Abstract
Most patients with first-time kidney stones undergo limited evaluations, and few receive preventive therapy. A prediction tool for the risk of a second kidney stone episode is needed to optimize treatment strategies. We identified adult first-time symptomatic stone formers residing in Olmsted County, Minnesota, from 1984 to 2003 and manually reviewed their linked comprehensive medical records through the Rochester Epidemiology Project. Clinical characteristics in the medical record before or up to 90 days after the first stone episode were evaluated as predictors for symptomatic recurrence. A nomogram was developed from a multivariable model based on these characteristics. There were 2239 first-time adult kidney stone formers with evidence of a passed, obstructing, or infected stone causing pain or gross hematuria. Symptomatic recurrence occurred in 707 of these stone formers through 2012 (recurrence rates at 2, 5, 10, and 15 years were 11%, 20%, 31%, and 39%, respectively). A parsimonious model had the following risk factors for recurrence: younger age, male sex, white race, family history of stones, prior asymptomatic stone on imaging, prior suspected stone episode, gross hematuria, nonobstructing (asymptomatic) stone on imaging, symptomatic renal pelvic or lower-pole stone on imaging, no ureterovesicular junction stone on imaging, and uric acid stone composition. Ten-year recurrence rates varied from 12% to 56% between the first and fifth quintiles of nomogram score. The Recurrence of Kidney Stone nomogram identifies kidney stone formers at greatest risk for a second symptomatic episode. Such individuals may benefit from medical intervention and be good candidates for prevention trials.
The prevalence of nephrolithiasis in the United States population is increasing, and 9% of men and 6% of women have had a symptomatic stone episode.1 After the first symptomatic kidney stone episode, knowledge regarding the risk of a second symptomatic episode would greatly help caregivers optimize prevention and management strategies. It is well known that patients with frequent symptomatic stone episodes are at increased risk for future episodes and need subspecialty care.2 It is less clear which, if any, patients who have had only one symptomatic stone episode need more than modest dietary and lifestyle recommendations. If the subset at high risk for another episode can be identified, subspecialty evaluation with 24-hour urine chemistries, radiographic monitoring for stones, medical therapy, or more intensive dietary counseling may be of benefit. Early effective interventions may spare such individuals the morbidity of painful stone episodes and potential long-term complications, such as kidney failure.3,4 Alternatively, those at low risk for a second episode would be spared the expense of subspecialty evaluations, potential harm from stone prevention medications, and a restrictive stone prevention diet (e.g., low dietary oxalate).
Several studies have identified predictors for recurrence after the first stone episode,5–9 although to our knowledge, no formal prediction tool has been developed for routine use in clinical care.10 Limitations of past studies include small sample size, inadequate ascertainment of potential predictors, and referral-based study populations (stone clinics). Previous studies combined symptomatic stone episodes with radiographic detection or growth of asymptomatic stones, but the latter is not a clinical event. It is the contribution of asymptomatic radiographic stones to the risk of symptomatic recurrence that is relevant to the patient. Patients seen in stone clinics have extensive laboratory evaluations but are also likely to have severe kidney stone disease that is recurrent. Conversely, most first-time symptomatic stone formers in the general population have a limited evaluation without urine chemistries.8
This lack of medical intervention among most first-time stone formers provides an opportunity to study the natural history of symptomatic recurrence.8 We performed a general population cohort study of all validated incident kidney stone formers in Olmsted County, Minnesota, from 1984 to 2003 and followed them for a second episode. Our objective was to develop a prediction tool for stone formers to estimate risk of a second symptomatic episode using only characteristics commonly available at the time of the first episode.
Results
On the basis of the International Classification of Diseases, Ninth Revision (ICD-9), codes, 4908 Olmsted County residents received a new diagnosis of kidney stones. Upon chart review we excluded the following: prevalent (episode before 1984 or residency) stone formers (19%), asymptomatic only (8%), suspected stone only (11%), no evidence of kidney stone disease (7%), age younger than 18 years (4%), no research authorization (4%), and never a resident of Olmsted County (1%). This left 2239 (46%) as validated first-time symptomatic stone formers. The first symptomatic stone episode resolved via observation of a voided stone in 48% of patients, surgery in 33%, and resolution of symptoms without observation of a stone in 8%; resolution was not documented in 12%. Validated stone formers were followed for a total 20,548 person-years (median follow-up from stone diagnosis, 11.2 years), and 707 patients had a second (recurrent) symptomatic stone during this interval. Symptomatic recurrence rates at 2, 5, 10, and 15 years were 11%, 20%, 31%, and 39%, respectively.
