Abstract
Hemodialysis catheter-related bacteremia is a common clinical problem with several management options. We performed a systematic review and meta-analysis to determine cure proportions with systemic antibiotics, antibiotic lock solution, and guidewire exchange. We searched databases and registries; conference proceedings from relevant medical societies; and article reference lists. Data regarding management approach, cure, follow-up, recurrence, complications, and microbiology were abstracted and pooled from 28 selected publications. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated from a mixed effects logistic regression model. In total, 1596 patients with tunneled hemodialysis catheter-related bacteremia were divided into groups on the basis of treatment with systemic antibiotics (n=697), antibiotic lock solution (n=546), or guidewire exchange (n=353). Antibiotic lock solution and guidewire exchange had similar cure proportions that were superior to systemic antibiotics alone (OR, 2.08; 95% CI, 1.25 to 3.45; P<0.01 for antibiotic lock solution; OR, 2.88; 95% CI, 1.82 to 4.55; P<0.001 for guidewire exchange versus systemic antibiotics). Cure proportions were highest for coagulase-negative staphylococci followed by gram-negative rods and Staphylococcus aureus. Among S. aureus infections, guidewire exchange led to a higher cure proportion than systemic antibiotics or antibiotic lock solution (OR, 3.33; 95% CI, 1.17 to 9.46; P=0.02; OR, 4.72; 95% CI, 1.79 to 12.46; P=0.002, respectively). Thus, results of this study suggest that tunneled hemodialysis catheter-related bacteremia should be treated with either guidewire exchange or antibiotic lock solution. Future studies should address prospectively whether one strategy is better than the other overall and for specific pathogens.
Keywords: hemodialysis, bacteremia, antibiotic lock solution, guidewire exchange
Central venous catheters (CVCs) are a common cause of nosocomial bacteremia among hemodialysis (HD)-dependent subjects. National surveillance data indicate that, among approximately 380,000 people requiring HD for ESRD in the United States in 2010, 18% of prevalent and 80% of incident dialysis patients used CVCs for access.1 Infection is the second leading cause of death among ESRD patients, and use of CVCs as access is a predictor of all-cause and infection-specific mortality.2
Treatment of catheter-related bacteremia (CRB) generally requires CVC removal in most patient populations. However, patients undergoing HD are different in several key aspects: (1) they have higher rates of venous thrombosis and stenosis that can lead to lack of future HD access sites, and (2) presence of a viable access site is literally a lifeline, especially in those patients who cannot tolerate long periods without dialysis. CVC removal, on one hand, may not allow reinsertion and thus, lead to loss of access site, and on the other hand, it may not actually have a better outcome in terms of reinfection with the same organism.3 Thus, there is a need to salvage access sites in these patients.
Management options for the hemodynamically stable patient with CRB with a goal for access site salvage include systemic antibiotics alone, use of an antibiotic lock solution, or guidewire exchange of the catheter. The latter two options also receive adjunctive systemic antibiotics. Antibiotic lock solution consists of high concentrations of antibiotic combined with anticoagulant instilled into the catheter lumen. Guidewire exchange is performed by inserting a guidewire through the infected catheter to the accessed vein followed by removal of the infected catheter and placement of a new catheter over the guidewire. Patients who are septic, are hemodynamically unstable, or have an exit site/tunnel infection generally undergo removal of the infected catheter and delayed placement of a new catheter. Catheter removal may be associated with difficulty in regaining venous access. There are additional costs associated with guidewire exchange or catheter removal/replacement procedures, which are a concern in the current era of an impetus for cost-effective management.
Guidelines for CRB management are variable in their recommendations. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) recommends guidewire exchange of a tunneled HD catheter when CRB occurs, whereas the European Renal Best Practice recommends use of guidewire exchange if removal and replacement of the catheter is not a feasible option, although it also states that the guidewire exchange procedure “is associated with a high failure rate.”4,5 The Infectious Diseases Society of America (IDSA) guidelines recommend HD catheter removal/replacement as a first-line approach to CRB, although guidewire exchange can be used if no alternative access is available.6 All three societies endorse antibiotic lock solution as an alternative to guidewire exchange. Despite these recommendations, some centers continue to use systemic antibiotics alone in the treatment of CRB caused by less virulent organisms (Saima Aslam, unpublished observations).
There is a lack of prospective randomized clinical trials (RCTs) that answer the question of the most effective management strategy for this relatively common clinical scenario. Additionally, there is no comprehensive systematic review and meta-analysis that assesses management options specifically for HD CRB. We hypothesized that use of systemic antibiotics alone, antibiotic lock solution, and guidewire exchange for hemodynamically stable patients with tunneled HD CRB would lead to similar cure proportions. We performed a systematic review and meta-analysis of clinical cohorts and interventional trials to compare cure proportions (outcomes) between these three management options (interventions) in patients with tunneled HD CRB (study population).
Results
Study Selection
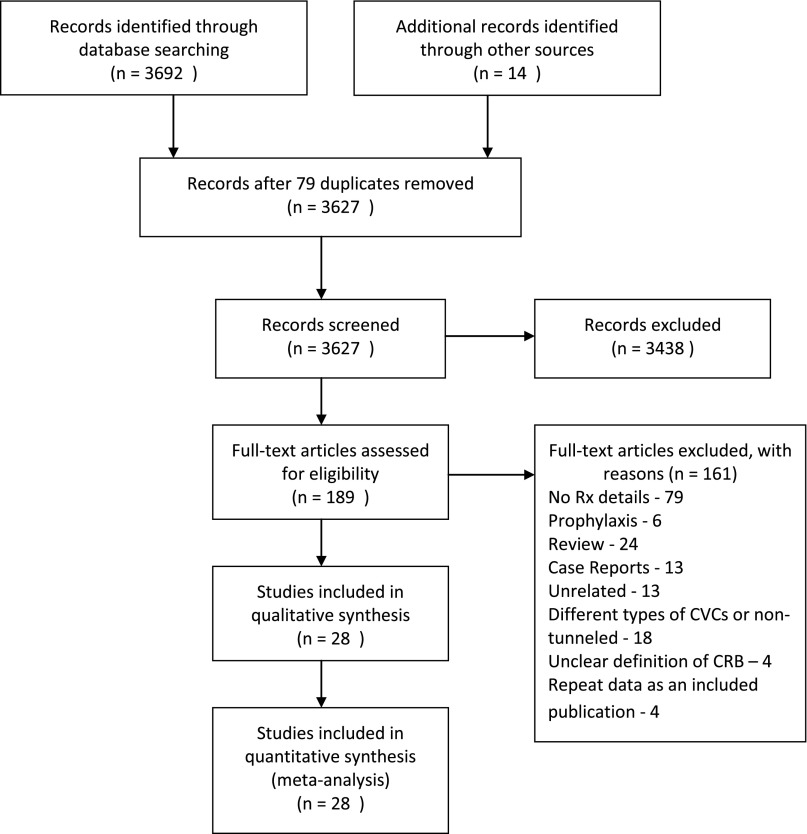
The selection process for the articles included in the systematic review and meta-analysis is presented in Figure 1. Twenty-eight publications fit criteria for inclusion. The database search and review of other sources identified 3706 records; 3438 records were excluded, because they were unrelated to the study question. Both reviewers conducted an independent PubMed search and agreed on the same full-text publications that were selected for the meta-analysis after exclusion of 161 full-text publications (including foreign language translations).
