Abstract
Most patients with type 1 diabetes (T1D) and proteinuria have poor glycemic control and a high risk of ESRD. We investigated whether long-term improvement of glycemic control reduces risk of ESRD in a prospective 7- to 15-year follow-up observation of 349 patients with CKD stages 1–3 enrolled in the Joslin Proteinuria Cohort of adults with T1D. All patients developed proteinuria between 1990 and 2004 and were followed until 2011 to ascertain onset of ESRD and deaths unrelated to ESRD. Furthermore, we analyzed data from 279 patients with ≥3 years of clinic follow-up available to assess the level of glycemic control after enrollment. Average HbA1c during the 5 years before study enrollment (prebaseline) was compared with HbA1c (postbaseline) averaged during the first half of follow-up (median, 5.1 years). Median prebaseline HbA1c was 9.3%, decreasing to 8.7% postbaseline. Cumulative risk of ESRD after 15 years was significantly lower for patients whose HbA1c decreased than for those whose HbA1c increased or remained poor (29% versus 42%; P<0.001). The difference between these groups was not visible at 5 years of follow-up but became visible at 10 and 15 years of follow-up. In multivariate Cox regression analysis of ESRD risk, the hazard ratio corresponding to a 1–percentage point improvement in postbaseline HbA1c was 0.76 (95% confidence interval, 0.63 to 0.91; P=0.003). In conclusion, results of this study suggest that long-term sustained improvement in HbA1c decelerates eGFR loss and delays the onset of ESRD in patients with T1D and proteinuria.
Keywords: end stage kidney disease, proteinuria, diabetes
Despite nearly universal treatment with renin-angiotensin system blockade and antihypertensive agents, the risk of ESRD in type 1 diabetes (T1D) remains high.1,2 This study revisited the role of improved glycemic control in delaying the onset of ESRD in patients with clinically established diabetic nephropathy. While urinary albumin has been the traditional index of nephropathy progression, it correlates imperfectly with renal function loss.3,4 Therefore, we used the rate of loss of GFR and time to ESRD onset as outcomes.5
Elevated glucose undoubtedly plays a causal role in the pathogenesis of diabetic nephropathy. Aggressive intervention to improve glycemia in healthy patients with T1D prevented onset of microalbuminuria and delayed development of impaired renal function in clinical trials.6,7 However, trials in patients with proteinuria have been neither long nor large enough to evaluate whether improved glycemic control reduces the risk of progression to ESRD. The few short-term studies in patients with T1D and proteinuria have yielded inconclusive results regarding the effect of improved glycemia on renal decline.8–11 Despite the shortcomings of published studies, this issue has not received attention. Instead, improved glycemic control in patients with proteinuria has been assumed to be too late to change renal outcomes. Unfortunately, natural history studies of diabetic nephropathy have not helped. Most were uninformative because glycated hemoglobin was evaluated during only one time period (such as baseline), and variation in renal function decline during follow-up could not be related to change in exposure to glycemia.1,2,12–14 This significant “research gap” regarding the effectiveness of glycemic control in preventing and treating various stages of diabetic nephropathy was recently reviewed.15
In this long-term follow-up study of patients with T1D and proteinuria, we characterized glycemia by mean hemoglobin A1c (HbA1c) values in two time intervals: before study entry (prebaseline) and again during follow-up (postbaseline). Their difference was used to assess whether improved glycemic control during the postbaseline period reduced the risk of ESRD after controlling for relevant confounders. This observational study was undertaken in a desperate effort to identify new therapies to reduce the risk of ESRD in T1D,1 such as the study by Fiorretto et al. That study showed 5–10 years of normoglycemia after pancreas transplantation was needed to see disappearance of morphologic lesions in diabetic kidneys of patients with T1D diabetes.16,17
The positive findings of our study highlight the importance of observational studies as a method of developing evidence-based clinical protocols in situations where clinical trials cannot be performed. In the case of the poor glycemic control that characterizes patients with T1D and proteinuria, a clinical trial is not likely to be performed if it involves withholding an intervention from controls who are patients at high risk of ESRD. Observational studies using rigorous methods may be the only avenue for evaluating the effectiveness of improved glycemic control as an intervention to reduce the risk of ESRD.
Results
Characteristics of the Study Group
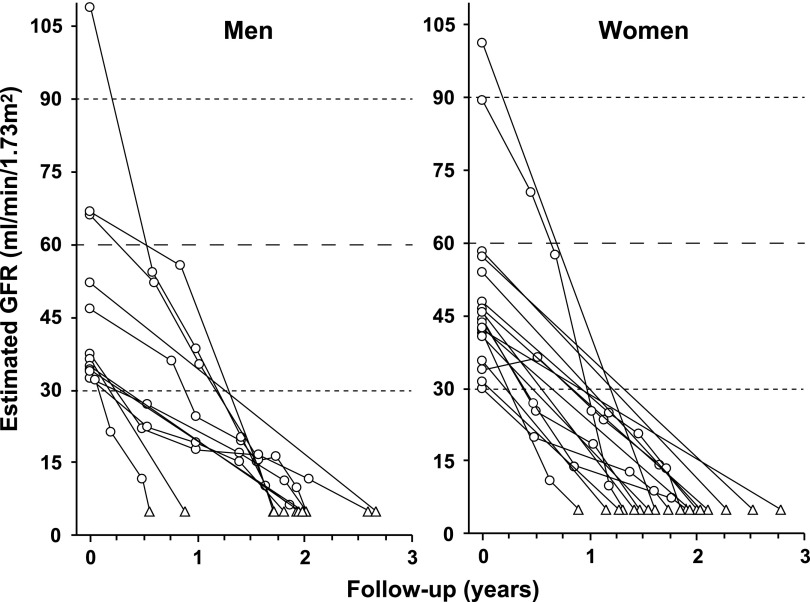
The study group comprises the 349 patients who had CKD stages 1–3 when enrolled in the Joslin Proteinuria Cohort, a cohort followed to describe the natural history of proteinuria and its progression to ESRD in T1D.1 Eligibility was ascertained by periodic monitoring of the computer files of the Joslin Clinic’s laboratory for values of the albumin creatinine ratio that qualified a patient for a diagnosis of proteinuria (see Concise Methods). The cohort was enrolled between 1991 and 2004 while attending clinic and was followed through 2011.5 Pre-enrollment glycemic control was assessed by retrieving all HbA1c measurements from the preceding 5-year interval (including that made at the enrollment examination). Multiple values within an individual year were averaged first, and then an average was taken of the years that had at least one value. To assess postenrollment glycemic control, we restricted the analysis to patients with a minimum of 3 years of subsequent clinic follow-up so that postbaseline measurements of HbA1c would be available (complete cohort: n=279). Postbaseline glycemic control was assessed by all HbA1c measurements during the first half of the follow-up interval. Multiple values within a year were averaged before averaging across years. The difference between pre- and postbaseline HbA1c was our measure of change in exposure. Those without a measure of change included 30 who progressed to ESRD within 3 years (and so had no opportunity to sustain improved glycemia) and 40 “nonattenders” who did not return to the clinic for other reasons (although still followed for events of ESRD or death). Trajectories of renal function decline for the 30 patients who reached ESRD within 3 years are displayed in Figure 1 to draw attention to these “rapid progressors” whose situation calls for particularly effective interventions. Characteristics of all three subgroups are summarized in Table 1.
