoring of antibiotic levels, and catheter replacement (where indicated). For patients requiring hospital admission, we determined (through blinded chart review using a priori definitions) whether the admission was directly attributed to the catheter (included hospitalization for catheter-related bacteremia or complications from the bacteremia [i.e., endocarditis, osteomyelitis, septic arthritis, and epidural abscess]) and complications related to catheter insertion.12
Study coordinators (blinded to treatment assignment) collected detailed information on hospital admissions for catheter-related bacteremia in all participating centers. Because costing methods differ across sites, the cost of admissions was based on data from the largest sites (Calgary and Edmonton, Alberta). However, recent studies suggest that hospital costs are similar to those within the sites in Ontario, Canada, where all other patients within this trial were admitted.22 The cost of inpatient care in Alberta was estimated using a microcosting method,23–25 combining allocation of all direct and overhead costs associated with an inpatient encounter during the entire hospital stay. The costs included represent the cost of treating the patient rather than charges or payments; the quality of the costing data has been ranked highly.24 A similar approach was taken for patients admitted for bleeding complications, the cost of which was considered in secondary analyses. The cost of physician’s services for all inpatient and outpatient encounters was recorded as per the Alberta provincial billing list in 2011.
Analytic Approach for Costing Analysis
In the primary analysis, we considered the cost of the study drugs (rt-PA and heparin) and all costs related to managing catheter malfunction and catheter-related bacteremia. Because there were few bleeding events with no significant difference in frequency between groups,13 we excluded the cost of bleeding in the primary analysis, but we did consider bleeding events in a secondary analysis.
Given the variable follow-up in the two study groups, and the expected non-Gaussian distribution of costs, there were unique issues we took into account when comparing costs across treatment groups.26,27 We used established methods to enable comparisons of mean costs because these are easily interpretable and relevant to health care payer. This included use of nonparametric bootstrap estimates to derive 95% CIs and mean cost differences between the treatment groups.28,29 Using 1000 bias-corrected bootstrap replications, and based on sampling with replacement from the original data, we estimated the distribution of a sampling statistic to derive 95% CIs.30 In sensitivity analyses, we used generalized linear models to compare total costs across groups.32 We built these models considering three family distributions (Gaussian, inverse Gaussian, and gamma) and specifying two link functions (identity and log). We studied the performance of these models and selected the final model considering the information criteria, goodness of fit, and graphical and formal tests based on residuals. We did analyses with Stata software, version 11 (StataCorp., College Station, TX). We repeated our costing analysis using United States cost estimates, acknowledging that the price of rt-PA is higher in the United States ($107 per 2 mg compared with Can$64 in Canada ($1=Can$1.01 in 2011), and that the cost of hospitalization and procedures is typically 50% higher in the United States than in Canada.14,32
Decision Analytic Modeling
Overview of Decision Analysis
Because the randomized trial was only 6 months in duration, we also used decision analysis to extrapolate the results from the trial to a 1-year time horizon. Using the results from the trial13 and the costing analysis described herein, we performed an incremental cost-effectiveness analysis comparing the study interventions. Model outcomes included cost, number of new catheters, and cases of catheter-related bacteremia.
Target Population
The target population included hemodialysis patients with a recently inserted catheter. To inform the decision analysis, we used costing and clinical data from randomly assigned patients from the time of enrollment throughout the 6-month study period.
Treatment Comparators
We modeled the interventions—rt-PA/heparin and heparin alone as locking solutions—as noted earlier.
Clinical and Costing Inputs
We derived most of the clinical and costing estimates from the Pre-CLOT trial13 and the costing analysis described herein (Table 3). The exception was the likelihood of transition from using a catheter to a fistula, which was informed by the trial results (22% of patients were transitioned to a fistula during the 6-month trial). No patients in the trial had an arteriovenous graft placed, which is consistent with current practice in Canada, where grafts are infrequently used33; as such, we only modeled transition to a fistula. Given the limited number of patients admitted for catheter-related bacteremia (and making the conservative assumption that the duration of hospitalization for catheter-related bacteremia would be similar irrespective of treatment assignment), for the decision analysis we based the mean cost of hospitalizations on all events, regardless of treatment arm.