Figure 1.
Flowchart showing the study selection process. Number of studies screened, assessed for eligibility, and included in the meta-analysis and reasons for exclusion of full-text articles.
Study Characteristics
Selected studies included only patients who underwent HD by means of tunneled cuffed catheters. We divided 1596 patients into three treatment groups: systemic antibiotics alone (SABX; n=697), antibiotic lock solution (ABL; n=546), and guidewire exchange (GWX; n=353) as detailed in Table 1. They were observational cohorts or chart reviews and included predominantly an adult ESRD population; 28% of patients were pediatric. There were no RCTs. Some studies had control groups, although most studies were single arm, which is noted in Table 1.3,7–33 Each publication contributed to one or more treatment group, and some contributed to two or more treatment groups.
Table 1.
Details of dataset for each of the three groups—SABX, ABL, and GWX
| Study | Study Period | No. of CRB | Type of Study/Approach | Patient Characteristics | Definition of Cure | Follow-Up (d) | Cure (%) | Recurrence with Same Organism (%) | Complications | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| SABX | ||||||||||
| Moss et al. 19907 United States | 1984–1988 | 16 | Retrospective chart review/systemic antibiotics alone | Not available for study subjects. 50% diabetic. Incident catheters | Clearance and lack of recurrent bacteremia | 28 | 4 (25) | — | None | |
| Swartz et al. 19948 United States | 1990–1993 | 29 | Prospective observational study/systemic antibiotics alone | Not available for study subjects. Incident catheters | Clinical resolution in 48 h+CVC salvage | ≥21 | 9 (31) | — | — | |
| Lund et al. 19969 United States | 1991–1992 | 22 | Retrospective chart review/systemic antibiotics alone | Not available for study subjects. Incident catheters. | Clinical resolution | 180 | 7 (31.8) | — | — | |
| Marr et al. 199710 United States | 1995–1996 | 38 | Prospective observational study/systemic antibiotics alone versus CVC removal | Median age=56 yr, 54% female. 38% diabetic. Prevalent catheters | Original CVC present at 3 mo or removal for noninfectious reason | 90 | 12 (31.6) | — | 5 (no subgroup details) | Persistent and recurrent bacteremia are in the same group—18 |
| Saad 199911 United States | 1995–1997 | 25 | Prospective observational study/systemic antibiotics alone versus GWX | Mean age=52.2 yr. 63.3% diabetic. Prevalent catheters | Lack of recurrence within 30 d | 30 | 11 (36.7) | 14 (56) | — | |
| Chawla and Nevins 200012 United States | 1986–1995 | 18 | Retrospective chart review/systemic antibiotics alone | Mean age=12.5 yr, 20% female. No patients with diabetes. Prevalent catheters | Clearance and CVC salvage | 120 | 6 (33.3) | 4 (22.2) | — | |
| Jean et al. 200213 France | 1994–1998 | 56 | Prospective observational study/systemic antibiotics alone | Mean age=59.8 yr, 30% female. 33% diabetic. Incident catheters. | Clinical resolution at 48 h and no recurrence | 90 | 29 (51.8) | — | 6 (3 shock/death, 1 spondylitis, 2 pneumonia) | |
| Lee et al. 200514 South Korea | 2001–2005 | 11 | Prospective observational study/systemic antibiotics alone versus ABL | Mean age=56 yr, 64% female. 45.5% diabetic. Prevalent catheters | Clinical resolution at 48 h and culture negative 1 wk after antibiotic completion | 21–28 | 6 (54.6) | — | — | |
| Mokrzycki et al. 20063 United States | 2000–2003 | 49 | Prospective observational study/systemic antibiotics alone versus GWX versus CVC removal and replacement versus CVC removal with alternate access | Mean age=55 yr, 44% female. 36% diabetic. Prevalent catheters | No recurrence/death in 90 d | 90 | 36 (73.5) | 10 (20.4) | 6 (no details) and 3 deaths | |
| Troidle and Finkelstein 200815 United States | 2001–2006 | 35 | Prospective observational study/removal versus GWX versus systemic antibiotics alone | Not available for study subjects. Prevalent catheters | No bacteremia/death in 60 d | 60 | 21 (60) | 5 (14.3) | 8 deaths | |
| Ashby et al. 200916 United Kingdom | — | 115 | Prospective observational study/systemic antibiotics alone versus CVC removal | Mean age=61.1 yr, 39% diabetic. Prevalent catheters | No recurrence/complication in 6 mo | 180 | 76 (66.1) | 38 (33) | 1 (no details) | Systemic antibiotics for 6 wk |
| Onder et al. 201317 United States | 1997–1998 | 95 | Retrospective chart review/systemic antibiotics alone | Mean age=14.4 yr, 48% female. Prevalent catheters | Clinical resolution at 48 ho+CVC salvage at 4 wk | 28 | 36 (37.9) | — | — | |
| Onder et al. 2013,17 2008,18 200719 United Statesa | 1999–2003 | 188 | Retrospective chart review/systemic antibiotics alone versus ABL | Mean age=13.9 yr, 56% female. Prevalent catheters | Clinical resolution at 48 h and no recurrence | 42 | 64 (34) | — | — | |
| ABL | ||||||||||
| Capdevila et al. 199320 Spain | 1990–1991 | 11b | Prospective observational study/ABL | Mean age=62.6 yr, 82% female. Incident catheters. | Clinical resolution 48–72 h+no recurrence within 6 wk | 42 | 9 (81.8) | 2 (18.2) | None | ABL: vanc/hep and cipro/hep |
| Bailey et al. 200221 United Kingdom | 2000 | 10 | Prospective observational study/ABL | Not available for study subjects. Prevalent catheters | Clinical resolution+negative cultures at 2 and 4 wk | 90 | 3 (30) | 2 (20) | 1 death | vanc/hep, gent/hep, ampho B/hep |
| Krishnasami et al. 200222 United States | 2000 | 62 | Prospective observational study/ABL versus historical catheter replacement control | Not available for study subjects. Prevalent catheters | Clinical resolution at 48 h+negative cultures at 4 wk+CVC salvage | 28 | 40 (64.5) | 0 | 11 (5 septic shock, 2 septic arthritis, 3 IE, 1 OM) | vanc/gent/hep, vanc/hep, gent/hep, cefaz/hep, cefaz/gent/hep |
| Vardhan et al. 200223 United Kingdom | — | 26 | Observational study/ABL | Not available for study subjects. Prevalent catheters | Clinical resolution at 72 h and negative cultures at 21 d | 21 | 16 (61.5) | 0 | None | vanc/gent/hep |