Univariable Analysis
Table 1 shows the candidate predictors in stone formers. Largest stone diameter had only a weak association with recurrence and was not considered further. Use of stone prevention medications, dietary alterations, and stone clinic evaluation were not considered in predictive models because these represent efforts that already target patients at high risk for recurrence (confounding by indication). Stone composition was available in 51% of patients at the time of the first episode, but only uric acid composition (all were >50% uric acid) was individually associated with symptomatic recurrence (P<0.001). Table 2 shows the unadjusted hazard ratios for each of the 31 remaining candidate predictors based on the Cox model. Cumulative symptomatic recurrence curves are presented for selected predictors in Figure 1.
Table 1.
Characteristics of the 2239 first-time symptomatic stone formers in Olmsted County, Minnesota (1984–2003), with and without symptomatic recurrence
| Characteristic | Symptomatic Recurrence | P Valuea | |
|---|---|---|---|
| No (n=1532) | Yes (n=707) | ||
| Clinical | |||
| Age (yr) | 42 (32, 54) | 41 (31, 51.0) | 0.03 |
| Men | 937 (61) | 462 (65) | 0.06 |
| White race | 1313 (86) | 659 (93) | <0.001 |
| Family history | 332 (22) | 243 (34) | <0.001 |
| BMI at nephrolithiasis diagnosis (kg/m2) | 27 (24, 31) | 28 (24, 31) | 0.38 |
| Patient pregnant during the episode | 34 (2.2) | 23 (3.3) | 0.15 |
| Cigarette smoking status | 0.50 | ||
| Current | 338 (22) | 170 (24) | |
| Former | 340 (22) | 146 (21) | |
| Never | 854 (56) | 391 (55) | |
| Prior incidental (asymptomatic) stone on imaging | 73 (4.8) | 51 (7.2) | 0.02 |
| Prior suspected stoneb | 70 (4.6) | 83 (12) | <0.001 |
| Pain | 0.27 | ||
| Renal colic | 1384 (90) | 644 (91) | |
| Atypical | 101 (6.6) | 36 (5.1) | |
| None | 47 (3.1) | 27 (3.8) | |
| Temperature>38°C | 100 (6.5) | 44 (6.2) | 0.78 |
| Urinary tract infection | 79 (5.2) | 33 (4.7) | 0.62 |
| Microscopic hematuria | 1203 (79) | 547 (77) | 0.54 |
| Gross hematuria | 304 (20) | 175 (25) | <0.01 |
| Imaging | |||
| Stone number | <0.001 | ||
| Missing: no imaging | 102 | 49 | |
| 0 | 233 (16) | 77 (12) | |
| 1 | 825 (58) | 288 (44) | |
| ≥2 | 372 (26) | 293 (45) | |
| Any nonobstructing stone | 404 (28) | 277 (42) | <0.001 |
| Any obstructing stone | 1083 (76) | 505 (77) | 0.61 |
| Staghorn stone | 19 (1.3) | 4 (0.6) | 0.14 |
| Symptomatic pelvic or lower-pole stone | 124 (8.7) | 145 (22) | <0.001 |
| Ureteropelvic junction stone | 76 (5.3) | 41 (6.2) | 0.40 |
| Ureter stone | 501 (35) | 242 (37) | 0.44 |
| Ureterovesicular junction stone | 467 (33) | 176 (27) | <0.01 |
| Largest stone diameter | 0.03 | ||
| <3 mm or reported as “tiny” or not reported | 957 (62) | 471 (66) | |
| 3–6 mm | 438 (29) | 164 (23) | |
| >6 mm | 137 (8.9) | 72 (10) | |
| Stone analysis | |||
| Composition groups | 0.04 | ||
| Unknown | 745 | 353 | |
| Mostly oxalate | 608 (77) | 256 (72) | |
| Mostly apatite | 132 (17) | 57 (16) | |
| Any brushite | 6 (0.8) | 4 (1.1) | |
| Any struvite | 7 (0.9) | 5 (1.4) | |
| Any uric acid | 26 (3.3) | 29 (8.1) | |
| Any calcium carbonate | 2 (0.3) | 1 (0.3) | |
| Any urate | 2 (0.3) | 0 (0.0) | |
| Any drug | 4 (0.5) | 2 (0.6) | |
| Comorbidities | |||
| CKD | 60 (3.9) | 21 (3.0) | 0.27 |
| Hypertension | 385 (25) | 167 (24) | 0.44 |
| Diabetes mellitus | 74 (4.8) | 38 (5.4) | 0.58 |
| Dyslipidemia | 244 (16) | 108 (15) | 0.69 |
| Gout | 41 (2.7) | 15 (2.1) | 0.43 |
| Hyperparathyroidism | 5 (0.3) | 3 (0.4) | 0.72 |
| Diarrhea | 145 (9.5) | 67 (9.5) | 0.99 |
| Lower urinary tract symptoms | 549 (36) | 236 (33) | 0.26 |
| Treatments | |||
| Stone surgery | 501 (33) | 214 (30) | 0.25 |
| Documented diet alteration | 272 (18) | 151 (21) | 0.04 |
| Stone clinic evaluation | 146 (9.5) | 94 (13) | <0.01 |
| Stone prevention medications | 42 (2.7) | 27 (3.8) | 0.17 |
Values are expressed as number (percentage) of patients or median (25th, 75th percentiles). BMI, body mass index.