Analytical Approach
In the baseline analysis, we used decision analysis to model the cost and consequences of using rt-PA/heparin or heparin as the locking solution in a simulated cohort of hemodialysis patients with a recently inserted central venous catheter, categorized into two age groups: those <65 years and those >65 years. The perspective of the economic evaluation was that of the health care purchaser, and we only considered access-related costs (cost of locking agent, cost of managing catheter-related complications, and the cost associated with fistula use in those who subsequently used a fistula).34 We did not include the cost of dialysis or transplantation because these costs were equal in both strategies.
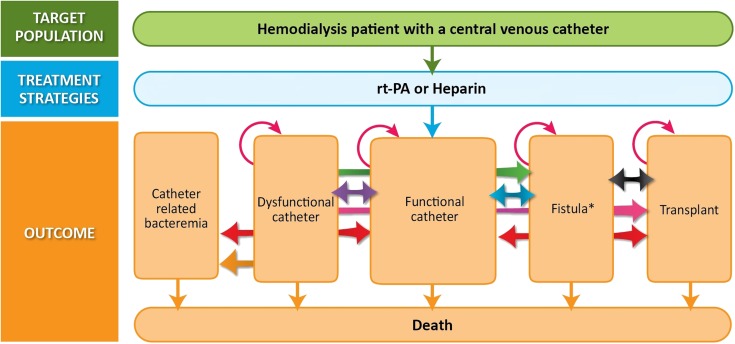
A Markov process was used to model transitions, over recurring 3-month cycles, between the different clinical states considered (Figure 2). Because the effect of rt-PA/heparin on quality of life and survival is uncertain, expressing the results as a cost per quality-adjusted life-year would add unnecessary uncertainty. Therefore, in addition to the cost of each strategy, we report outcomes that are relevant for patients, including the expected number of new catheter insertions and catheter-related bacteremias over a 2-year time horizon. To facilitate interpretation, we present the analyses for a cohort of 100 hemodialysis patients with a recently inserted catheter. We discounted costs and clinical outcomes at an annual rate of 5%.34 Costs were calculated based on 2011 Canadian dollars (1Can$=$1.006). Decision analysis was performed with the use of TreeAge Pro 2012 (TreeAge Software, Inc.).
Figure 2.
Markov model structure.
Consistent with published guidelines,34 we established face, internal, and predictive validity of our model comparing model outputs (a function of both input variable and model structure) with reported data from the trial.
Sensitivity Analysis
We performed extensive sensitivity analyses to test the robustness of our findings. These analyses assessed the effects of varying baseline estimates within the 95% CI observed within the trial, or within clinically plausible ranges for other estimates on access-related cost and clinical outcome, including the duration of benefit of rt-PA. In addition, because the risk of catheter-related bacteremia varies across dialysis units from 0.5 to 8 episodes per 1000 catheter-days,19 this estimate was also varied widely.
Disclosures
B.J.M.: Investigator-initiated research grant from Hoffman La Roche. N.S.D.: Research grants from Hoffman La Roche, Janssen, Inc., Chief Medical, and Amgen; honorarium for lectures from Takeda, Hoffman La Roche, Chief Medical. M.T.: Investigator-initiated research grants from Abbott and Baxter. M.D.: Speaker/consultant fee and/or research funds from Amgen, Gambro, Baxter, Merck, Novartis. R.H.: Investigator-initiated research grant from Amgen. L.M.: Investigator-initiated research grant from Hoffman La Roche; honorarium for lectures or advisory boards from Hoffman La Roche. B.R.H.: Investigator-initiated research grant from Hoffman La Roche.