| Poole et al. 200424 United States | 2001–2002 | 47 | Prospective interventional study/ABL versus historical control | Mean age=54 yr, 62% female. 51% diabetic. Prevalent catheters | Clinical resolution 48 h+negative cultures at 4 wk | 28 | 33 (70.2) | 5 (10.6) | 3 (1 shock, 1 empyema, 1 infected thrombus) | vanc/ceftaz/hep, vanc/hep, or ceftaz/hep |
| Lee et al. 200514 South Korea | 2001–2005 | 18 | Prospective observational study/systemic antibiotics alone versus ABL | Mean age=61.3 yr, 56% women. 93.7% diabetic. Prevalent catheters. | Clinical resolution at 48 h and culture negative 1 wk after antibiotic completion | 21–28 | 16 (88.9) | — | — | vanc/hep, imi/hep, cipro/hep, ceftaz/hep |
| Maya et al. 200725 United States | 2002–2006 | 113 | Retrospective review of prospective database/ABL (S. aureus bacteremia only) | Mean age=52.3 yr, 55% women. 46.3% diabetic. Prevalent catheters | Clinical resolution and no recurrence in 90 d | 90 | 46 (40.7) | 27 (23.9) | 11 (5 IE, 2 OM, 1 septic arthritis, 1 shock, 1 infected thrombus, 1 chest wall abscess) | vanc/hep and cefaz/hep |
| Peterson et al. 200926 United States | 2004–2007 | 64 | Retrospective review of prospective database/ABL (VSE only) | Mean age=54.5 yr, 64% female. 66% diabetic. Prevalent catheters | Clinical resolution at 48 h and no recurrence 90 d | 90 | 39 (60.9) | 15 (23.4) | 4 (1 IE, 3 OM) | vanc/hep lock |
| Onder et al. 2013,17 201025 United Statesa | 2004–2006 | 149 | Retrospective chart review/ABL | Mean age=12.4 yr, 57% women. Prevalent catheters | CRB clearance, CVC salvage, and no infection within 6 wk | 42 | 80 (53.7) | — | — | vanc/hep, vanc/TPA, tobra/hep, tobra/TPA |
| Joshi and Hart 201225 United States | — | 46 | Prospective observational study/ABL | Mean age=47 yr, 38% women. 50% diabetic. Prevalent catheters | CRB clearance, CVC salvage, and no infection within 6 wk | 21–35 | 27 (58.7) | 0 | 1 IE | various abx mixed with either hep or citrate (amikacin, ampho B, cefaz, cefaz + gent, ceftaz, gent, gent + vanc, vanc) |
| GWX | ||||||||||
| Shaffer 199529 United States | 1992–1994 | 12 | Observational study/GWX | Not available for study subjects. Prevalent catheters | No bacteremia within 90 d | 90 | 10 (83.3) | — | None | |
| Robinson et al. 199830 United States | 1996–1997 | 23 | Prospective observational study/GWX | Mean age=59 yr, 100% women. Percent diabetic not available. Prevalent catheters | No bacteremia within 90 d | 90 | 19 (82.6) | 2 (8.7) | 1 diskitis | |
| Saad 199911 United States | 1995–1997 | 43 | Prospective observational study/systemic abx alone versus GWX | Mean age=52 yr. 58.1% diabetic. Prevalent catheters | Lack of recurrence within 30 d | 30 | 35 (81.4) | 6 (14) | — | |
| Beathard 199931 United States | 1996–1997 | 77 | Prospective observational study/GWX versus delayed placement | Mean age=53.8 yr. 48% patients with diabetes. Incident catheters. | Lack of recurrence within 45 d | 45 | 48 (62.3) | 5 (6.5) | None | |
| Tanriover et al. 200032 United States | 1997–1998 | 31 | Retrospective review of prospective database/GWX versus delayed placement | Mean age=52 yr, 58% female. 35% diabetic. Prevalent catheters | Infection-free survival | ≥21 (up to 180) | 15 (48.4) | — | 7 (no details) | |
| Mokrzycki et al. 20063 United States | 2000–2003 | 35 | Prospective observational study/systemic antibiotics alone versus GWX versus CVC removal and replacement versus CVC removal with alternate access | Mean age=57 yr, 54% female. 57% diabetic. Prevalent catheters. | No recurrence/death in 90 d | 90 | 34 (97.1) | 1 (2.9) | 1 | |
| Troidle and Finkelstein 200815 United States | 2001–2006 | 36 | Prospective observational study/removal versus GWX versus systemic abx alone | Not available for study subjects. Prevalent catheters | No bacteremia/death in 60 d | 60 | 21 (58.3) | 1 (2.8) | 8 deaths | |
| Langer et al. 201133 United States | 2001–2008 | 96 | Retrospective chart review/GWX in staphylococcal versus nonstaphylococcal bacteremiac | Mean age=57.5 yr, 51% female. 52.5% diabetic. Prevalent catheters | No bacteremia/death in the exchanged catheter | ≥21 | 55 (57.3) | — | — |
VSE, vancomycin sensitive enterococci; IE, infective endocarditis; OM, osteomyelitis; vanc, vancomycin; hep, heparin; cipro, ciprofloxacin; gent, gentamicin; ampho B, amphotericin B; cefaz, cefazolin; ceftaz, ceftazidime; imi, imipenem; TPA, tissue plasminogen activator; tobra, tobramycin.
Two or more publications contribute to each group, because the publications had overlapping information for the same patient populations. However, the years of patient enrollment are specific and distinguish the groups. There is no overlap of CRB cases between the Onder groups.
Study actually quotes data on 13 episodes of CRB. However, two of them are recurrences that occurred within the follow-up period of the initial episode and thus, have been counted as failures.
We grouped the staphylococcal and nonstaphylococcal infections into one group.
All studies defined CRB as a positive blood culture along with clinical signs/symptoms and no other potential source. The definitions of cure were variable (Table 1), although they fulfilled our definition of cure. The median follow-up period was 45 days (range=21–180 days) from CRB diagnosis. Six articles also noted results of patients that had catheter removal/replacement. These patients’ clinical pictures were similar to the patients’ clinical pictures who underwent guidewire exchange in one study,32 but in other studies, it was clearly reserved for those patients with sepsis or severe disease10,16,31 or was unclear.3,15
Individual study characteristics are described in Table 1. We report on the study location, study time period, number of CRB cases, type of study, patient demographics, definition of cure, percent cured, recurrence, follow-up duration, complications, and type of antibiotic lock (when used). Details of individual antibiotic lock solutions are presented in Table 2.
Table 2.