From chi-squared or rank-sum test.
Characteristic renal colic attributed to a stone but no stone seen on imaging or voided.
Table 2.
Univariate hazard ratios for recurrence of symptomatic stones
| Predictor | Hazard Ratio | P Value |
|---|---|---|
| Age, per decade | 0.92 | 0.002 |
| Male sex | 1.15 | 0.07 |
| White race | 1.31 | 0.07 |
| Family history of kidney stones | 1.64 | <0.001 |
| Body mass index | 1.01 | 0.27 |
| Pregnant | 1.40 | 0.11 |
| Current smoker | 0.95 | 0.45 |
| Prior incidental (asymptomatic) stone on imaging | 1.53 | 0.004 |
| Prior suspected stonea | 2.27 | <0.001 |
| Renal colic | 1.000 | >0.99 |
| Fever | 0.97 | 0.84 |
| Urinary tract infection | 1.06 | 0.75 |
| Microscopic hematuria | 0.97 | 0.77 |
| Gross hematuria | 1.29 | 0.004 |
| ≥2 stones on imaging | 1.72 | <0.001 |
| Any nonobstructing stone | 1.81 | <0.001 |
| Any obstructing stone | 1.07 | 0.45 |
| Staghorn stone | 0.59 | 0.29 |
| Symptomatic pelvic or lower-pole stone | 2.34 | <0.001 |
| Ureteropelvic junction stone | 1.19 | 0.29 |
| Ureter stone | 1.09 | 0.29 |
| Ureterovesicular junction stone | 0.79 | 0.007 |
| Any known uric acid composition | 2.11 | <0.001 |
| CKD | 0.99 | 0.97 |
| Hypertension | 0.94 | 0.45 |
| Diabetes mellitus | 1.14 | 0.42 |
| Dyslipidemia | 1.00 | 0.99 |
| Gout | 0.84 | 0.51 |
| Hyperparathyroidism | 1.07 | 0.91 |
| Diarrhea | 0.97 | 0.79 |
| Lower urinary tract symptoms | 0.88 | 0.12 |
Data from n=2239 patients; 707 episodes.
Characteristic renal colic attributed to a stone but no stone seen on imaging or with voiding.
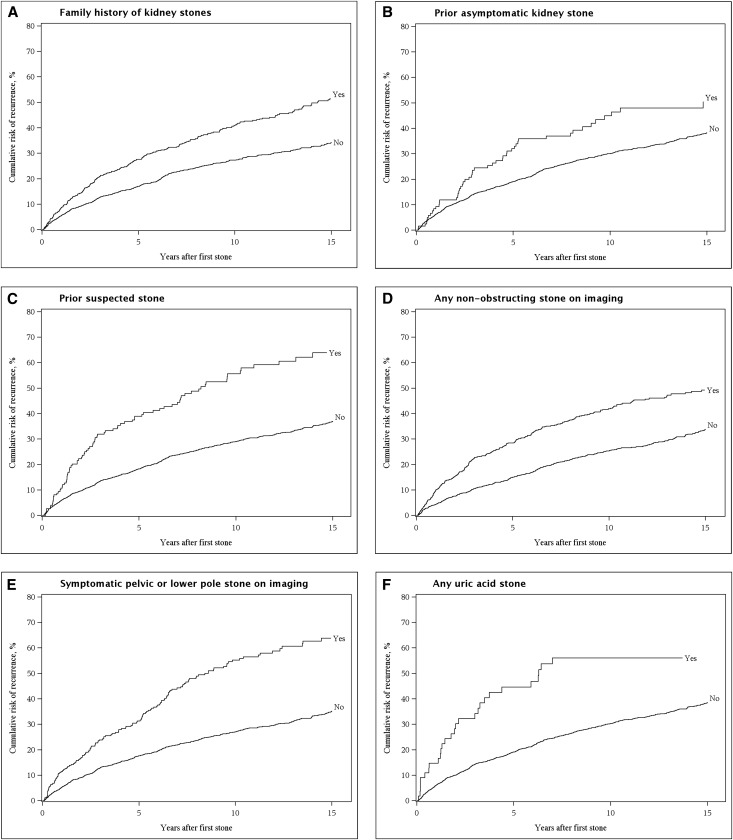
Figure 1.