Figure 1.
Individual trajectories of renal decline in rapid progressors. eGFR trajectories are plotted for 30 patients who developed ESRD within 3 years from the study entry. eGFR measurements are represented by circles and ESRD events by triangles.
Table 1.
Characteristics of the study group of proteinuric patients with T1D and CKD stages 1–3 according to availability of a measure of postbaseline glycemia
| Characteristic | Complete Cohort (n=279) | Rapid Progressors (n=30) | Nonattenders (n=40) |
|---|---|---|---|
| Women, n (%) | 124 (44.4) | 18 (60.0) | 15 (37.5) |
| Age (yr) | 38 (32, 44) | 37 (33, 43) | 36 (33, 42) |
| Diabetes duration (yr) | 24 (19, 31) | 22 (18, 26) | 25 (20, 32) |
| Duration of care at Joslin Clinic (yr) | 19 (10, 28) | 16 (10, 23) | 18 (13, 24) |
| ACR (mg/g) | 687 (441, 1244) | 2665 (883, 3944) | 809 (535, 1560) |
| Serum creatinine (mg/dl) | 1.00 (0.79, 1.28) | 1.65 (1.38, 1.95) | 1.12 (0.93, 1.45) |
| eGFR (ml/min per 1.73 m2) | 85 (64, 110) | 41 (36, 51) | 77 (55, 98) |
| Systolic BP (mmHg) | 130 (120, 142) | 133 (120, 144) | 133 (128, 145) |
| Diastolic BP (mmHg) | 78 (70, 84) | 79 (70, 85) | 82 (77, 86) |
| Treatment with ACEI or ARBa | 194 (69.5%) | 18 (60.0%) | 28 (70.0%) |
| Prebaseline HbA1c (%)b | 9.3 (8.4, 10.4) | 10.1 (8.9, 11.6) | 8.8 (7.8, 9.7) |
| Postbaseline HbA1c (%)c | 8.7 (7.8, 9.8) | – | – |
| No. of prebaseline HbA1c measures per patient | 7 (3, 10) | 6 (2, 11) | 2 (1, 5) |
| No. of postbaseline HbA1c measures per patient | 7 (2, 12) | – | – |
| Follow-up (yr) | 10.2 (7.8, 14.5) | 1.9 (1.5, 2.0) | 9.6 (6.5, 11.2) |
| Incidence rate of ESRD/100 patient-years (n) | 2.7 (77) | 55.5 (30) | 4.9 (18) |
| Non-ESRD mortality/100 patient-years (n) | 0.8 (23) | 0 (0) | 1.6 (6) |
| No. of eGFR values per patient | 11 (5, 23) | 3 (2, 10) | 2 (2, 3) |
| eGFR values per year | 1.2 (0.5, 2.3) | 2.0 (1.0, 5.8) | 0.3 (0.2, 0.4) |
Data are median (first, third quartiles) or number (percentage). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker.
Prevalence of renoprotection was 55% in patients entering study in 1991–1995, 61% in 1996–2000, and 82% in 2001–2004.1
Time-weighted average HbA1c during 5 years up to and including enrollment examination.
Time-weighted average HbA1c during first half of each individual’s follow-up interval. The median (first, third quartiles) length of these half-intervals of follow-up was 5.1 years (3.9, 7.3).
At study entry, the group of patients with measured pre- and postbaseline HbA1c (complete cohort) had been receiving care at the Joslin Clinic for a median of 19 years, and proteinuria had been present <3 years for the majority. By design, their albumin-to-creatinine ratio (ACR) was within the proteinuric range. Their median eGFR at enrollment was 85 ml/min per 1.73 m2. BP was close to the therapeutic target (median, 130/78 mmHg). Although the 69.5% prevalence of treatment with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers at study entry appears low by current practice, this reflects the long history of the cohort. Roughly one third of the study group was recruited in the early 1990s, when renoprotective treatment was only just coming into vogue (see Table 1 footnote).
The present report considers only the first 15 years of observation because few patients had longer follow-up. Median follow-up was 10.2 years, with first and third quartiles of 7.8 and 14.5 years, respectively. The subgroup with measured postbaseline HbA1c accumulated 2898 person-years of follow-up. ESRD developed in 77 patients and was registered in the US Renal Data System ([USRDS]; incidence rate, 2.6/100 patient-years). In addition, 23 patients died of causes unrelated to ESRD (mortality rate, 0.8/100 patient-years). ESRD developed in all of the rapid progressor subgroup by definition. Among nonattenders, the incidence rate of ESRD and mortality rate due to causes unrelated to ESRD were 4.9 and 1.6/100 person-years, twice as high as in patients with measured postbaseline HbA1c.
During follow-up, rapid progressors and patients with measured postbaseline HbA1c had multiple determinations of eGFR (an average of 1.2–2.0 per year) for tracing eGFR trajectories, while nonattenders had very few.
Pre- and Postbaseline Levels of Glycemic Control
Only the subgroup of 279 patients with measured postbaseline HbA1c were useful for investigating whether long-term improvement of glycemic control in patients with T1D and proteinuria reduces their risk of ESRD. At enrollment, most patients had poor glycemic control. Median (first, third quartiles) prebaseline HbA1c was 9.3% (8.4%, 10.4%). As noted in our previous report on this study group, the prebaseline HbA1c of later-entering patients trended downward (0.04 percentage point per calendar year; P=0.04).1 This trend was tiny compared with the decrease to postbaseline HbA1c: median (first, third quartiles), 8.7% (7.8%, 9.8%).