Composition of antibiotic lock solutions used in the included studies of the meta-analysis
| Study | Antibiotic Lock Solutions |
|---|---|
| Capdevila et al. 199320 | Vancomycin or ciprofloxacin 100 μg/ml+5% sodium heparin. Both antibiotics with heparin were used in one patient. |
| Bailey et al. 200221 | Vancomycin 100 μg/ml, gentamicin 20 μg/ml, or amphotericin B 2.5 mg/ml+heparin saline 5000 units/ml. Antibiotic combinations were not used. |
| Krishnasami et al. 200222 | Vancomycin 2.5 mg/ml, gentamicin 1 mg/ml, cefazolin 5 mg/ml+heparin 2500 units/ml (gentamicin could be used alone or with heparin or another antibiotic could be added to the combination). |
| Vardhan et al. 200223 | Vancomycin 100 μg/ml+gentamicin 20 μg/ml+heparin 5000 units/ml. |
| Poole et al. 200424 | Vancomycin 2.5 mg/ml, ceftazidime 5 mg/ml, cefazolin 5 mg/ml+heparin 2500 units/ml. Each drug could be used alone with heparin or in combination. |
| Lee et al. 200514 | Vancomycin 8.3 mg/ml, imipenem 32.3 mg/ml, ciprofloxacin 1.3 mg/ml, or ceftazidime 12.9 mg/ml+heparin 1774–4355 units/ml. Antibiotics were not combined. |
| Maya et al. 200725 | Vancomycin 2.5 mg/ml or cefazolin 5 mg/ml+heparin 5000 units/ml. |
| Peterson et al. 200926 | Vancomycin 2.5 mg/ml+heparin 5000 units/ml. |
| Onder et al. 2013,17 201027 (1/2004–6/2006) | Tobramycin 5 mg/ml or vancomycin 5 mg/ml+tissue plasminogen activator 1 mg/ml or heparin 5000 units/ml. Antibiotics were not combined. |
| Joshi and Hart 201228 | Amikacin 25 mg/ml, amphotericin B 2.5 mg/ml, cefazolin 5 mg/ml, ceftazidime 5 mg/ml, gentamicin 1 mg/ml, or vancomycin 1 mg/ml+heparin 2500 units/ml. Gentamicin could also be combined with either vancomycin or cefazolin (in addition to heparin). |
The concentrations cited are the final concentrations in the lock solutions.
Quality Assessment and Risk of Bias
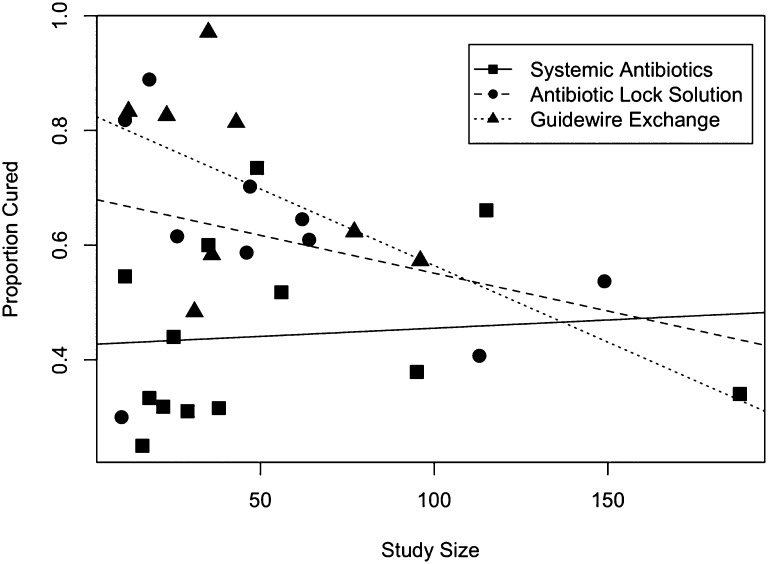
Quality assessment by the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies (Supplemental Appendix) revealed that most studies had low or moderate risk of bias, whereas none of the included studies had a high risk of bias (Supplemental Table 1). When risk of bias was introduced as a covariate in the model for proportion cured, its effect was not significant: odds ratio (OR) of cure for moderate versus low risk of bias, 1.39; 95% confidence interval (95% CI), 0.20 to 9.9; P=0.18. There was large statistically significant heterogeneity of the cure proportions within each treatment group, although the heterogeneity was similar for all treatment groups: SABX: Q statistic (Q), Q=62.4 degrees of freedom (df), (df=12), P<0.001, I2=80.1; ABL: Q=31.5 (df=9), P<0.001, I2=71.4; GWX: Q=33.4 (df=7), P<0.001, I2=79.0; overall: Q=127.2 (df=28), P<0.001, I2=80.0. These differences were accounted for in subsequent analysis. The funnel plot (Figure 2) showed that the cure proportion did not vary between small and large studies for SABX, indicating that there is no evidence of a study selection bias. However, for ABL and GWX, the cure rates tend to be smaller for larger studies than smaller studies. This result indicates the possibility of selection bias, with smaller studies with lower cure rates possibly not published. This heterogeneity was accounted for in the analysis as well. Because the follow-up time differed between studies, we performed a sensitivity analysis, where follow-up time was introduced as a covariate in the model for proportion cured. Follow-up time was not significant (P=0.28), suggesting that studies with longer follow-up time did not display larger cure proportions. We also did not note a bias in the results when we conducted sensitivity analyses by year of publication (P=0.84).
Figure 2.
Funnel plot for the three groups combined in one plot. The funnel is on the side, with the spout (larger sample sizes) toward the right side of the plot.
Results of Individual Studies
Details of patient groups and cure proportions from individual studies are noted in Table 1.
Primary End Point: Cure Proportions for Infection
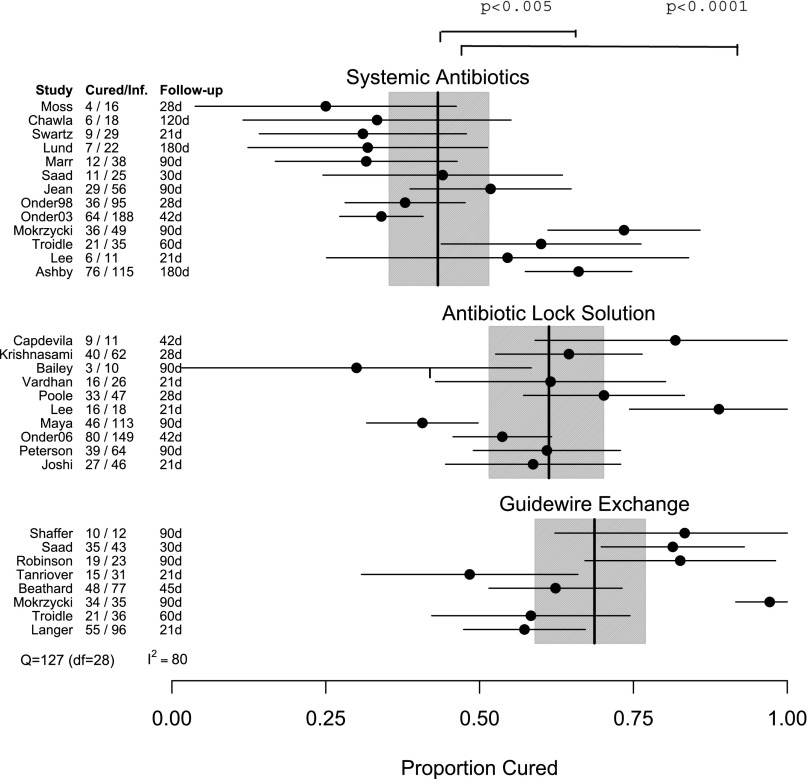
Cure proportions for individual studies in each treatment are plotted in Figure 3, and the results of the metaregression are in Table 2. The overall cure proportions were SABX=45% (range=25%–73.5%, cured/total=317/697), ABL=57% (range=30%–88.9%, cured/total=309/546), and GWX=67% (range=48.4%–97.1%, cured/total=237/353). There were significant differences in cure proportions between ABL and SABX (OR, 2.08; 95% CI, 1.25 to 3.45; P<0.01) and between GWX and SABX (OR, 2.88; 95% CI, 1.82 to 4.55; P<0.001). The difference between GWX and ABL was not significant (OR, 1.39; 95% CI, 0.78 to 2.46; P=0.27). These comparisons take the between-study heterogeneity into account.