Supplementary Material
Acknowledgments
B.J.M., M.T., and B.R.H. are supported by Alberta Innovates–Health Solutions (formerly Alberta Heritage Foundation for Medical Research) salary awards. B.J.M., P.R., B.R.H., and M.T. were supported by an alternative funding plan from the government of Alberta and the universities of Alberta and Calgary. This research was supported by an unrestricted grant from Roche Canada. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050463/-/DCSupplemental.
References
- 1.Moist LM, Trpeski L, Na Y, Lok CE: Increased hemodialysis catheter use in Canada and associated mortality risk: Data from the Canadian Organ Replacement Registry 2001-2004. Clin J Am Soc Nephrol 3: 1726–1732, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 21: 721–728, 2006 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health. Incidence, prevalence, patient characteristics and modality, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 4.Canadian Organ Replacement Register. 2010 CORR Report–treatment of end-stage organ failure in Canada, 1999 to 2008, 2010. Available at: https://secure.cihi.ca/free_products/corr_annual_report_2010_e.pdf
- 5.Develter W, De Cubber A, Van Biesen W, Vanholder R, Lameire N: Survival and complications of indwelling venous catheters for permanent use in hemodialysis patients. Artif Organs 29: 399–405, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Little MA, O’Riordan A, Lucey B, Farrell M, Lee M, Conlon PJ, Walshe JJ: A prospective study of complications associated with cuffed, tunnelled haemodialysis catheters. Nephrol Dial Transplant 16: 2194–2200, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Oliver MJ, Verrelli M, Zacharias JM, Blake PG, Garg AX, Johnson JF, Pandeya S, Perl J, Kiss AJ, Quinn RR: Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 27: 810–816, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, Donaldson C: Cost analysis of ongoing care of patients with end-stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis 40: 611–622, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, Radkevich V, Murphy B: Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J Am Soc Nephrol 16: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Lok CE, Appleton D, Bhola C, Khoo B, Richardson RM: Trisodium citrate 4%—an alternative to heparin capping of haemodialysis catheters. Nephrol Dial Transplant 22: 477–483, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Grudzinski L, Quinan P, Kwok S, Pierratos A: Sodium citrate 4% locking solution for central venous dialysis catheters—an effective, more cost-efficient alternative to heparin. Nephrol Dial Transplant 22: 471–476, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hemmelgarn BR, Moist L, Pilkey RM, Lok C, Dorval M, Tam PY, Berall MJ, LeBlanc M, Toffelmire EB, Manns BJ, Scott-Douglas N, Canadian Hemodialysis Catheter Working Group : Prevention of catheter lumen occlusion with rT-PA versus heparin (Pre-CLOT): Study protocol of a randomized trial [ISRCTN35253449]. BMC Nephrol 7: 8, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmelgarn BR, Moist LM, Lok CE, Tonelli M, Manns BJ, Holden RM, LeBlanc M, Faris P, Barre P, Zhang J, Scott-Douglas N, Prevention of Dialysis Catheter Lumen Occlusion with rt-PA versus Heparin Study Group : Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. N Engl J Med 364: 303–312, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan V, Chiu EJ, Thomas JT, Khan A, Dolson GM, Darouiche RO. Healthcare costs associated with hemodialysis catheter-related infections: A single-center experience. Infect Control Hosp Epidemiol 28: 606–609, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Ravani P, Gillespie BW, Quinn RR, MacRae J, Manns B, Mendelssohn D, Tonelli M, Hemmelgarn B, James M, Pannu N, Robinson BM, Zhang X, Pisoni R: Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol 24: 1668–1677, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rooden CJ, Schippers EF, Guiot HF, Barge RM, Hovens MM, van der Meer FJ, Rosendaal FR, Huisman MV: Prevention of coagulase-negative staphylococcal central venous catheter-related infection using urokinase rinses: A randomized double-blind controlled trial in patients with hematologic malignancies. J Clin Oncol 26: 428–433, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Moran J, Sun S, Khababa I, Pedan A, Doss S, Schiller B: A randomized trial comparing gentamicin/citrate and heparin locks for central venous catheters in maintenance hemodialysis patients. Am J Kidney Dis 59: 102–107, 2012 [DOI] [PubMed] [Google Scholar]