There are multiple predictors of symptomatic recurrence. Cumulative risk of recurrence after the first symptomatic kidney stone with family history of stones (A), prior asymptomatic stone (B), prior suspected stone (C), any nonobstructing stone on imaging (D), symptomatic pelvic or lower pole stone on imaging (E), or any known uric acid for stone composition (F).
Multivariable Analysis
Because number of stones on imaging was highly correlated with the presence of any nonobstructing stone, this variable was dropped from subsequent models. Hyperparathyroidism, with a prevalence <1%, was also eliminated. A multivariable model including all 29 remaining candidate predictors had a C-statistic of 0.670. We felt that a model with fewer parameters would be clinically more useful, and a reduced model using just 11 of the predictors had a slightly smaller C-statistic of 0.661 (Table 3). Significant predictors in this final model included younger age, male sex, family history, prior suspected stone, any nonobstructing stones, symptomatic pelvic or lower-pole stone, and known uric acid composition. With bootstrapping, the final model C-index corrected for optimism was 0.647. There was good agreement between observed and predicted 10-year recurrence risk (Supplemental Figure 1).
Table 3.
Final model for predicting symptomatic recurrence using all stone formers and the subset with CT imaging
| Predictor | All Stone Formers (n=2239, C Statistic=0.661) | Stone Formers with CT Imaging (n=765, C Statistic=0.687) | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age, per decade | 0.89 (0.84 to 0.94) | <0.001 | 0.95 (0.86 to 1.05) | 0.31 |
| Male sex | 1.29 (1.09 to 1.52) | 0.003 | 1.45 (1.07 to 1.97) | 0.02 |
| White | 1.32 (0.97 to 1.80) | 0.07 | 1.34 (0.75 to 2.43) | 0.33 |
| Family history of stones | 1.57 (1.34 to 1.86) | <0.001 | 1.73 (1.26 to 2.37) | <0.001 |
| Prior asymptomatic stone on past imaging | 1.34 (0.99 to 1.81) | 0.06 | 1.46 (0.86 to 2.48) | 0.16 |
| Prior suspected stone episodea | 1.93 (1.51 to 2.46) | <0.001 | 1.96 (1.26 to 3.05) | 0.003 |
| Gross hematuria | 1.08 (0.90 to 1.29) | 0.42 | 1.43 (1.02 to 1.99) | 0.04 |
| Any nonobstructing stone | 1.66 (1.41 to 1.94) | <0.001 | 2.07 (1.54 to 2.77) | <0.001 |
| Symptomatic pelvic or lower-pole stone | 2.02 (1.67 to 2.45) | <0.001 | 1.69 (1.17 to 2.45) | 0.006 |
| Symptomatic ureterovesicular junction stone | 0.87 (0.73 to 1.04) | 0.12 | 0.93 (0.69 to 1.26) | 0.64 |
| Any known uric acid composition | 2.37 (1.60 to 3.50) | <0.001 | 3.15 (1.43 to 6.92) | 0.004 |
CI, confidence interval.
Characteristic renal colic attributed to a stone but no stone seen on imaging or voided.
Stone imaging technology has changed over time; computed tomography (CT) scans made up 1% of the images from 1984 to 1988 but 76% of the images from 2000 to 2003. After the final model was refit to include only patients with CT imaging, the C-statistic was slightly higher (0.687 versus 0.661). The CT imaging model had about one third the sample size with much shorter follow-up time, but the predictor hazard ratios (HRs) were similar to the “all stone formers” model for the imaging predictors (Table 3). A model stratifying on any nonobstructing stone also slightly increased the C-statistic (0.670 versus 0.661), and most predictors did not change or were attenuated in patients with any nonobstructing stone (Table 4). Only 17 patients (2%) had a second symptomatic episode attributed to the same stone that caused the first symptomatic episode; their exclusion had no substantive effect on the predictor HRs (data not shown) or the C-statistic (0.663 versus 0.661).
Table 4.