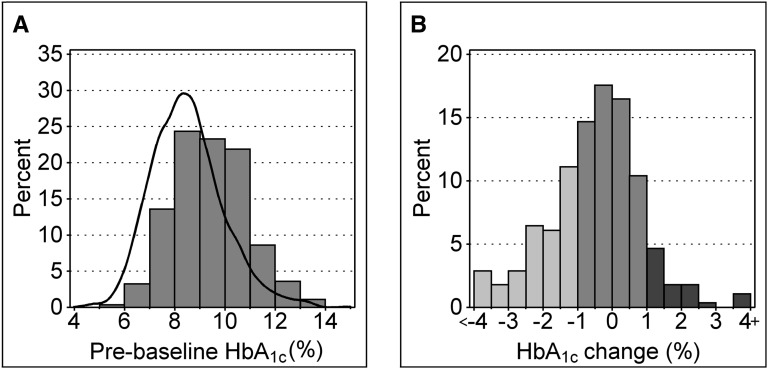
In Figure 2A, the distribution of prebaseline HbA1c is compared with a smoothed representation of the distribution of a similar measure of HbA1c in the reference cohort of 1412 adults with T1D without proteinuria recruited into the Joslin Study on Natural History of Microalbuminuria in the early 1990s. As expected, the distribution of prebaseline HbA1c in the complete cohort (median [first, third quartiles], 9.3% [8.4%, 10.4%]) was shifted higher by 0.9 percentage point (P<0.001) than in the reference cohort (median [first, third quartiles], 8.4% [7.5%, 9.5%].18 The relative odds of proteinuria for a 1–percentage point higher HbA1c was 1.41 (95% confidence interval [95% CI], 1.31 to 1.53; P<0.001). In Figure 2B, the changes in glycemic exposure after study entry are represented as a histogram. Values are negative if postbaseline HbA1c decreased (glycemia improved) and positive if it increased (glycemia worsened). Change was small (<1 percentage point in absolute value) for 165 patients; HbA1c increased ≥1 percentage points for 27 and decreased ≥1 percentage points for 87.
Figure 2.
Pre- and postbaseline HbA1c in the complete cohort. (A) Distribution of prebaseline HbA1c in 279 patients with CKD stages 1–3 from the Joslin Proteinuria Cohort. The black contour outlines distribution of HbA1c (5-year average) in 1412 patients from the Joslin Clinic without proteinuria. (B) Distribution of changes between pre- and postbaseline HbA1c (postbaseline level measured over median of 5.1 years). Improvements >1 percentage point are light gray, deteriorations >1 percentage point are dark gray, and changes within a 1% boundary are depicted in intermediate shade.
Another way of representing changes in HbA1c after the enrollment is a joint array of pre- and postbaseline HbA1c values. For this purpose, we divided each HbA1c measure into three categories (<8.0%, ≥8.0 to ≤9.5%, and >9.5%), which we labeled fair, poor, and very poor, respectively (Table 2). During follow-up, 157 (56.3%) patients remained in the same category (cells denoted with “a” footnote in Table 2), 30 (10.7%) patients moved to a worse category (cells below those denoted with “a” footnote), and 92 (33.0%) moved to a better category (cells above those denoted with “a” footnote). We do not attach particular significance to the values of these HbA1c cutpoints other than their convenience for describing the complete cohort. We note, however, that the lower cutpoint corresponds to an apparent threshold for microalbuminuria risk we reported previously,18 and the upper is the 75th percentile of the distribution of HbA1c in the reference cohort.
Table 2.
Distribution of complete cohort according to pre- and postbaseline HbA1c together with incidence rate of ESRD per 100 patient-years and median change between pre- and postbaseline HbA1c
| Postbaseline HbA1c | Prebaseline HbA1c | ||
|---|---|---|---|
| Fair (n=48) | Poor (n=105) | Very Poor (n=126) | |
| Fair: <8.0% | n=40 0.92 (4/434) 0.0a | n=28 2.47 (7/283) −1.0 | n=18 0.50 (1/199) −2.6 |
| Poor: ≥8.0% and <9.5% | n=8 2.10 (2/95) 0.9 | n=55 2.51 (15/597) 0.0a | n=46 2.65 (13/490) −1.9 |
| Very poor: ≥9.5% | n=0 – | n=22 4.96 (10/202) 1.1 | n=62 4.19 (25/597) −0.2a |
Values are number of patients at risk, incidence rate (events/patient-years), and median change in HbA1c between pre and postbaseline.
During follow-up, 157 (56.3%) patients remained in the same category; 30 (10.7%) patients moved to a worse category (cells below those denoted with footnote “a”), and 92 (33.0%) moved to a better category (cells above those denoted with footnote “a”).
Risk of ESRD according to Pre- and Postbaseline HbA1c
Table 2 shows the incidence rates of ESRD for patients in each cell. For patients who remained in the same category (main diagonal), ESRD risk increased strongly with HbA1c category, roughly doubling between fair and poor and again between poor and very poor. Relative to patients whose HbA1c remained unchanged, the ESRD incidence rate was higher in the 30 patients whose HbA1c increased (although not to a statistically significant extent: P=0.12), while it was dramatically lower in the 92 patients whose HbA1c decreased (P=0.03). The benefit from improved glycemia was most clearly manifest in the group with the worst prebaseline glycemic control. The incidence rate of ESRD was 4.2 per 100 patient-years in patients in whom glycemic control was unchanged but decreased to 2.7 and 0.5 per 100 patient-years in those whose glycemic control improved to poor and fair, respectively (Table 2).
Characteristics of patients according to patterns of pre- and postbaseline glycemia described in Table 2 are shown in Table 3. Subgroup 1 comprises the patients whose pre- and postbaseline glycemia were fair; subgroup 2 comprises those whose postbaseline glycemia has improved over prebaseline; and subgroup 3 comprises patients whose pre- and postbaseline glycemia were poor or very poor. The comparison of subgroup 2 versus 3 is considered first because it is critical to answer the main question of this study. Both subgroups were similar at enrollment with regard to demographic characteristics such as sex, age, calendar date of enrollment (median 1997 versus 1998), and duration of diabetes. They were also similar with regard to all the other characteristics in Table 3 except for a higher prevalence of treatment with an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker in subgroup 2 (improved glycemic control). Postbaseline HbA1c differed by design. In contrast, patients with fair pre- and postbaseline glycemic control (subgroup 1) had lower levels of important prognostic markers, such as ACR and neutrophil gelatinase–associated lipocalin at baseline, evidence of less exposure to kidney injury before study entry.
Table 3.