Figure 3.
Success rates and 95% CIs for individual studies in the three treatment groups. The vertical lines give the mean success rates (and 95% confidence bands; shaded areas) for each group, taking into account within-group variation. We also provide the numbers cured and total infected for each study and the duration of follow-up in days. There were significant differences in cure proportions between ABL and SABX (OR, 2.08; 95% CI, 1.25 to 3.45; P<0.01) and between GWX and SABX (OR, 2.88; 95% CI, 1.82 to 4.55; P<0.001). The difference between GWX and ABL was not significant (OR, 1.39; 95% CI, 0.78 to 2.46; P=0.27).
Cure Proportions for Specific Bacterial Categories
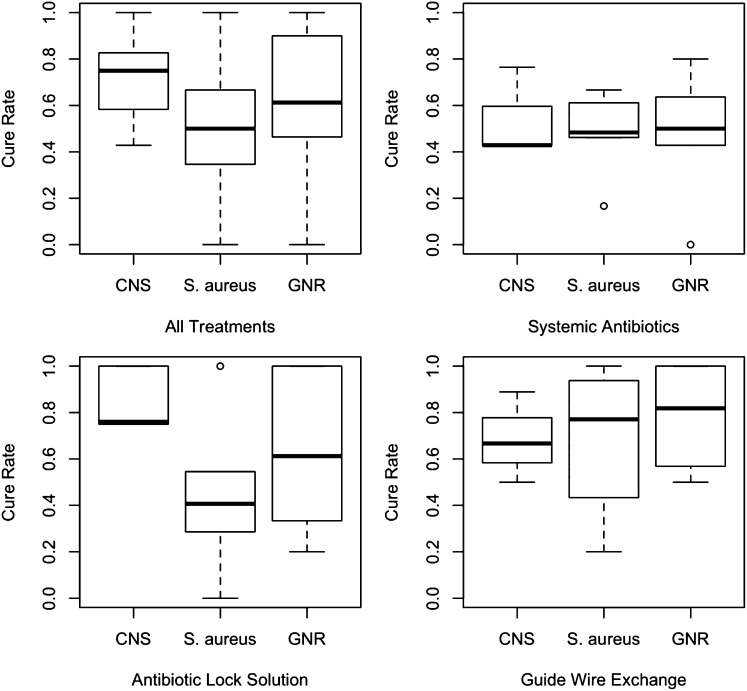
Among studies that reported microbiological details (SABX, 11 of 13; ABL, 9 of 10; GWX, 6 of 8), 23.4% (n=324) of CRB was caused by coagulase-negative staphylococci (CNS), 25.9% (n=359) was caused by Staphylococcus aureus, and 22% (n=305) was caused by gram-negative rods (GNRs) (Supplemental Table 2). We assessed cure proportions overall by type of organism as well as subgroup analysis by organism type and each treatment separately. Figure 4 shows the distribution of cure proportions for CNS, S. aureus, and GNR overall and each treatment separately. For CNS, no heterogeneity in cure proportion was found for any of the three treatments (SABX, P=0.18; ABL, P=0.94; GWX, P=0.81); the findings were similar for S. aureus for SABX (P=0.50) and ABL (P=0.44), but significant heterogeneity was found for GWX (P=0.04). For GNR, significant study-to-study heterogeneity in the cure proportions was found for all three treatments (SABX, P=0.01; ABL, P=0.02; GWX, P=0.001). We adjusted for this heterogeneity in our analysis.
Figure 4.
Box plots of success rates stratified by the type of infecting pathogen, overall (upper left panel), and by type of treatment. Circles denote outliers. The highest cure proportion was for CNS followed by GNR and S. aureus. The ORs of a cure were CNS versus GNR, 1.71 (95% CI, 0.99 to 2.97; P=0.06); CNS versus S. aureus, 3.13 (95% CI, 1.73 to 5.67; P<0.001); and GNR versus S. aureus, 1.83 (95% CI, 1.13 to 2.97; P=0.02).
After taking type of treatment into account, we found differences in cure proportions of various bacteria, with the highest cure for CNS followed by GNR and S. aureus. The ORs of a cure were CNS versus GNR, 1.71; 95% CI, 0.99 to 2.97; P=0.06; CNS versus S. aureus, 3.13; 95% CI, 1.73 to 5.67; P<0.001; GNR versus S. aureus, 1.83; 95% CI, 1.13 to 2.97; P=0.02.
Among S. aureus infections, GWX achieved significantly higher cure proportion than both SABX and ABL (OR, 3.33; 95% CI, 1.17 to 9.46; P=0.02; OR, 4.72; 95% CI, 1.79 to 12.46; P=0.002).
Finally, for each type of infection, we examined whether the distribution of bacteria differed between the three treatments. We found that all three treatments were equally likely for various bacteria (CNS P value=0.80, S. aureus P value=0.90, GNR P value=0.60). Hence, there was no evidence of selection bias of a treatment toward a particular type of bacteria.
Other Characteristics
Recurrence with the same organism as the initial infecting pathogen was reported in five of 13 studies in the SABX group with a weighted mean of 29.3%, eight of 10 studies in the ABL group with a weighted mean of 13.5%, and five of eight studies in the GWX group with a weighted mean of 7.03% (SABX versus ABL: OR, 4.85; 95% CI, 1.61 to 14.63; P<0.01; ABL versus GWX: OR, 1.30; 95% CI, 0.41 to 4.13; P=0.65; SABX versus GWX: OR, 6.33; 95% CI, 2.87 to 13.94; P<0.001).
Complications (including death) were reported in six of 13 studies in the SABX group with a weighted mean of 9.4%, eight of 10 studies in the ABL group with a weighted mean of 8.2%, and six of eight studies in the GWX group with a weighted mean of 7.9%. No significant differences between the three treatments regarding rate of complications were found (SABX versus ABL: OR, 1.45; 95% CI, 0.42 to 5.03; P=0.56; SABX versus GWX: OR, 1.79; 95% CI, 0.79 to 4.06; P=0.17; ABL versus GWX: OR, 1.23; 95% CI, 0.34 to 4.45; P=0.75).
The length of time that the CVC had been in place before infection was infrequently reported. A variety of antimicrobials was used in the preparation of antibiotic lock solutions, including vancomycin, aminoglycosides, cephalosporins, quinolones, imipenem, and amphotericin B. Heparin was commonly used as the anticoagulant in the lock solution, although citrate and tissue plasminogen activator were used in one study each. The concentrations of drugs used for each antibiotic lock solution are described in Table 2.
Discussion
We provide systematic evidence that use of antibiotic lock solution or guidewire exchange of the infected catheter is superior to the use of systemic antibiotics alone in the treatment of HD CRB. Guidewire exchange seems to have a higher cure proportion than antibiotic lock solution (67% versus 57%), although this difference was significant only for S. aureus.