Model predicting symptomatic recurrence stratified on any nonobstructing stone
| Predictor | Nonobstructing Stone Absent (n=1407; 381 Events) | Nonobstructing Stone Present (n=681; 277 Events) | P Value | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | ||
| Age, per decade | 0.86 (0.79 to 0.93) | <0.001 | 0.93 (0.86 to 1.02) | 0.11 | 0.17 |
| Male sex | 1.37 (1.09 to 1.71) | <0.01 | 1.24 (0.96 to 1.59) | 0.10 | 0.59 |
| White | 1.74 (1.07 to 2.84) | 0.03 | 1.02 (0.68 to 1.52) | 0.94 | 0.07 |
| Family history of stones | 1.47 (1.18 to 1.82) | <0.001 | 1.61 (1.24 to 2.09) | <0.001 | 0.54 |
| Prior asymptomatic stone on past imaging | 0.59 (0.30 to 1.14) | 0.11 | 1.77 (1.24 to 2.53) | 0.002 | 0.004 |
| Prior suspected stone episodea | 2.51 (1.80 to 3.51) | <0.001 | 1.57 (1.09 to 2.27) | 0.02 | 0.09 |
| Gross hematuria | 1.21 (0.95 to 1.54) | 0.12 | 0.97 (0.73 to 1.28) | 0.81 | 0.24 |
| Symptomatic pelvic or lower-pole stone | 2.79 (2.14 to 3.65) | <0.001 | 1.56 (1.19 to 2.05) | 0.001 | <0.01 |
| Symptomatic ureterovesicular junction stone | 0.86 (0.69 to 1.08) | 0.18 | 0.87 (0.66 to 1.16) | 0.35 | 0.88 |
| Any known uric acid composition | 2.28 (1.42 to 3.66) | <0.001 | 2.78 (1.34 to 5.76) | <0.01 | 0.60 |
C statistic=0.670. CI, confidence interval.
Characteristic renal colic attributed to a stone but no stone seen on imaging or voided.
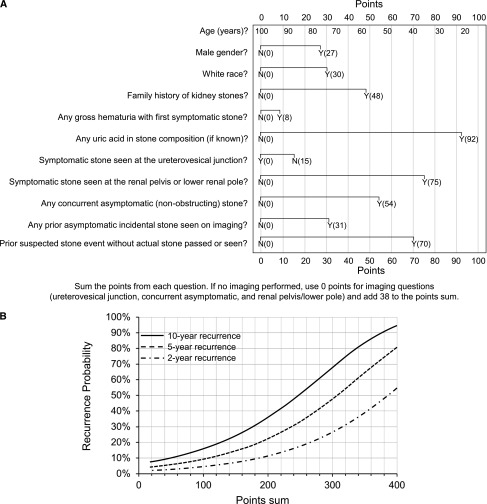
Nomogram
The linear predictors (Cox model coefficients) from the final model were used to develop the Recurrence of Kidney Stone (ROKS) nomogram. Once total points are summed (Figure 2A), Figure 2B can be used to estimate the risk of symptomatic recurrence. The median (25th, 75th percentiles) for points assigned was 163 (129, 203). Symptomatic recurrence at 10 years across point quintiles ranged from 12% to 56% (Supplemental Figure 2). Because imaging was not done in 6.6% (n=151) of patients, the final model included only the 2088 with imaging. Additional analysis confirmed that risk prediction for patients without imaging could consider the imaging variable points as “missing at random” (data not shown). Thus, the nomogram for patients without imaging assigns 0 points for the three imaging variables (ureterovesicular junction stone, concurrent asymptomatic (nonobstructing) stone, renal pelvic or lower-pole stone) and adds 38 (the average contribution among stone formers with imaging) to the total points.
Figure 2.
The Recurrence of Kidney Stone (ROKS) nomogram can be easily applied in first time symptomatic stone formers. First, determine the total points based on the sum of 11 predictors (A). Second, estimate recurrence risk at 2 years, 5 years, and 10 years based on the total points (B). Risk of recurrence at 2, 5, and 10 years is 1–αexp(−1.2797+0.00942×points), where α=0.936, 0.871, and 0.785, respectively. An electronic version of the ROKS nomogram is available on the QxMD app “Calculate” (iOS: http://qx.md/qx; Android: http://qx.md/android; and web tool: http://qxmd.com/ROKS). N, no; Y, yes.
Surgery was weakly associated with a decreased risk of symptomatic recurrence when added as an additional predictor to the final model (HR, 0.82; P=0.03) but had a trivial improvement on discrimination (C statistic=0.662 versus 0.661). The presence of “incidental” nonobstructing stones influenced the effect of surgery on recurrence risk. Among the 681 patients with any nonobstructing stone on imaging, adding surgery to the final model was associated with a decreased risk of recurrence (HR, 0.65; P=0.002). However, among the 1407 patients without any nonobstructing stone on imaging, adding surgery to the final model was not associated with a decreased risk of recurrence (HR, 1.01; P=0.94). Nevertheless, we could not consistently determine from the surgical reports whether nonobstructing stones were removed in addition to the obstructing stone. Thus, we did not include surgery in the final nomogram.
Example Case
A 30-year-old (82 points) white (30 points) woman presents with flank pain from her first symptomatic kidney stone. Her father also had kidney stones (48 points). She had a similar flank pain episode 5 years previously that resolved on its own, but no stone was ever seen (70 points). She also presents with gross hematuria (8 points). A 10-mm renal pelvic stone is surgically removed (75 points+15 points for the symptomatic stone not being at the ureterovesicular junction) that is 100% calcium oxalate. She also has an 8-mm upper-pole nonobstructing stone (54 points). Her nomogram total points are 382. Her individualized risk of symptomatic recurrence at 2, 5, and 10 years is 49%, 75%, and 91%, respectively.
