Abstract
Parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) enhance phosphate excretion by the proximal tubule of the kidney by retrieval of the sodium-dependent phosphate transporters (Npt2a and Npt2c) from the apical plasma membrane. PTH activates adenylyl cyclase (AC) through PTH 1 receptors and stimulates the cAMP/PKA signaling pathway. However, the precise role and isoform(s) of AC in phosphate homeostasis are not known. We report here that mice lacking AC6 (AC6−/−) have increased plasma PTH and FGF-23 levels compared with wild-type (WT) mice but comparable plasma phosphate concentrations. Acute activation of the calcium-sensing receptor or feeding a zero phosphate diet almost completely suppressed plasma PTH levels in both AC6−/− and WT mice, indicating a secondary cause for hyperparathyroidism. Pharmacologic blockade of FGF receptors resulted in a comparable increase in plasma phosphate between genotypes, whereas urinary phosphate remained significantly higher in AC6−/− mice. Compared with WT mice, AC6−/− mice had reduced renal Npt2a and Npt2c protein abundance, with approximately 80% of Npt2a residing in lysosomes. WT mice responded to exogenous PTH with redistribution of Npt2a from proximal tubule microvilli to intracellular compartments and lysosomes alongside a PTH-induced dose–response relationship for fractional phosphate excretion and urinary cAMP excretion. These responses were absent in AC6−/− mice. In conclusion, AC6 in the proximal tubule modulates cAMP formation, Npt2a trafficking, and urinary phosphate excretion, which are highlighted by renal phosphate wasting in AC6−/− mice.
Parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) are the primary regulators of renal phosphate (Pi) excretion by the proximal tubule and critically involved in the regulation of Pi balance and maintenance of plasma Pi. PTH acts on the proximal tubule through the Gs protein-coupled PTH 1 receptor (PTH1R) to stimulate adenylyl cyclase (AC) and, thus, the synthesis of cAMP and consecutive activation of protein kinase A (PKA).1–3 After activation of PTH1R, the sodium-phosphate cotransporters Npt2a and Npt2c (approximately 70% and approximately 30% reabsorption of filtered Pi, respectively) are retrieved from the apical plasma membrane, resulting in increased Pi excretion.4–6 In addition, cAMP-independent effects of PTH have been reported,7 which may involve PTH1R action on phosphoinositide-specific phospholipase C (PLC)8,9 and mitogen-activated protein kinases.10,11 FGF-23 mediates its action in the proximal tubule through the FGF receptor/Klotho complex, which downregulates the expression of Npt2a and Npt2c.12,13 Immunohistochemistry identified Npt2a to be localized predominantly in the apical plasma membrane of S1–S3 segments of the proximal tubule.6,14,15 Npt2c has a similar cellular distribution with greater abundance in S1 segments.5,16,17
Genetic defects in Gsα result in impaired PTH-induced retrieval of Npt2a, resulting in a failure to respond with increased urinary Pi excretion: a disease commonly referred to as pseudohypoparathyroidism type Ia or Ib (depending on the presence or absence of skeletal defects, respectively).18–20 In comparison, less is known about the role of AC proteins in mediating the signaling between PTH1R and Npt2a and/or Npt2c. Generation of cAMP involves the activation of ACs, of which nine different membrane-bound isoforms have been identified (AC1–AC9).21 Studies on AC isoform mRNA expression in rat kidney showed that, except for AC1 and AC8, all AC isoforms are expressed.22–24 In mice where a green fluorescent protein reporter gene was expressed under the control of the AC6 promoter, green fluorescent protein-positive cells were found in cells of the proximal tubules, thick ascending limbs, distal tubules, and collecting ducts.25 Functional studies have shown a role of AC6 in the thick ascending limb, distal tubule, and collecting duct insofar as mice lacking AC6 have Bartter syndrome26 and nephrogenic diabetes insipidus (NDI).27,28 In a collecting duct-specific AC6 knockdown model, vasopressin-stimulated epithelial sodium channel open probability was abolished.29
Based on studies indicating that vasopressin V2 receptors use the AC6–cAMP signaling pathway26–30 and the relatively high abundance of AC6 in the proximal tubule,23,30,31 we proposed that AC6 may contribute to PTH-stimulated cAMP formation, Pi excretion, and thus, Pi homeostasis. In the present study, we examined Pi homeostasis, PTH-induced renal excretion of Pi and cAMP, and trafficking and expression of Npt2a and Npt2c in mice lacking AC6 (AC6−/−). The results show that AC6−/− mice have renal phosphate wasting, and thus, AC6 has an essential contribution to PTH actions, cAMP formation, and renal Pi excretion in vivo.