Characteristics of subgroups of the complete cohort defined according to prebaseline HbA1c changes during follow-up
| Characteristic | Study Subgroups according to Glycemic Control | |||
|---|---|---|---|---|
| 1: Fair Both Times | 2: Postbaseline Improved over Prebaseline | 3: Poor or Very Poor Both Times | P Value for Subgroup 2 versus Subgroup 3 | |
| Patients (n) | 40 | 92 | 139 | – |
| Women | 14 (35.0) | 40 (43.5) | 66 (47.5) | 0.55 |
| Age (yr) | 41 (36, 49) | 36 (30, 42) | 38 (33, 43) | 0.05 |
| Diabetes duration (yr) | 29 (22, 34) | 23 (17, 30) | 25 (19, 31) | 0.36 |
| ACR (mg/g) | 600 (396, 1018) | 687 (488, 1225) | 702(434, 1356) | 0.99 |
| Serum creatinine (mg/dl) | 1.09 (0.84, 1.38) | 1.00 (0.79, 1.30) | 0.98 (0.78, 1.28) | 0.93 |
| eGFR (ml/min per 1.73 m2) | 82 (57, 99) | 90 (66, 108) | 82 (62, 112) | 0.53 |
| Systolic BP (mmHg) | 130 (120, 142) | 133 (120, 142) | 129 (119, 141) | 0.44 |
| Diastolic BP (mmHg) | 76 (70, 82) | 80 (71, 88) | 78 (70, 83) | 0.26 |
| Treatment with ACEI or ARB | 32 (80.0) | 71 (77.2) | 86 (61.9) | 0.01 |
| Total cholesterol (mg/dl) | 176 (157, 211) | 206 (176, 233) | 207 (187, 240) | 0.28 |
| Statin treatment | 8 (20.0) | 21 (22.8) | 29 (20.9) | 0.72 |
| Smoking (ever) | 22 (55.0) | 48 (52.2) | 80 (57.5) | 0.42 |
| Smoking (present) | 8 (20.0) | 23 (25.0) | 38 (27.3) | 0.69 |
| Urinary NGAL/creatinine (ng/mg) | 8.7 (4.4, 28.2) | 13.1 (7.7, 35.8) | 16.7 (6.0, 44.9) | 0.94 |
| Serum CRP (mg/L) | 0.11 (0.05, 0.23) | 0.16 (0.07, 0.40) | 0.14 (0.06, 0.34) | 0.66 |
| Prebaseline HbA1c (%) | 7.3 (7.0, 7.7) | 10.0 (9.1, 10.7) | 9.3 (8.8, 10.5) | 0.03 |
| Postbaseline HbA1c (%) | 7.2 (6.8, 7.7) | 8.0 (7.7, 8.9) | 9.7 (8.8, 10.5) | <0.001 |
Unless otherwise noted, values are expressed as number (percentage) or median (first, third quartiles). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; NGAL, neutrophil gelatinase–associated lipocalin; CRP, C-reactive protein.
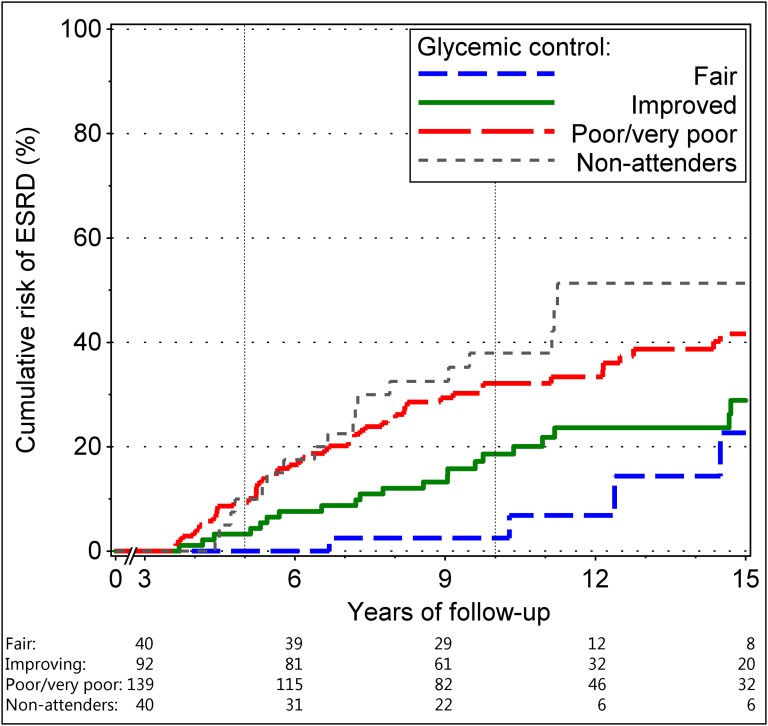
The cumulative risk of ESRD in the study subgroups described in Table 3 and the nonattenders in Table 1 is shown in Figure 3. There was a large (P<0.001) difference in ESRD risk between patients with fair and stable and poor/very poor glycemic control: 0% versus 9% at 5 years, 3% versus 32% at 10 years, and 23% versus 42% at 15 years. Patients whose HbA1c improved postbaseline had intermediate risk: 5%, 19%, and 29% at 5, 10, and 15 years of follow-up, respectively. These patients had significantly less risk (P<0.01) than those who remained with poor/very poor HbA1c all the time. Interestingly, the difference increased with follow-up time: It was not significant at 5 years (5% versus 9%; P=0.38) but was significant at 10 years (19% versus 32%; P=0.02) and onward. The risk of ESRD in nonattenders was almost identical to that in patients who had poor or very poor glycemic control all the time.
Figure 3.
Cumulative risk of ESRD according to pre- and postbaseline HbA1c. Risk in 40 patients with fair (≤8%) pre- and postbaseline HbA1c is represented by blue dotted line, risk in 92 patients in whom poor or very poor prebaseline HbA1c improved is shown as green solid line, and risk in 139 patients with poor (>8% to ≤9.5%) and very poor (>9.5%) pre- or postbaseline HbA1c is shown as red interrupted line. The risk in 40 nonattenders is represented by gray dotted line. Number of patients remaining in the risk set is shown at the bottom.