CRB arises from a biofilm present on the catheter surface. Biofilms consist of organisms that are adherent to the catheter as well as each other and encased within an extracellular polysaccharide matrix. Antibiotics have poor activity against biofilm-embedded bacteria and minimum inhibitory concentrations that are up to 500–1000 times higher than the minimum inhibitory concentration for the same organism when in a planktonic phase.34–36 Despite this finding, systemic antibiotics alone continue to be used clinically in many instances of HD CRB treatment, especially when caused by less virulent organisms, such as CNS. In this study, we showed that systemic antibiotics alone for treatment of HD CRB leads to a poor cure proportion, irrespective of the type of infecting pathogen. Thus, use of systemic antibiotics alone for treatment of HD CRB should not be pursued.
Antibiotic lock solution targets the luminal biofilm associated with CRB, and guidewire exchange removes the infected focus altogether. Antibiotic lock solution worked particularly well for CNS as well as GNR infections but not S. aureus infections. The addition of an antibiofilm agent to an antibiotic lock solution may offer some benefit over that of a traditional antibiotic lock.37 There is some concern that increased use of antibiotic lock solution may affect antimicrobial resistance patterns seen in health care systems. Specifically, gentamicin resistance has been noted in centers that used gentamicin-heparin locks in HD catheters for prevention of CRB.38,39 However, this risk is expected to be minimized when the antibiotic in question is used for a short and limited duration of time in individual patients for the treatment of a specific infection. Guidewire exchange of the infected catheter had a high success rate overall, irrespective of the type of infecting pathogen, which is a reflection of the removal of the infection at its source.
Overall cure proportion for S. aureus CRB was lower than other categories, which underscores the virulence of the organism as well as the high morbidity/mortality seen with S. aureus infections.40 The IDSA guidelines state that the HD catheter should always be removed for patients with CRB caused by S. aureus, Pseudomonas species, or Candida species and that a temporary nontunneled CVC should be placed in a different site.6 The use of guidewire exchange is only recommended if no alternative access site is available. This practice leads to increased medical costs because of prolonged hospitalization and the need for multiple procedures. Several studies report success rates between 66% and 89% when CRB was treated with removal and subsequent replacement of the HD catheter.3,15,16,32 The two strategies (guidewire exchange and removal/replacement) do not show a difference in outcome in two studies,3,32 whereas removal/replacement showed a higher success rate in one study,15 although they are all nonrandomized observational studies. The results of this meta-analysis suggest that the use of guidewire exchange for S. aureus or GNR bacteremia may be a reasonable up-front treatment option. Langer et al.33 did not find a difference in cure rates when guidewire exchange was used to treat staphylococcal versus nonstaphylococcal bacteremia (subgroup analysis for S. aureus specifically did not find a difference as well). The cost of a guidewire exchange procedure is expected to be significantly higher than antibiotic lock solution. Thus, it may be more cost-effective to treat certain organisms, such as CNS, with antibiotic lock solution upfront and use guidewire exchange primarily for certain organisms, such as GNRs or S. aureus. This use may lead to cost savings as well as securing access sites in HD patients.
We acknowledge several limitations of our study. The foremost limitation of the results of this meta-analysis is that none of the included studies were RCTs. This finding highlights the lack of RCTs in the field of HD CRB treatment, despite the fact that it is a common clinical scenario. Furthermore, observational cohorts and uncontrolled trials have inherent biases on the basis of the method and nature of data collection. We noted significant heterogeneity of cure proportions within each treatment group, although did adjust for it in our analysis (overall: Q=127.2 [df=28], P<0.001, I2=80.0). We minimized selection bias by using a predefined search strategy with independent data selection and extraction by two independent reviewers without any language restrictions. Data abstractors were not blinded to the authors, affiliations, and journals, and thus, there is potential for selection bias. There was no evidence of publication bias for studies included in the SABX group by the funnel plot. However, for ABL and GWX groups, cure rates tended to be smaller for larger studies than smaller studies, indicating publication and/or selection bias, with smaller studies with lower cure rates possibly not published. We weighted the analysis by study size so that any single study would not be overrepresented. The data were collected from numerous types of studies, including some studies with a primary purpose that was not to describe treatment outcomes or all three treatments that are under consideration in the meta-analysis; thus, there is concern for potential reporting bias. Assessment of study quality did not reveal any study that had a high risk of bias, although about one half of the studies fell in the moderate risk category. Follow-up time differed between studies, although it did not significantly affect the results on the basis of sensitivity analysis.
Despite the above limitations, this study is the only meta-analysis that we are aware of that included data only from ESRD patients with tunneled HD CRB and hence, is very relevant to the target population and question that we are trying to answer. There is a great need for evidence-based practice in the clinical management of CRB in HD patients, and this meta-analysis highlights the need for head-to-head trials between the use of antibiotic lock solution and guidewire exchange as well as elucidation of the role that the infecting pathogen plays in the success of a particular management strategy. Additional questions that require answers include information on the use of sequential and/or combinations of different therapies as well as optimal timing of an intervention, such as guidewire exchange.
We conclude that use of antibiotic lock solution or guidewire exchange leads to significantly higher success than systemic antibiotics alone for treatment of CRB in ESRD patients undergoing HD through a tunneled catheter. We were unable to show a difference between the use of antibiotic lock solution and guidewire exchange, most likely because of the heterogeneity within the included studies; however, the studied patient populations did mirror clinical situations and therefore, would be applicable to most ESRD patients that develop HD CRB. Use of guidewire exchange is superior to the other two modalities for treatment of S. aureus bacteremia. RCTs in this field are urgently needed.
Concise Methods
We followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines for reporting.41 Supplemental Table 3 details the MOOSE checklist as relevant to this study.
Literature Search
We conducted comprehensive searches in PubMed (1966 to July of 2012), the Excerpta Medica database (1950 to October of 2012), Cumulative Index to Nursing and Allied Health Literature plus with full text (no date restriction), Indmed (no date restriction), Index Medicus for the South East Asian Region (no date restriction), KoreaMed (no date restriction), World Health Organization International Clinical Trials Registry (no date restriction), Clinicaltrials.gov (no date restriction), and Complete Retrieval of Information on Scientific Projects (no date restriction) databases. The search strategy included the terms “hemodialysis,” “catheter,” “infection,” and “bacteremia.” Supplemental Material shows the complete search strategy. We also hand-searched conference proceedings from 2010 to 2012 for the IDSA, Interscience Conference on Antimicrobial Agents and Chemotherapy, and American Society of Nephrology meetings. We reviewed the reference lists of relevant articles, reviews, and guidelines for additional references. When needed, we contacted authors of included publications by email for further clarification or data. Our search strategy was not limited by language (when needed, foreign language papers were translated into English). Every effort was made to include all possible relevant studies.