Results
Basal Analysis of AC6−/− Mice with Free Access to Control Diet and Fluid
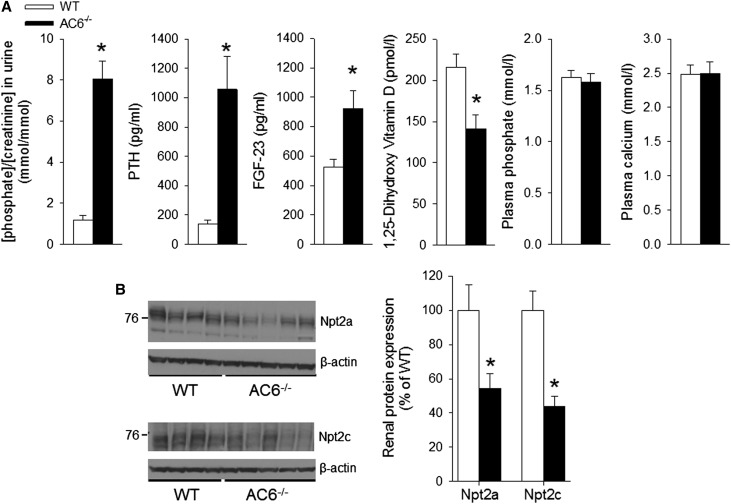
Urinary Pi/creatinine was significantly higher on control diet (0.6% Pi, 0.8% Ca2+) in AC6−/− versus wild-type (WT) mice in spontaneously voided urine, which was associated with an approximately 7-fold higher plasma PTH and an approximately 2-fold higher plasma FGF-23 in AC6−/− versus WT mice. Plasma 1,25-dihydroxy vitamin D [1,25 (OH2)D] was significantly lower (approximately 0.7-fold) in AC6−/− versus WT mice. Plasma Pi and Ca2+ were not different between genotypes (Figure 1A). Under basal conditions, higher urinary Pi/creatinine was associated with significantly reduced renal abundance of Npt2a and Npt2c (Figure 1B) and mRNA expression levels (Npt2a: 0.71±0.1 and Npt2c: 0.46±0.1 versus WT, P<0.05).
Figure 1.