To estimate the association of the risk of ESRD with HbA1c change as a continuous variable, we used multivariate Cox regression models and two glycemia variables: prebaseline HbA1c (Figure 1A) and change between pre- and postbaseline HbA1c (Figure 1B). This representation of them in the model separates the association of postbaseline HbA1c change from that of prior glycemic exposure. The ESRD hazard ratio for an increase in prebaseline HbA1c of 1% was 1.72 (95% CI, 1.38 to 2.14; P<0.001). The hazard ratio for a 1% postbaseline HbA1c decrease was 0.76 (95% CI, 0.63 to 0.91; P=0.003). Inclusion of relevant baseline covariates in the model did not materially change these estimates (Table 4). The association of decreased risk with improved postbaseline HbA1c was independent of the effect of BP or renoprotective treatment or calendar year of enrollment into the study. To further exclude the possibility that the association of improved HbA1c was confounded by increased treatment with renoprotective agents, we stratified the analysis according to presence of these treatments at study entry. The parameter estimates for HbA1c remained similar and statistically significant (data not shown).
Table 4.
Summary of associations of patient characteristics with risk of ESRD in multivariate Cox regression analysis
| Characteristic | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Prebaseline HbA1c (per 1–percentage point increase) | 1.72 (1.38 to 2.14) | <0.001 |
| HbA1c change (per 1–percentage point improvement) | 0.76 (0.63 to 0.91) | 0.003 |
| Male sex | 1.71 (1.04 to 2.82) | 0.03 |
| Duration of diabetes (per 10-yr increase) | 1.11 (0.80 to 1.54) | 0.52 |
| ACR (doubling) | 1.68 (1.33 to 2.12) | <0.001 |
| Baseline eGFR (per 10-ml/min per 1.73 m2 increase) | 0.71 (0.65 to 0.78) | <0.001 |
| Systolic BP (per 10-mmHg increase) | 0.97 (0.86 to 1.10) | 0.64 |
| Renoprotective treatment at baseline (yes) | 0.79 (0.48 to 1.31) | 0.36 |
| Calendar year of enrollment (per 1 yr later) | 0.97 (0.91 to 1.02) | 0.21 |
Similar estimates were obtained when the analysis was stratified by the median baseline eGFR (85 ml/min per 1.73 m2) and when stratified according to the presence at baseline of treatment with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers (194 yes; 85 no).
Effect of Changes in HbA1c on Trajectories of eGFR
A more comprehensive examination of the association of HbA1c change with diabetic nephropathy than either of the preceding analyses (which relied only on the observed times to ESRD) is a joint longitudinal-survival analysis. It incorporates the follow-up estimates of eGFR based on serum creatinine together with the occurrences of ESRD to model eGFR trajectories regardless of whether ESRD was observed (see Concise Methods and Supplemental Material). The model estimates individual trajectories of eGFR change, constraining the trajectories by the time to ESRD if known. Follow-up time was included as a quadratic function to allow for acceleration or deceleration of trajectories (Table 5).
Table 5.
Summary of association of changes in postbaseline HbA1c with features of trajectories of eGFR in joint longitudinal-survival analysis
| Feature | Estimate (95% CI) | P Value |
|---|---|---|
| Intercept | 52.44 (30.29, 74.59) | <0.001 |
| Prebaseline HbA1c (%) | 3.28 (0.95, 5.61) | <0.01 |
| Time (yr) | 6.34 (1.66, 11.01) | <0.01 |
| Prebaseline HbA1c × time | −1.17 (-1.66, -0.67) | <0.001 |
| Time2 | 0.017 (-0.406, 0.441) | 0.94 |
| Prebaseline HbA1c × time2 | −0.000 (-0.047, 0.046) | 0.99 |
| Decrease in HbA1c × time2 | 0.083 (0.034, 0.132) | <0.001 |
| Increase in HbA1c × time2 | −0.032 (-0.114, 0.050) | 0.44 |
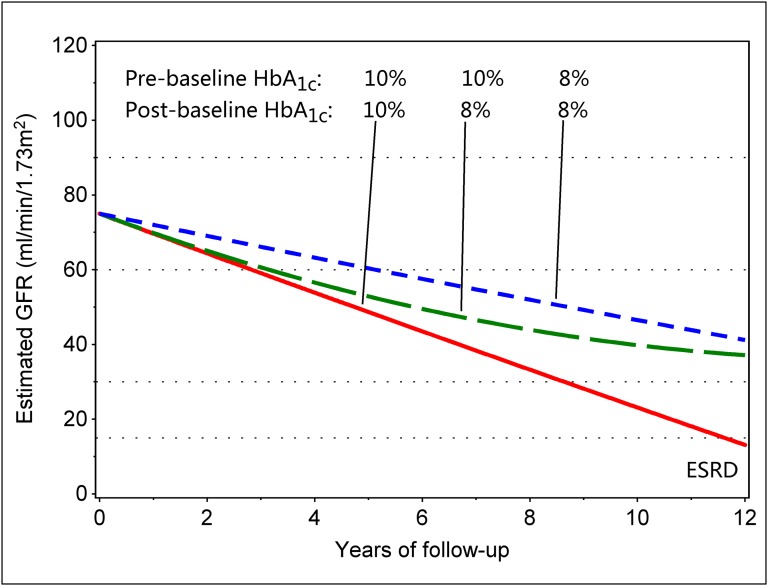
The trajectories were linear for patients whose postbaseline HbA1c change was zero, and the slope of decline steepened with each increase of 1 percentage point in prebaseline HbA1c by 1.2 (95% CI, 0.7 to 1.7) ml/min per 1.73 m2 per year (P<0.001). Independent of prebaseline HbA1c, a postbaseline decrease of 1 percentage point in HbA1c was associated with a deceleration of the decline by 0.17 (95% CI, 0.07 to 0.26) ml/min per 1.73 m2 per year of follow-up (P<0.001). Although the numeric value of the deceleration appears small, it is compounded annually and accumulates. A postbaseline increase of 1 percentage point in HbA1c may accelerate the slope, but the small number of patients in this category was insufficient for drawing any conclusion. To visually illustrate the fitted model, we plotted predicted eGFR trajectories in Figure 4. Red and blue lines depict trajectories for individuals with a zero postbaseline change (i.e., stable HbA1c) and prebaseline HbA1c of 8% and 10%. The green interrupted line depicts an individual with a prebaseline HbA1c of 10% and postbaseline HbA1c of 8%, an improvement of 2 percentage points. The estimated deceleration of eGFR decline is sufficient to postpone the onset of ESRD beyond the time horizon of this study.
Figure 4.
Sample trajectories of renal function decline according to pre- and postbaseline HbA1c. The trajectories were predicted with quadratic joint longitudinal-survival model in patients with different pre- and postbaseline HbA1c: straight lines were estimated in cases where HbA1c remained unchanged. The line curved upward illustrates sustained improvement of HbA1c by 2 percentage points (from 10% to 8%).