Study Selection and Relevance
Two investigators (S.A. and M.R.; both infectious disease physicians) independently screened the title and abstract of retrieved citations and reviewed the full text of potentially eligible publications to identify studies for inclusion. We included studies that detailed management of bacteremia arising from tunneled HD catheters in patients with ESRD. Studies that incorporated different patient populations were excluded. Several publications by a single group of authors had overlapping information regarding the same group of patients—they were combined into a single dataset so that information was not repeated (the investigators combined data independently; differences were resolved by discussion). Selected studies included interventional clinical trials, observational cohorts, and case series reporting ≥10 CRB episodes. Case reports and abstracts were excluded. Unpublished studies were excluded if we were unable to get complete study methodology and data.
Data Extraction
Two investigators (S.A. and M.R.) independently abstracted data regarding management, cure, follow-up, recurrence, complications, and microbiological details by a standardized form on the basis of clinical relevance to the study hypothesis. Differences were settled by discussion. We contacted authors of included publications for clarifications/additional information when needed (publications were not included if there were unanswered questions).
Assessment of Study Quality and Heterogeneity
Study quality was assessed by the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies, which evaluates patient selection, comparability, and outcome.42 On the basis of this tool, the risk of bias for each included study was categorized as low, medium, or high. The specific form used is in the Supplemental Appendix. Quality assessors were not blinded. Study heterogeneity was assessed by the Cochrane Q test of heterogeneity and I2 statistic as described below in detail. We examined the presence of publication bias by producing a funnel plot of cure rates versus study size for each treatment, and the plots were combined in a single plot.
Definitions
All included studies fulfilled the following requirements on the basis of NKF KDOQI guidelines.4 CRB was defined by a positive blood culture in a symptomatic HD patient with no alternative source for bacteremia. Cure was defined as clinical resolution with no recurrent bacteremia within ≥3 weeks of follow-up from treatment initiation. Patients in all groups received systemic antibiotics for ≥2 weeks. If cure proportions were available for two different follow-up periods, we included cure proportion for the longer duration.
Data Synthesis and Analyses
We divided patients into three treatment groups: SABX, ABL, and GWX. If patients underwent sequential treatment after failure of the first approach, we only included the initial treatment category.
Direct comparison of treatments was not possible for most studies, and therefore, we synthesized the study results by comparing the proportion cured between the three methods, with each study contributing to only the treatment or treatments that were considered in that study. We used a mixed effects logistic regression model with a study-specific normal random effect that modified the overall log odds of cure for the particular treatment(s) to which the study contributed. The test of homogeneity (Q) and the measure of inconsistency (I2) were modifications of the standard Q and I2 statistics as follows. The Pearson chi-squared statistics for the three treatment groups (Q1, Q2, and Q3) test the homogeneity of the cure proportion within each treatment. Q1, Q2, and Q3 have degrees of freedom (k1−1, k2−1, and k3−1), where k1, k2, and k3 are the numbers of studies reporting on each treatment. Q=Q1+Q2+Q3 is an overall test of homogeneity, with degrees of freedom (k−3), where k is the number of studies. The treatment-specific measures of inconsistency are IT2=max(0, {QT−(kT−1)]/Q} and T=1, 2, 3, with overall inconsistency measured by I2=max(0, {Q−(k−3)]/Q}.
Our primary end point (cure proportion for each treatment) was computed as the ratio of cured infections to total infections. For each treatment, we examined the homogeneity of cure proportions between studies. Because these cure proportions were heterogeneous, we also compared cure proportions between the three treatments using a mixed effects logistic regression model. Thus, the log odds of curing the infection depend on type of treatment (SABX versus ABL versus GWX) included as a fixed effect in addition to a study-specific normal random effect that modeled the heterogeneity between studies. For each treatment, every study was weighted by the number of subjects receiving treatment. The differences in cure proportions between the three treatments were evaluated using ORs as the principle summary measure, 95% CIs for the OR, and P values from the Wald test associated with this mixed effects logistic regression model (Table 3). The ORs were adjusted to take into account differences in cure proportions because of between-study heterogeneity. Each of the three pairwise treatment comparisons (ABL versus SABX, GWX versus SABX, and GWX versus ABL) were of primary interest into themselves and considered separately at the 0.05 level. Accordingly, no adjustment for multiple comparisons was done.
Table 3.
The results of the mixed effects logistic regression model with a study-specific normal random effect that modifies the overall log odds of cure for the particular treatment(s) to which the study contributes
| Group | Proportion Cured | OR (95% CI) | P Value |
|---|---|---|---|
| SABX | 317 of 697 | Reference | — |
| ABL | 309 of 546 | 2.08 (1.25 to 3.45) | <0.01 |
| GWX | 237 of 353 | 2.88 (1.82 to 4.55) | <0.001 |
These comparisons take the between-study heterogeneity into account.
Secondary outcomes were cure proportions by organism, recurrence, and complications. We examined cure proportions separately for three microbiological categories: CNS, S. aureus, and GNR. For each bacterial category and treatment, we checked for homogeneity of cure proportions between studies using the Fisher exact test. To test differences in cure proportions between types of bacteria and differences between treatments in curing different types of bacteria, we fit mixed effects logistic regression models, in which the log odds of cure depend on the type of bacteria, treatment, and possibly, their interactions (as fixed effects) and study (as a random effect). We examined the hypothesis of certain treatments having better cure proportions, preferentially for certain types of bacteria, by testing for the presence of an interaction between bacterial category and treatment in the aforementioned model using the likelihood ratio test. In the absence of an interaction effect, we fit a model with additive effects for bacterial category and treatment and tested for differences using the Wald test. Finally, we examined the association between the proportion of infections of a certain bacterial type out of the total number of infections in a given study and the treatment (SABX versus ABL versus GWX) using a mixed effects logistic regression model with a fixed treatment effect and a random study effect, allowing for heterogeneity between studies.
Infection recurrence and complications were compared between the three groups using a mixed effects logistic regression model using the same methodology as used earlier.
We used the R statistical platform (Vienna, Austria) for analysis.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. J.W. Seo of Hallym University, Korea for his English translation of several Korean publications, including one selected for the meta-analysis.
This work was supported by funding from National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant 7K23-DK078828 (to S.A.).
A portion of the data was presented at the 18th International Conference on Continuous Renal Replacement Therapies in San Diego, CA, February 12–15, 2013.
The funding agency was not involved in any aspect of data collection, analysis, or manuscript writing.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013091009/-/DCSupplemental.