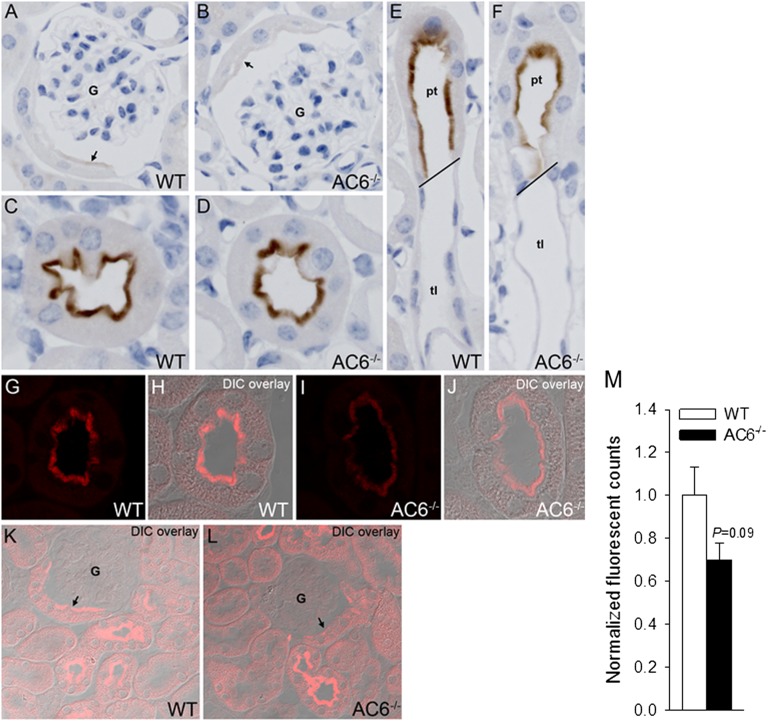
Increased PTH and FGF-23 levels are associated with reduced renal Npt2a and Npt2c expression in AC6−/− mice with free access to food and water. (A) In contrast to WT mice, significantly higher urinary phosphate/creatinine, PTH, and FGF-23 levels were detected in AC6−/− mice, whereas plasma 1,25 (OH2)D levels were suppressed. No significant differences between AC6−/− and WT mice were detected in plasma phosphate or calcium concentrations. (B) Analysis of Npt2a and Npt2c abundances in membrane fractions of renal cortex by Western blotting; densitometric analysis of Western blots was performed using β-actin expression as a reference. Increased urinary phosphate in AC6−/− mice is associated with lower amounts of Npt2a and Npt2c compared with WT. For physiologic parameters, n=9–11/genotype; for protein abundance, n=5/genotype. *P<0.05 versus WT.
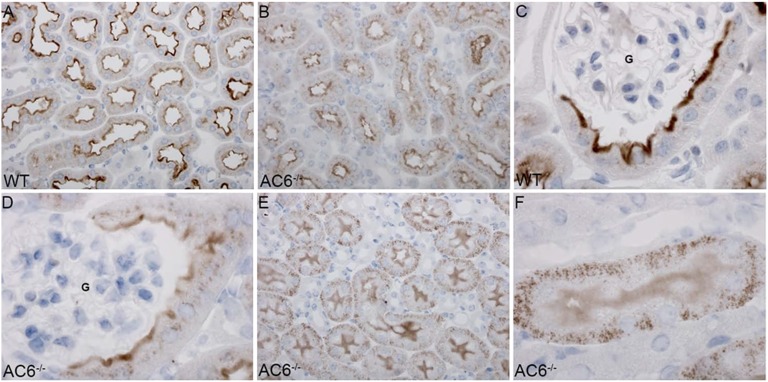
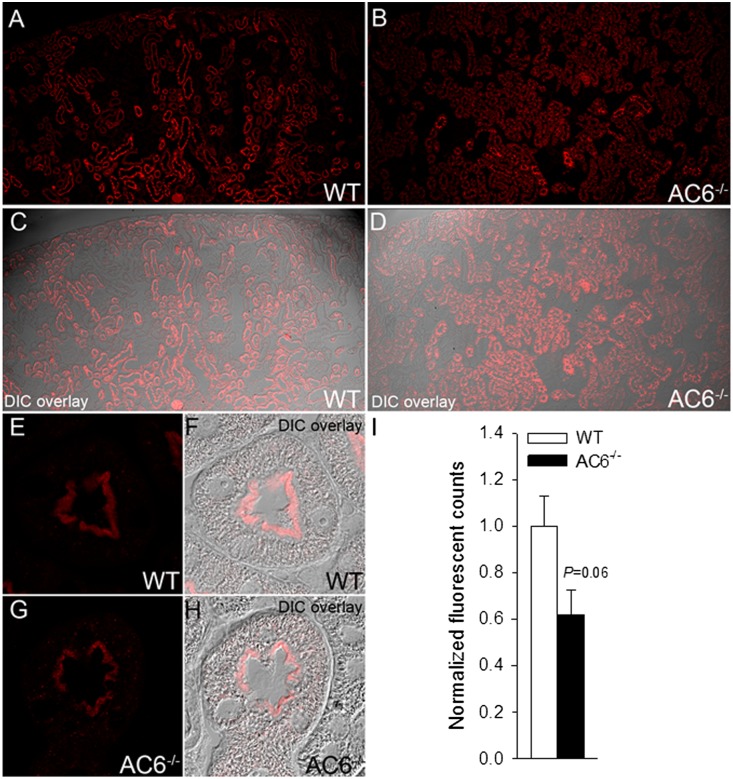
Immunohistochemistry (Figure 2) detected Npt2a throughout the proximal tubule of WT mice, with greater abundance in the early S1 segment. Npt2a staining was predominantly localized to the microvilli. In AC6−/− mice, the tubular distribution of Npt2a was similar to WT mice, but Npt2a staining was generally weaker and observed, in addition to microvilli, intracellularly in a punctate distribution. In AC6−/− mice, Npt2a labeling in the S3 segment was predominantly in distinct intracellular structures, with diffuse labeling of the microvilli. Semiquantitative laser-scanning confocal microscopy (Figure 3) confirmed the observed tubular and intracellular distributions of Npt2a in both genotypes.
Figure 2.