Discussion
Most patients with T1D who develop proteinuria have poor glycemic control and a high risk of ESRD.1,2 Our long-term follow-up observation of the Joslin Proteinuria Cohort examined the relationship of progression to ESRD to the levels of glycemic control before and after study entry. Two distinct patterns emerged. One pattern, manifested by about 10% of the patients, is a very rapid decline of renal function that leads to ESRD within 3 years, leaving no time to achieve a stable change in glycemia (Figure 1). The eGFR slopes were linear and equally steep in men and women. The second pattern, present in the rest of the patients, is a broad distribution of less-steep declines to ESRD (half of them beyond 10 years). While the slope of renal decline in these patients is strongly associated with past glycemic control, it is significantly influenced by subsequent changes in HbA1c. Sustained improvement is associated with significantly reduced slopes of renal decline. The magnitude of this reduction is large in patients with the poorest control in the past (HbA1c between 9.5% and 14% in our study), and it decreases as the severity of past glycemia diminishes. While one may conjecture that the process responsible for this association parallels the morphologic reversal of kidneys lesions described by Fioretto et al. after T1D was cured by a pancreas transplantation,16,17 we have no morphologic material to confirm this. Moreover, in that study pretransplant glycemic control was not as poor as the poorest control in our study, and few of those patients had proteinuria.
Fioretto et al. observed a 5- to 10-year lag time between pancreas transplantation and reversal of morphologic lesions in kidneys. The findings of our study seem to be consistent with such a lag between improved glycemia and its effect on the risk of ESRD, but the size of our study does not allow its precise estimation. Nevertheless, this evidence for a lag may explain the failure of several short-term trials designed to find that improved glycemia slowed eGFR loss in patients with proteinuria.8–11 Our findings, in contrast, justify optimism that prolonged improvement in glycemic control by ordinary clinical tools may be an effective treatment for most patients with T1D and proteinuria, a population that remains at extremely high risk of ESRD despite antihypertensive and renoprotective treatment.1,2 However, a different strategy is needed for the minority of patients with T1D who rapidly progress to ESRD.
Poor glycemic control in T1D is an important determinant of the onset of proteinuria, so patients with very high HbA1c are consistently over-represented in populations with proteinuria, as illustrated by our Joslin Proteinuria Cohort and the FinnDiane study.1,2 The substantial reduction in HbA1c in a significant subset of our cohort followed the diagnosis of proteinuria and was perhaps motivated by heightened awareness of dire consequences. In addition, publications from the Diabetes Control and Complication Trial during our recruitment may have been influential for some patients.19 The absence of any identifiable clinical differences between patients whose HbA1c decreased and those whose HbA1c remained unchanged supports a claim that the HbA1c change was not related to prognostic factors.
The strengths and weaknesses of our study must be acknowledged so that investigators can properly interpret the findings when identifying future research needs. Important strengths include (1) ascertainment of study patients as proteinuria developed while under the care of the Joslin Clinic rather than a collection of referrals to the clinic because proteinuria had developed; (2) very long follow-up (median, 10 years) that was nearly complete; (3) the availability of two temporally independent measures of exposure to hyperglycemia (past exposure and exposure during follow-up); (4) two objective outcome measures (eGFR trajectories and time to onset of ESRD); and (5) analysis with models allowing simultaneous evaluation of the two outcomes that are, in fact, measures of a single underlying process: the progressive loss of renal function until ESRD is reached.
The first weakness of our study is the absence of randomization in the assignment of HbA1c changes. This would eliminate the possible effect of unaccounted differences between the study participants with regard to their amenability to improved glycemic control. A second weakness is the lack of consistency in spacing of HbA1c measurements and their paucity in some patients. These decrease the precision of the estimated exposures, but this loss of precision only favors the null hypothesis; more precise estimates could only improve the estimates of benefit, not nullify them.
The prospect of improving the prognosis for patients with a risk of ESRD as high as patients with T1D and proteinuria by improving glycemia, particularly in those with the worst hyperglycemia, demands specific pursuits in clinical research and patient care. This includes investigation strategies to motivate and assist patients with proteinuria to achieve and sustain improvements in glycemia. For example, financial incentives could be offered as motivation. If successful, the incentives could be substantial before their costs would equal the cost of RRT. It is important to ascertain what HbA1c value must be sustained to be effective. For example, our data suggest that values around 8%–9% may suffice. Because almost 80% of patients with T1D and proteinuria have poor or very poor glycemic control, the goal should be to achieve improvement for all of them. Perhaps new clinical protocols to accomplish this goal need to be developed specifically for these patients. For example, an injectable and acid-degradable polymer involving glucose-mediated release of insulin is under development for the self-regulated delivery of insulin.20 In mice with T1D, a single injection stabilized blood glucose at around 200 mg/dl for up to 10 days. In humans, this would correspond to sustaining an approximate HbA1c level at 8%. For now, this level of glycemia is considered unsatisfactory for patients without proteinuria, but would it be sufficient for the needs of patients with T1D and proteinuria? Special consideration should be given to patients whom we called rapid progressors. Most had very poor prebaseline glycemic control, and the majority entered observation in CKD stage 3, so they had only a short way to travel to reach ESRD. Had the effort to improve glycemic control started when their renal function was normal, they would have had a longer interval in which to improve glycemic control. Alternatively, improved glycemic control in this subset of patients might have a minimal effect on changing trajectories of eGFR loss. If so, they might be a target population for new experimental therapies.
The final pursuit is to confirm our results in another study. Although a clinical trial would be more desirable than another observational study, serious obstacles impede the design and conduct of a clinical trial aimed to test the effectiveness of improved glycemic control in proteinuric patients with T1D. About 5000 new cases of ESRD develop every year from an even larger pool of patients (about 80,000) who would be eligible for the study.1 However, what agency would fund a trial that requires at least a 10-year follow-up? Although HbA1c values in the 9%–11% range persist for years in many patients, would it be allowed to enroll a group of controls that would intentionally be left without an effective attempt to improve their glycemic control? Is there an alternative to the current clinical trial paradigm for developing treatment recommendations for high-risk patients with T1D?