Discussion
The primary clinical concern for patients after their first symptomatic kidney stone is the prevention of another painful episode. Given the relatively low risk of second symptomatic episode for many patients, evaluation and treatment are often limited following the first symptomatic kidney stone.8 Thus, many patients do not receive metabolic evaluations (including 24-hour urine studies) or medications (only 3% of our cohort) until they have had multiple painful episodes. A more accurate assessment of recurrence risk could inform decisions regarding the benefits of a more extensive evaluation. The ROKS nomogram provides such a clinical tool for helping physicians estimate the risk of symptomatic recurrence that is also applicable in the era of CT imaging. Instead of using the commonly cited 50% risk of recurrence at 10 years based on high-risk patients seen in urology clinics,8,9,11,12 the nomogram can individualize the risk of symptomatic recurrence at 10 years for most patients (ranging from 12% for the lowest-risk quintile to 56% for the highest-risk quintile). Treatment decisions between patient and physician will be better informed.
A population-based study of Olmsted County performed 35 years ago identified a similar 10-year risk of recurrence of 35% among first-time stone formers (compared with 31% in this current study).7 However, this prior study did not evaluate for factors that increased this risk, other than male sex.7 Indeed, only a few long-term studies besides ours have identified predictors for symptomatic recurrence, and these studies were referral-based, had limited ascertainment of risk factors for recurrence, and had incomplete follow-up of patients for symptomatic recurrence. For example, first-time stone formers seen in a subspecialty “stone clinic” were followed for a mean of 7.5 years, and the only predictors found for recurrence were younger age and higher urine pH.6 The sample consisted of only 195 patients with 52 recurrence episodes, and the study did not distinguish between asymptomatic radiographic stones and symptomatic stone episodes. We believe this distinction is important because radiographic stone formation and growth is not a clinical event. Radiographic stones can pass with minimal symptoms that do not lead to clinical care or stabilize for decades without ever passing. Further, radiographic stone recurrence can be biased by differential follow-up CT imaging between patients. Another study followed 233 patients with calcium oxalate stones for recurrence. Younger age, male sex, family history of stones, low fluid intake, and high-protein diet were all independent risk factors for recurrence. The recurrence rate of 62% by 5 years was very high and was attributed to selection bias in a stone clinic.5 The increased risk of recurrence in men has been shown in many prior studies.8 Our findings of younger age, male sex, and family history of stones being independent predictors of recurrence are consistent with this prior work.
The predictors for symptomatic recurrence in our final model are biologically plausible. Male sex, white race, and family history of kidney stones associate with a higher prevalence of stone disease,1,13–15 probably because of genetic factors. Genetic factors that also contribute to recurrence. Younger age may also reflect a genetic component that causes an earlier manifestation of stones and their recurrence. Patients with prior suspected stone episodes are also at increased risk for a second episode, most likely because they are actually already recurrent symptomatic stone formers at the time of their first confirmed episode. The location of an obstructing stone also informs recurrence risk. Presence of a stone at the renal pelvis or lower pole suggests a predisposition to form a stone too large to be passed into the ureter; thus, affected patients are more likely to have a symptomatic recurrence. Conversely, patients who form smaller stones that pass to the ureterovesicular junction by the time of imaging may not always have symptoms with future stone passage if obstruction does not occur. Finally, the chemistry and biology of uric acid stone disease differ from those of calcium stone disease, and this likely influence recurrence risk.16
Patients with nonobstructing stones on imaging at the time of their first symptomatic stone have already demonstrated a propensity to form multiple stones. This explains their increased risk for symptomatic recurrence even with stone surgery. Separate models for patients with versus those without any nonobstructing stone improved the model fit by <1% and thus were not used for the final nomogram. Most predictors were attenuated in patients with any nonobstructing stone (consistent with the nonobstructing stone capturing much of the risk).
Incidentally detected nonobstructing (asymptomatic) stones are relatively common.17 Overall, an asymptomatic stone on past imaging was an independent predictor for symptomatic recurrence. However, any prior asymptomatic stone on past imaging trended toward lower risk in patients with nonobstructing stones absent at first episode (possibly the patients passed the same stone and now are stone free) but higher risk in patients with nonobstructive stones present at the first episode (the same stone might still be present, to pass in the future). If stone surgery is not performed to remove these nonobstructing stones, they can later become obstructing and lead to symptomatic recurrence.18 We did not include surgery as a variable in the final nomogram. However, this study does suggest that if surgery is performed to remove a symptomatic stone, further removal of nonobstructing stones helps prevent future symptomatic episodes.