Npt2a in AC6−/− mice is found more intracellularly in a punctate distribution. Immunohistochemical analysis of Npt2a in WT and AC6−/− mice. (A) In WT mice, Npt2a was detected throughout the proximal tubule, with greater abundance in the early (S1) segment. Npt2a staining was predominantly localized to the apical microvilli. (B) In AC6−/− mice, Npt2a was detected throughout the proximal tubule, with greater abundance in the early (S1) segment. Npt2a staining was weaker compared with WT mice and observed in the apical microvilli and intracellular in a punctate distribution. (C and D) Higher magnification of the punctate intracellular distribution of Npt2a in the initial proximal tubule of AC6−/− mice. G, glomerulus. (E and F) In AC6−/− mice, Npt2a labeling in the late proximal tubule (S3) segment was predominantly in distinct intracellular structures, with diffuse labeling of the apical brush border.
Figure 3.
Quantitative immunofluorescent labeling identifies reduced Npt2a in AC6−/− mice. Immunofluorescent labeling of Npt2a and quantitative confocal laser-scanning microscopy. A and B show representative images of cortical kidney sections from WT and AC6−/− mice under control conditions. (C and D) Differential interference contrast (DIC) overlay to show kidney structure and localization of imaging. (E and F) At high magnification, Npt2a staining in WT mice was predominantly localized to the apical microvilli. (G and H) In AC6−/− mice, Npt2a staining was weaker compared with WT mice and observed in the apical microvilli and intracellular in a punctate distribution. (I) Quantification of Npt2a labeling intensity (mean fluorescent signal) shows that total Npt2a labeling intensity is reduced in AC6−/− mice compared with WT (n=4/genotype).