- 18.James MT, Conley J, Tonelli M, Manns BJ, MacRae J, Hemmelgarn BR, Alberta Kidney Disease Network : Meta-analysis: Antibiotics for prophylaxis against hemodialysis catheter-related infections. Ann Intern Med 148: 596–605, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lok CE, Mokrzycki MH: Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 79: 587–598, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Venditto M, du Montcel ST, Robert J, Trystam D, Dighiero J, Hue D, Bessette C, Deray G, Mercadal L: Effect of catheter-lock solutions on catheter-related infection and inflammatory syndrome in hemodialysis patients: Heparin versus citrate 46% versus heparin/gentamicin. Blood Purif 29: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Power A, Duncan N, Singh SK, Brown W, Dalby E, Edwards C, Lynch K, Prout V, Cairns T, Griffith M, McLean A, Palmer A, Taube D: Sodium citrate versus heparin catheter locks for cuffed central venous catheters: A single-center randomized controlled trial. Am J Kidney Dis 53: 1034–1041, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kosa D, Joarder M, Gafni A, Lok C: Analysis of the Hemodialysis Infection Prevention with Polysporin Ointment (Hippo) study. Presented at the National Kidney Foundation Spring Clinical Meeting, Orlando, FL, April 2–6, 2013
- 23.Canadian Institute for Health: I. Guidelines for Management Information Systems in Canadian Health Service Organizations: Report. Ottawa, Ontario, Canada, Canadian Institute for Health, 1999 [Google Scholar]
- 24.McKillop I: A Research Project to Examine the Costing Methodologies Recommended in the MIS Guidelines [Dissertation/Thesis]. Ottawa, Ontario, Canada, Canadian Institute for Health, 1995
- 25.McKillop I, Pink GH, Johnson LM: The financial management of acute care in Canada: A review of funding, performance monitoring and reporting practices. March 2001. Available at: https://secure.cihi.ca/estore/productSeries.htm?locale=en&pc=PCC53. Accessed January 2012
- 26.Afifi AA, Kotlerman JB, Ettner SL, Cowan M: Methods for improving regression analysis for skewed continuous or counted responses. Annu Rev Public Health 28: 95–111, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Mihaylova B, Briggs A, O’Hagan A, Thompson SG: Review of statistical methods for analysing healthcare resources and costs. Health Econ 20: 897–916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber JA, Thompson SG: Analysis of cost data in randomized trials: An application of the non-parametric bootstrap. Stat Med 19: 3219–3236, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Desgagné A, Castilloux AM, Angers JF, LeLorier J: The use of the bootstrap statistical method for the pharmacoeconomic cost analysis of skewed data. Pharmacoeconomics 13: 487–497, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Davison AC, Hinkley DV: Bootstrap methods and their application: Some bootstrapping techniques. Stata J 4: 312–328, 2004 [Google Scholar]
- 31.Moran JL, Solomon PJ, Peisach AR, Martin J: New models for old questions: Generalized linear models for cost prediction. J Eval Clin Pract 13: 381–389, 2007 [DOI] [PubMed] [Google Scholar]
- 32.The World Bank : World development indicators 2000, Washington, DC, The World Bank, 2001 [Google Scholar]
- 33.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, Canaud BJ, Pisoni RL: Vascular access use and outcomes: An international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 23: 3219–3226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canadian Agency for Drugs and Technologies in Health: Guidelines for the economic evaluation of health technologies: Canada, 3rd Ed. http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. Accessed January 2012
- 35.Canadian Institute for Health Information: Canadian Organ Replacement Register Treatment of End-Stage Organ Failure in Canada, 2000 to 2009, Ottawa, Ontario, Canada, Canadian Institute for Health Information, 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.