References
- 1.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Ishani A, Collins AJ, Herzog CA, Foley RN: Septicemia, access and cardiovascular disease in dialysis patients: The USRDS Wave 2 study. Kidney Int 68: 311–318, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Mokrzycki MH, Zhang M, Cohen H, Golestaneh L, Laut JM, Rosenberg SO: Tunnelled haemodialysis catheter bacteraemia: Risk factors for bacteraemia recurrence, infectious complications and mortality. Nephrol Dial Transplant 21: 1024–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation : KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis 48[Suppl 1]: S1–S322, 2006. 17045862 [Google Scholar]
- 5.Vanholder RCB, Fluck R, Jadoul M, Labriola L, Marti-Monros A, Tordoir J, Van Biesen W: Diagnosis, prevention and treatment of haemodialysis catheter-related bloodstream infections (CRBSI): A position statement of European Renal Best Practice (ERBP). Nephrol Dial Transplant Plus 3: 234–246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK: Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49: 1–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss AH, Vasilakis C, Holley JL, Foulks CJ, Pillai K, McDowell DE: Use of a silicone dual-lumen catheter with a Dacron cuff as a long-term vascular access for hemodialysis patients. Am J Kidney Dis 16: 211–215, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Swartz RD, Messana JM, Boyer CJ, Lunde NM, Weitzel WF, Hartman TL: Successful use of cuffed central venous hemodialysis catheters inserted percutaneously. J Am Soc Nephrol 4: 1719–1725, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Lund GB, Trerotola SO, Scheel PF, Jr., Savader SJ, Mitchell SE, Venbrux AC, Osterman FA, Jr.: Outcome of tunneled hemodialysis catheters placed by radiologists. Radiology 198: 467–472, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB: Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med 127: 275–280, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Saad TF: Bacteremia associated with tunneled, cuffed hemodialysis catheters. Am J Kidney Dis 34: 1114–1124, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Chawla PG, Nevins TE: Management of hemodialysis catheter-related bacteremia—a 10-year experience. Pediatr Nephrol 14: 198–202, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Jean G, Charra B, Chazot C, Vanel T, Terrat JC, Hurot JM, Laurent G: Risk factor analysis for long-term tunneled dialysis catheter-related bacteremias. Nephron 91: 399–405, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lee HR, Lee YK, Song YL, Kim SJ, Joo MH, Kim SG, Oh JE, Seo JW, Koo JR, Kim HJ, Noh JW, Shin SJ: Treatment of catheter-related bacteremia with an antibiotic lock protocol in hemodialysis patients. Korean J Nephrol 24: 903–911, 2005 [Google Scholar]
- 15.Troidle L, Finkelstein FO: Catheter-related bacteremia in hemodialysis patients: The role of the central venous catheter in prevention and therapy. Int J Artif Organs 31: 827–833, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Ashby DR, Power A, Singh S, Choi P, Taube DH, Duncan ND, Cairns TD: Bacteremia associated with tunneled hemodialysis catheters: Outcome after attempted salvage. Clin J Am Soc Nephrol 4: 1601–1605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onder AM, Billings AA, Chandar J, Nield L, Francoeur D, Simon N, Abitbol C, Zilleruelo G: Antibiotic lock solutions allow less systemic antibiotic exposure and less catheter malfunction without adversely affecting antimicrobial resistance patterns. Hemodial Int 17: 75–85, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Onder AM, Chandar J, Billings AA, Simon N, Diaz R, Francoeur D, Abitbol C, Zilleruelo G: Comparison of early versus late use of antibiotic locks in the treatment of catheter-related bacteremia. Clin J Am Soc Nephrol 3: 1048–1056, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onder AM, Chandar J, Saint-Vil M, Lopez-Mitnik G, Abitbol CL, Zilleruelo G: Catheter survival and comparison of catheter exchange methods in children on hemodialysis. Pediatr Nephrol 22: 1355–1361, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Capdevila JA, Segarra A, Planes AM, Ramírez-Arellano M, Pahissa A, Piera L, Martínez-Vázquez JM: Successful treatment of haemodialysis catheter-related sepsis without catheter removal. Nephrol Dial Transplant 8: 231–234, 1993 [PubMed] [Google Scholar]
- 21.Bailey E, Berry N, Cheesbrough JS: Antimicrobial lock therapy for catheter-related bacteraemia among patients on maintenance haemodialysis. J Antimicrob Chemother 50: 615–617, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, Allon M: Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int 61: 1136–1142, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Vardhan A, Davies J, Daryanani I, Crowe A, McClelland P: Treatment of haemodialysis catheter-related infections. Nephrol Dial Transplant 17: 1149–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Poole CV, Carlton D, Bimbo L, Allon M: Treatment of catheter-related bacteraemia with an antibiotic lock protocol: Effect of bacterial pathogen. Nephrol Dial Transplant 19: 1237–1244, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Maya ID, Carlton D, Estrada E, Allon M: Treatment of dialysis catheter-related Staphylococcus aureus bacteremia with an antibiotic lock: A quality improvement report. Am J Kidney Dis 50: 289–295, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Peterson WJ, Maya ID, Carlton D, Estrada E, Allon M: Treatment of dialysis catheter-related Enterococcus bacteremia with an antibiotic lock: A quality improvement report. Am J Kidney Dis 53: 107–111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onder AM, Billings A, Chandar J, Francoeur D, Simon N, Abitbol C, Zilleruelo G: PREFABL: Predictors of failure of antibiotic locks for the treatment of catheter-related bacteraemia. Nephrol Dial Transplant 25: 3686–3693, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Joshi AJ, Hart PD: Antibiotic catheter locks in the treatment of tunneled hemodialysis catheter-related blood stream infection. Semin Dial 26: 223–226, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Shaffer D: Catheter-related sepsis complicating long-term, tunnelled central venous dialysis catheters: Management by guidewire exchange. Am J Kidney Dis 25: 593–596, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Robinson D, Suhocki P, Schwab SJ: Treatment of infected tunneled venous access hemodialysis catheters with guidewire exchange. Kidney Int 53: 1792–1794, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Beathard GA: Management of bacteremia associated with tunneled-cuffed hemodialysis catheters. J Am Soc Nephrol 10: 1045–1049, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Tanriover B, Carlton D, Saddekni S, Hamrick K, Oser R, Westfall AO, Allon M: Bacteremia associated with tunneled dialysis catheters: Comparison of two treatment strategies. Kidney Int 57: 2151–2155, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Langer JM, Cohen RM, Berns JS, Chittams J, Cooper ET, Trerotola SO: Staphylococcus-infected tunneled dialysis catheters: Is over-the-wire exchange an appropriate management option? Cardiovasc Intervent Radiol 34: 1230–1235, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Aslam S: Effect of antibacterials on biofilms. Am J Infect Control 36: e9–e11, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Costerton JW, Stewart PS, Greenberg EP: Bacterial biofilms: A common cause of persistent infections. Science 284: 1318–1322, 1999 [DOI] [PubMed] [Google Scholar]
- 36.El-Azizi M, Rao S, Kanchanapoom T, Khardori N: In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann Clin Microbiol Antimicrob 4: 2, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aslam S, Trautner BW, Ramanathan V, Darouiche RO: Pilot trial of N-acetylcysteine and tigecycline as a catheter-lock solution for treatment of hemodialysis catheter-associated bacteremia. Infect Control Hosp Epidemiol 29: 894–897, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Landry DL, Braden GL, Gobeille SL, Haessler SD, Vaidya CK, Sweet SJ: Emergence of gentamicin-resistant bacteremia in hemodialysis patients receiving gentamicin lock catheter prophylaxis. Clin J Am Soc Nephrol 5: 1799–1804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbas SA, Haloob IA, Taylor SL, Curry EM, King BB, Van der Merwe WM, Marshall MR: Effect of antimicrobial locks for tunneled hemodialysis catheters on bloodstream infection and bacterial resistance: A quality improvement report. Am J Kidney Dis 53: 492–502, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Lowy FD: Staphylococcus aureus infections. N Engl J Med 339: 520–532, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Wells GASB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 3, 2014
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.