The ROKS nomogram has several limitations. First, the nomogram is intended only for use in first-time symptomatic stone formers, not recurrent symptomatic stone formers. Having prior stone episodes is highly predictive of future episodes.2,19 Second, the nomogram is not intended for rare kidney stone compositions, which are inadequately represented here (e.g., brushite, struvite, cystine) or rare genetic disorders (e.g., adenine phosphoribosyltransferase deficiencies, Dent disease, primary hyperoxaluria), and such patients should be managed as being at high risk for recurrence. Third, urine volume and chemistries may improve the prediction of recurrence,9,20 but these tests are not routinely obtained in our target population of first-time stone formers. Fourth, stone composition was unknown in 49% of the first-time stone formers. Nevertheless, known uric acid composition was such a potent predictor of recurrence that we included it in the nomogram. These data show the importance of determining stone composition in first-time stone formers. Finally, this nomogram needs to be externally evaluated in other community-based settings with a larger proportion of nonwhite residents.
In conclusion, this study identified risk factors for symptomatic recurrence among first-time symptomatic stone formers in the general population. On the basis of these data, a ROKS nomogram was developed to aid physicians in identifying patients at high versus low risk for recurrence. With an estimate of the risk of recurrence, physicians and patients can make more informed decisions on dietary and medical interventions. Currently, few (3%) receive medication to prevent stones after their first episode. There is randomized clinical trial evidence for the efficacy of thiazide diuretics, potassium citrate, and allopurinol for decreasing stone recurrence compared with placebo.21 However, future studies are needed to determine whether treatment decisions based specifically on this nomogram will reduce symptomatic episodes. The ROKS nomogram may be useful in clinical trials targeting high-risk stone formers, and this would help further determine the clinical utility of the nomogram.
Concise Methods
Setting and Participants
After institutional review board approval, all Olmsted County, Minnesota, adult residents with a first-time symptomatic kidney stone between 1984 and 2003 were identified and followed for evidence of symptomatic recurrence in a historical cohort study design. All data were obtained through the Rochester Epidemiology Project, a resource that provides access to medical records of nearly all providers for residents of the County.22 Diagnostic codes dating back to 1935 are indexed, and residents with their first care for stone disease in 1984–2003 were identified using ICD-9 codes 592, 594, and 274.11. Residents who did not provide the Minnesota Research Authorization23 and those with any diagnostic code for kidney stones before 1984 were excluded. Of the remaining patients, charts were carefully reviewed by two dedicated nurse abstractors under the supervision of two nephrologists (A.D.R., J.C.L.) and a urologist (A.E.K.). The first kidney stone episode and any second (recurrent) episode through 2012 were validated (Supplemental Methods).
Candidate Predictors
Complete (inpatient and outpatient) medical records were reviewed for the entire duration of each patient’s residency in Olmsted County (from birth to death). Any past asymptomatic kidney stones, bladder stones, or “suspected stone” episodes were identified if they occurred before the first episode. Other candidate predictors for symptomatic recurrence were required to be identified prior to or up to 90 days after the first episode. Race was analyzed as white versus nonwhite. Recurrent or frequent urinary tract infections before the first stone episode were recorded.
A diagnosis of diabetes mellitus required the presence of two fasting glucose levels ≥126 mg/dl or use of an antidiabetic medication. Hypertension required two consecutive BP readings >140/90 mmHg (prior to the first stone episode because pain can elevate BP) or use of medication to treat hypertension. Diarrhea included loose stools, prior gastric bypass, ileostomy, or colostomy. Stones directly attributed to a systemic metabolic disorder (e.g., primary hyperparathyroidism) or related to a family history of kidney stones were also identified. ICD-9 codes were only used to identify gout: 274.0, 274.81, 274.82, 274.89, or 274.9. Symptoms recorded at the first stone episode included pain, gross hematuria, fever (>38.0°C), and lower urinary tract symptoms (urgency or frequency). Stone composition by infrared spectroscopy was grouped into mutually exclusive categories: unknown; majority calcium oxalate with or without hydroxyapatite; majority hydroxyapatite with or without calcium oxalate; any uric acid; any struvite; any brushite; any urate, any carbonate, or drug stone.