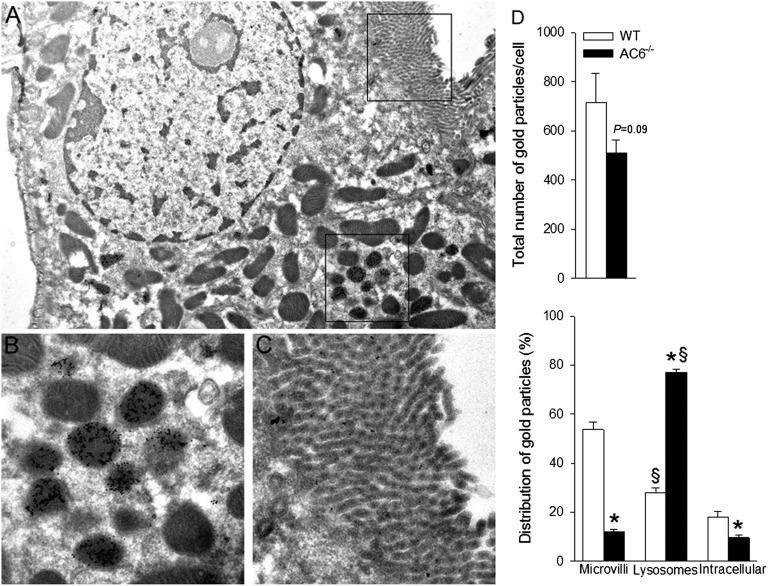
Immunogold electron microscopy of Npt2a (Figure 4) indicated that, in contrast to WT mice (Supplemental Figure 1), gold particles in AC6−/− mice were abundantly observed in structures morphologically resembling secondary lysosomes (Supplemental Figure 2 shows double labeling with various markers), with little labeling associated with microvilli or intracellularly. Semiquantitative assessment of total gold particles showed a different distribution of Npt2a between the genotypes; AC6−/− mice had significantly lower numbers of gold particles in microvilli and higher numbers in lysosomes compared with WT. There was a trend for decreased numbers of total gold particles in AC6−/− (Figure 4).
Figure 4.
The majority of Npt2a resides in lysosomes within proximal tubule cells of AC6−/− mice. (A) Low-magnification immunogold electron microscopy image showing the structure of the proximal tubule cell and the areas highlighted in B and C. At low magnification, gold particles can be observed in defined intracellular structures. (B) At higher magnification, gold particles representing Npt2a are abundantly observed in lysosomes. (C) Little Npt2a labeling was associated with the apical microvilli. Gold particles are 10 nm in diameter. (D) The distribution of colloidal gold particles representing Npt2a was counted within individual proximal tubule cells. Distribution of gold particles was subdivided into the apical microvilli, intracellular compartments, and lysosomes (n=4/genotype). *P<0.05 versus WT. §P<0.05 versus microvilli and intracellular same genotype.
Npt2c labeling by immunohistochemistry was weak in WT mice in the early S1 segment, with staining predominantly localized to the microvilli (Figure 5). Npt2c labeling intensity increased in late proximal tubules from both genotypes and was often detected in the microvilli, with a clear transition to thin descending limbs of Henle’s loop. Labeling intensity of Npt2c in early proximal tubules was weaker in AC6−/− versus WT mice, with no observable differences in late proximal segments. Laser-scanning confocal microscopy (Figure 5) confirmed the observed tubular and intracellular distributions of Npt2c in both genotypes and showed a trend for reduced Npt2c abundance in AC6−/− mice.
Figure 5.
Weaker Npt2c labeling in early proximal tubules of AC6−/− mice. Immunohistochemical and quantitative confocal laser-scanning microscopy analysis of Npt2c in WT and AC6−/− mice. (A and B) Weak Npt2c labeling was observed in the early (S1) segment of the proximal tubule, with staining predominantly localized to the apical microvilli (arrows). (C and D) Npt2c labeling intensity increased in the late proximal tubule in both WT and AC6−/− mice and was detected in the apical microvilli. (E and F) Npt2c labeling was observed in the late proximal tubule (pt; S3) segment with clear transitions to unlabeled descending thin limbs of Henle’s loop (tl). G–J show representative quantitative laser-scanning confocal microscopy images of Npt2c in WT and AC6−/− mice and corresponding differential image contrast (DIC) overlays. Npt2c was localized to the apical brush border in WT and AC6−/− mice. (K and L) In general, Npt2c labeling intensity in early proximal tubules (arrows) was greater in WT compared with AC6−/− mice, with no observable difference in late proximal segments. (M) Quantification of total Npt2c labeling intensity (mean fluorescent signal) in proximal tubules of WT and AC6−/− mice (n=4/genotype).