Finally, the findings from our study in T1D may not be generalizable to patients with type 2 diabetes. When proteinuria develops in patients with type 2 diabetes, their past glycemic control is better than that in patients with T1D. Two large clinical trials examined the effectiveness of improved glycemic control (average HbA1c, from 7.3% to 6.5% in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation [ADVANCE] trial and from 7.0%–7.9% to <6.0% in the Action to Control Cardiovascular Risk in Diabetes [ACCORD] trial) in reducing the occurrence of diabetic complications. Whereas the ADVANCE trial found a significantly reduced risk of ESRD,21 the ACCORD trial found no change in risk of any kidney outcome.22 While the results of these two large clinical trials are discrepant at this time, our study suggests that a larger effect of improved glycemic control might have been seen if the trials had lasted longer. The durations of both clinical trials were too short to allow us to see beyond the lag in manifestation of the effect.
Concise Methods
Study Group
Patients for this study about the effect of glycemic control on risk of ESRD were drawn from the Joslin Proteinuria Cohort. Briefly, patients in the Joslin Proteinuria Cohort (n=423) were recruited between 1991 and 2004 from among the population of adults with T1D (approximately 3500 individuals). To eliminate selection/referral biases, only patients who were receiving long-term care at the Joslin Clinic in Boston, Massachusetts, were enrolled.1 This cohort was originally assembled for studies on genetics of diabetic nephropathy. All these patients developed persistent proteinuria between 1990 and 2004 while attending the Joslin Clinic. Proteinuria was diagnosed using measurements of the ACR in spot urine. Methods for ACR determination and the definition of proteinuria were reported previously.18,23 Briefly, persistent proteinuria was diagnosed when the ACR (in mg of albumin/g of creatinine) in two of three consecutive urine samples taken at least 3 months apart was ≥250 for men or ≥355 for women. The concentration of urinary creatinine is lower in women than men (on average); hence, the ratio is higher for an equivalent albumin excretion rate. ACRs were calibrated to timed measurements of the albumin excretion rate, and these sex-specific ACR criteria are both equivalent to an albumin excretion rate of 300 µg/min.1,23
Patients were recruited and examined within a few years of onset of persistent proteinuria (median, 2.9 years; 25th, 75th percentiles: 1 year, 5 years). Once enrolled in the study, they were examined every 2 years during routine clinic visits or at their homes if they stopped coming to the clinic. Descriptions of baseline and follow-up examinations, ascertainment of ESRD cases through the USRDS, and ascertainment of deaths unrelated to ESRD were described in our previous publication on the natural history of ESRD in T1D, which included the experience of the Joslin Proteinuria Cohort through the end of 2008.1 For the present report, follow-up was extended through the end of 2011, and all patients with CKD stages 1–3 at enrollment were considered (n=349). Cohort members with CKD stages 4–5 at enrollment were excluded. Longitudinal measurements of HbA1c and serum creatinine from study examinations were supplemented with similar data from clinic visits.
Variation of HbA1c
Glycemic control was evaluated separately during pre- and postbaseline periods to evaluate whether a change occurred. For prebaseline HbA1c, all HbA1c measurements were extracted from the Joslin clinical laboratory’s computer archives from the 5 years up to and including the enrollment examination, for a total of 2763 determinations. The number per patient ranged from two to 15 (median, 7). To represent prebaseline exposure, multiple values within an individual year were averaged before the average of the years having at least one value were averaged.
For analyzing postbaseline HbA1c data, we distinguished three subgroups of patients. The first comprised patients who progressed rapidly to ESRD within 3 years (n=30) and did not have an opportunity to achieve sustained improvement in glycemic control. The second comprised nonattenders, patients who stopped coming to the clinic within 3 years and could not be examined at their homes (n=40). For these two groups combined, the total number of postbaseline HbA1c determinations was 122, with most patients having zero or one measurement. Thus, no good measure of postbaseline HbA1c exists for them. The third subgroup (n=279) comprised patients who continued to attend the clinic or participated in the study by being examined at home and had postbaseline measurements of HbA1c for assessing a change in glycemic control. Postbaseline HbA1c was represented by the HbA1c measurements during the first half of follow-up. The distribution of follow-up intervals was truncated at 15 years, and the individual intervals were halved. Because of the expected lag-time between a change in exposure and its effect, measurements during the second half of follow-up were disregarded. The number of postbaseline HbA1c determinations in the first half-interval ranged from two to 18, with a median (first, third quartiles) of seven (2, 12). Multiple values within a year were averaged before averaging across years to represent postbaseline HbA1c. The length of this postbaseline half-interval ranged from 3.0 to 7.5 years, with a median (first, third quartiles) of 5.1 years (3.9, 7.3).
To place the distribution of prebaseline HbA1c values for our complete cohort in context, we obtained a similar measure from a previously described reference cohort of 1412 patients with T1D comprising a 50% random sample of those without proteinuria attending the Joslin Clinic in 1991–1992.18 All HbA1c measurements for these patients during 5 years after entry were summarized as for the complete cohort.
HbA1c is measured in the Joslin clinical laboratory for routine visits, as well as for research purposes. The methods used during the last 20 years were calibrated to Diabetes Complications and Control standards: in the 1990s, Bio-Rad HPLC analyzer (Bio-Rad, Hercules, CA); in 2001–2005, Tosoh 2+2 HPLC analyzer; and in 2006 and beyond, Tosoh G7 HPLC analyzer (Tosoh Bioscience, South San Francisco, CA).
Trajectories of Renal Function Decline
During follow-up of the Joslin Proteinuria Cohort, serum samples were collected at special follow-up examinations, as previously described.1,5 Serum creatinine in samples from baseline and follow-up examinations were measured at the University of Minnesota with the Roche enzymatic assay (product no. 11775685) Q8 on a Roche/Hitache Mod P analyzer (Indianapolis, IN), calibrated to be traceable to an isotope dilution mass spectrometry (IDMS) reference assay.5 In addition, we retrieved serum creatinine measurements by the Joslin clinical laboratory for routine visits that were assayed with the Jaffe modified picrate method on a Ciba Corning Express Plus Chemistry Analyzer (Medfield, MA). Of these, 1113 were assayed in blood from the same draw as that sent to the reference laboratory. As described elsewhere,5 the duplicated measurements were used to calibrate Joslin clinical laboratory results to the IDMS-traceable method. We traced trajectories of renal function changes in the 349 patients using 4118 serum creatinine measurements, of which 1085 were directly validated by the IDMS-traceable method. The number of serum creatinine measurements per patient ranged from two to 27, with a median (first, third quartiles) of 10 (4, 20). The eGFR was calculated with the CKD-Epidemiology Collaboration formula.24
Definitions of ESRD and ESRD-Unrelated Death
Enrolled patients were followed for the development of ESRD and death by matching the study roster against the USRDS and the National Death Index (NDI). In the United States, persons who develop ESRD are eligible for Medicare support, and the USRDS tracks requests to Medicare for RRT. During the years of this study, the criteria for acceptance into this program and for priority for transplantation have not changed for this age group with T1D. The onset of ESRD was recorded as the date of first renal transplant or renal dialysis. The most recent query took place in October 2013 and covered all incident cases of ESRD between 1991 and the end of 2011.