Laboratory tests recorded were spot urine microhematuria and 24-hour urine volume and chemistries. As expected, 24-hour urine studies within 6 months of the first stone episode were missing in most (only 31% had volume, 28% had calcium, and 27% had oxalate) and were not considered further. Imaging findings at the first stone episode by CT, excretory urography, abdominal radiography, or ultrasonography were abstracted for number of stones, largest stone diameter, stone location, and upper tract dilatation. The presence of any nonobstructing kidney stones in addition to symptomatic obstructing stones was identified. Surgery (ureteroscopic, percutaneous, or open lithotomy or shockwave lithotripsy) or care at a stone clinic for prevention of symptomatic recurrence was noted. Any diet alterations or stone prevention medications initiated within 3 months of the first stone episode were recorded. The resolution of the first episode (voided stone, surgery, symptoms resolved, or unknown) was determined.
Statistical Analyses
Analyses were done with SAS (SAS Institute Inc., Cary, NC). Multivariable models to predict symptomatic recurrence that considered all the candidate predictors were developed (Supplementary Methods). A simplified nomogram based on the final statistical model was developed to predict the probability of symptomatic recurrence at 2, 5, and 10 years.24
Disclosures
None.
Supplementary Material
Acknowledgments
This project was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (Mayo Clinic O’Brien Urology Research Center, DK100227 and DK83007) and made possible by the Rochester Epidemiology Project (AG034676) from the National Institutes of Health, US Public Health Service.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “A Nomogram for the Prediction of Kidney Stone Recurrence,” on pages 2685–2687.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013091011/-/DCSupplemental.
References
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parks JH, Coe FL: An increasing number of calcium oxalate stone events worsens treatment outcome. Kidney Int 45: 1722–1730, 1994 [DOI] [PubMed] [Google Scholar]
- 3.El-Zoghby ZM, Lieske JC, Foley RN, Bergstralh EJ, Li X, Melton LJ, 3rd, Krambeck AE, Rule AD: Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol 7: 1409–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC, Tonelli M, Alberta Kidney Disease Network : Kidney stones and kidney function loss: A cohort study. BMJ 345: e5287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khedr MS, Abdel-Hamid AS, Feldman HI: Risk factors of stone recurrence in idiopathic renal calculi. Suez Canal Univ Med J 3: 41–48, 2000 [Google Scholar]
- 6.Trinchieri A, Ostini F, Nespoli R, Rovera F, Montanari E, Zanetti G: A prospective study of recurrence rate and risk factors for recurrence after a first renal stone. J Urol 162: 27–30, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT: Renal stone epidemiology: A 25-year study in Rochester, Minnesota. Kidney Int 16: 624–631, 1979 [DOI] [PubMed] [Google Scholar]
- 8.Uribarri J, Oh MS, Carroll HJ: The first kidney stone. Ann Intern Med 111: 1006–1009, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Sutherland JW, Parks JH, Coe FL: Recurrence after a single renal stone in a community practice. Miner Electrolyte Metab 11: 267–269, 1985 [PubMed] [Google Scholar]
- 10.Matlaga BR: The need for better decision tools in managing stone disease. J Urol 188: 698–699, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Williams RE: Long-term survey of 538 patients with upper urinary tract stone. Br J Urol 35: 416–437, 1963 [PubMed] [Google Scholar]
- 12.Ljunghall S: Renal stone disease. Studies of epidemiology and calcium metabolism. Scand J Urol Nephrol 1–96, 1977 [PubMed] [Google Scholar]
- 13.Pak CYC, Britton F, Peterson R, Ward D, Northcutt C, Breslau NA, McGuire J, Sakhaee K, Bush S, Nicar M, Norman DA, Peters P: Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med 69: 19–30, 1980 [DOI] [PubMed] [Google Scholar]
- 14.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC: Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 63: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Sarmina I, Spirnak JP, Resnick MI: Urinary lithiasis in the black population: an epidemiological study and review of the literature. J Urol 138: 14–17, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Millman S, Strauss AL, Parks JH, Coe FL: Pathogenesis and clinical course of mixed calcium oxalate and uric acid nephrolithiasis. Kidney Int 22: 366–370, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Bansal AD, Hui J, Goldfarb DS: Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol 4: 680–684, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glowacki LS, Beecroft ML, Cook RJ, Pahl D, Churchill DN: The natural history of asymptomatic urolithiasis. J Urol 147: 319–321, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Strauss AL, Coe FL, Deutsch L, Parks JH: Factors that predict relapse of calcium nephrolithiasis during treatment: A prospective study. Am J Med 72: 17–24, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Strauss AL, Coe FL, Parks JH: Formation of a single calcium stone of renal origin. Clinical and laboratory characteristics of patients. Arch Intern Med 142: 504–507, 1982 [PubMed] [Google Scholar]
- 21.Worcester EM, Coe FL: Clinical practice. Calcium kidney stones. N Engl J Med 363: 954–963, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA: Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 41: 1614–1624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melton LJ, 3rd: The threat to medical-records research. N Engl J Med 337: 1466–1470, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Iasonos A, Schrag D, Raj GV, Panageas KS: How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26: 1364–1370, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.