The NDI (http://www.cdc.gov/nchs/ndi.htm) is a central index of death records on file in the vital statistics offices of the United States. In addition to information from the NDI, vital status and circumstances surrounding death were abstracted from medical records. Cause of death was coded by International Classification of Diseases (ICD), Ninth and Tenth Revisions (depending on date of death), provided by the NDI database. Matching with the NDI is complete through the end of 2011.
Living patients according to NDI matching who were free of ESRD as of the end of 2011 were contacted in 2012 and 2013 to establish their status as of the end of 2011. We were able to ascertain the status (alive, ESRD, or death) for 92% of the study group as of the end of 2011. This includes the subgroup of nonattenders described earlier.
Medical records of patients who died before diagnosis of ESRD were reviewed. Eleven were reclassified as having reached ESRD before dying on the basis of the last eGFR before death and other information recorded at death. In addition, five patients had CKD stage 5 at the last clinic visit in 2011. We classified these patients as reaching ESRD, although this was not yet confirmed in the USRDS. Deaths were assigned to cardiovascular causes according to codes recorded in the NDI. For deaths before 1999, these were ICD-9 codes 401–450; for deaths after 1998, they were ICD-10 codes I10–I74.
Statistical Analyses
Characteristics of these patients were summarized by percentages or medians and quartiles. The pre- and postbaseline HbA1c values were compared with a signed-rank test, and HbA1c values between proteinuric and nonproteinuric patients were compared with a rank-sum test.
Only the events of ESRD that occurred between the fourth and 15th years of follow-up were used in the analyses. The incidence rate of ESRD was stratified according to categories defined by pre- and postbaseline HbA1c. Cox regression models of ESRD risk included prebaseline HbA1c and the pre- to postbaseline change in HbA1c. Proportional hazard assumptions were checked by testing interactions of these covariates with time.
To investigate the effect of HbA1c change on eGFR trajectories during the 15 years of follow-up, we used a joint longitudinal-survival model proposed by Henderson et al.25 (also see Supplemental Material). In this model, a linear mixed-effects component is augmented with a time-to-ESRD (survival) submodel to account for informative dropout due to onset of ESRD. The eGFR trajectories in the mixed-effects component were modeled as a quadratic function to allow for acceleration or deceleration of decline. Computations were performed using SAS PROC NLMIXED, as presented by Guo and Carlin (SAS Institute, Cary, NC).26
Statistical significance was set at P<0.05. Analyses were conducted in SAS for Windows, version 9.3.
Disclosures
None.
Supplementary Material
Acknowledgments
This project was supported through Juvenile Diabetes Research Foundation research grant 1-2008-1018 (A.S.K.), National Institutes of Health grant DK41526 (A.S.K.), and Juvenile Diabetes Research Foundation fellowship grant 3-2009-397 (J.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013091002/-/DCSupplemental.
References
- 1.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS: Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22: 545–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forsblom C, Harjutsalo V, Thorn LM, Wadén J, Tolonen N, Saraheimo M, Gordin D, Moran JL, Thomas MC, Groop PH, FinnDiane Study Group : Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 22: 537–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS: In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int 77: 57–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skupien J, Warram JH, Smiles AM, Niewczas MA, Gohda T, Pezzolesi MG, Cantarovich D, Stanton R, Krolewski AS: The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int 82: 589–597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group : Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290: 2159–2167, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B, DCCT/EDIC Research Group : Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viberti GC, Mackintosh D, Bilous RW, Pickup JC, Keen H: Proteinuria in diabetes mellitus: Role of spontaneous and experimental variation of glycemia. Kidney Int 21: 714–720, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Ciavarella A, Vannini P, Flammini M, Bacci L, Forlani G, Borgnino LC: Effect of long-term near-normoglycemia on the progression of diabetic nephropathy. Diabete Metab 11: 3–8, 1985 [PubMed] [Google Scholar]
- 10.van Ballegooie E, de Jong PE, Donker AJ, Sluiter WJ: The effect of continuous subcutaneous insulin infusion on renal function in type I diabetic patients with and without proteinuria. Proc Eur Dial Transplant Assoc Eur Ren Assoc 21: 722–724, 1985 [PubMed] [Google Scholar]
- 11.Bending JJ, Viberti GC, Watkins PJ, Keen H: Intermittent clinical proteinuria and renal function in diabetes: Evolution and the effect of glycaemic control. Br Med J (Clin Res Ed) 292: 83–86, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alaveras AE, Thomas SM, Sagriotis A, Viberti GC: Promoters of progression of diabetic nephropathy: The relative roles of blood glucose and blood pressure control. Nephrol Dial Transplant 12[Suppl 2]: 71–74, 1997 [PubMed] [Google Scholar]
- 13.Mulec H, Blohmé G, Grände B, Björck S: The effect of metabolic control on rate of decline in renal function in insulin-dependent diabetes mellitus with overt diabetic nephropathy. Nephrol Dial Transplant 13: 651–655, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH: Progression of diabetic nephropathy. Kidney Int 59: 702–709, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Goel G, Perkins BA: Can improved glycemic control slow renal function decline at all stages of diabetic nephropathy? Semin Nephrol 32: 423–431, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Fioretto P, Mauer SM, Bilous RW, Goetz FC, Sutherland DE, Steffes MW: Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet 342: 1193–1196, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH: Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 332: 1251–1255, 1995 [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, Cheng H, Langer RS, Anderson DG: Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 7: 4194–4201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M, Mogensen CE, Poulter N, Mancia G, Cass A, Patel A, Zoungas S, ADVANCE Collaborative Group : Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 83: 517–523, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Ismail-Beigi F, Craven TE, O’Connor PJ, Karl D, Calles-Escandon J, Hramiak I, Genuth S, Cushman WC, Gerstein HC, Probstfield JL, Katz L, Schubart U, ACCORD Study Group : Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int 81: 586–594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warram JH, Gearin G, Laffel L, Krolewski AS: Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 7: 930–937, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson R, Diggle P, Dobson A: Joint modelling of longitudinal measurements and event time data. Biostatistics 1: 465–480, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Carlin BP: Separate and joint modeling of longitudinal and event time data using standard computer packages. Am Stat 58: 16–24, 2004 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.