Response to Calcium-Sensing Receptor Activation, Dietary Phosphate Restriction, and FGF Receptor Blockade in AC6−/− Mice
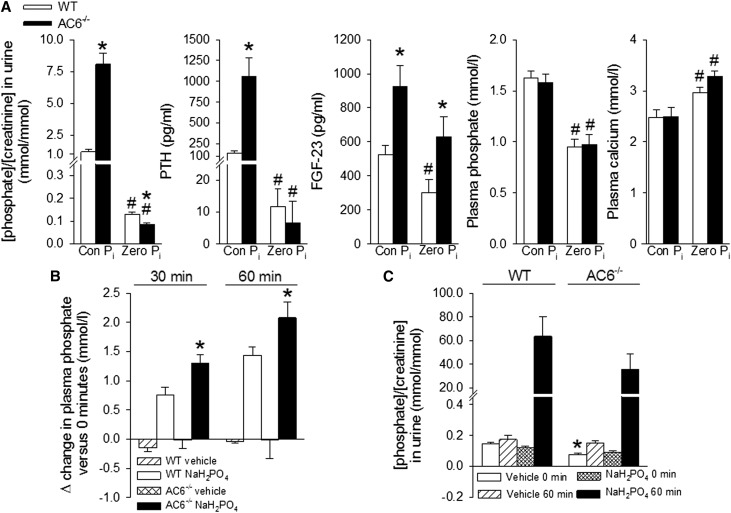
At baseline, PTH was significantly higher in AC6−/− versus WT mice (255±60 versus 39±6 pg/ml, P<0.05), and acute oral treatment with the calcimimetic NPS R-568 suppressed plasma PTH after 30 minutes to comparable low levels (AC6−/−: 14±2 versus WT: 14±1 pg/ml, both P<0.05 versus the basal same genotype), indicating secondary hyperparathyroidism and not primary hypersecretion of PTH. To test if AC6−/− mice can respond to a reduction in dietary Pi, mice were placed for 7 days on a zero Pi diet. Both genotypes reduced urinary Pi/creatinine, plasma Pi, and PTH (Figure 6A). Of note, zero Pi intake was the only condition where urinary Pi/creatinine was significantly lower in AC6−/− versus WT mice. Plasma FGF-23 was reduced in both genotypes compared with control diet; however, FGF-23 remained significantly higher in AC6−/− versus WT mice. Blocking FGF receptors by PD173074 for 6 days resulted in a comparable increase in plasma Pi (Δ increase in WT: 0.8±0.1 and AC6−/−: 0.9±0.2 mmol/L), whereas cumulative urinary Pi/creatinine was significantly higher and Npt2c expression tended to be lower in AC6−/− mice (Supplemental Figures 3 and 4).
Figure 6.
AC6−/− mice reduce urinary phosphate and plasma PTH in response to a zero phosphate diet; however, they show greater increases in plasma phosphate after acute oral phosphate loading. (A) One week of zero phosphate intake (Zero Pi) significantly reduced urinary P/creatinine and plasma PTH levels in both genotypes (for comparison, values of the control diet [Con Pi] are shown from Figure 1). Plasma FGF-23 levels remained significantly higher in AC6−/−, whereas plasma phosphate was reduced to comparable levels in WT and AC6−/− mice. (B) Effects of acute oral phosphate loading. AC6−/− mice show a significantly greater increase in plasma phosphate after 30 and 60 minutes compared with WT mice. (C) Urinary phosphate/creatinine tended to be lower 60 minutes after phosphate loading in AC6−/− versus WT mice. For diets, n=9–11/genotype; for acute oral phosphate loading, n=4–8/group. *P<0.05 versus WT. #P<0.05 versus basal same genotype.
Response of AC6−/− Mice to Acute Hyperphosphatemia
To assess the ability of AC6−/− mice to excrete an acute Pi load, mice were given 0.5 mol/L sodium phosphate by oral gavage after 7 days of zero dietary Pi intake (to induce maximum Pi absorption from the intestine32 and prevent differences in intestinal absorption). After 30 and 60 minutes, plasma Pi increased to significantly higher levels in AC6−/− versus WT mice (Figure 6B). The increase in urinary Pi/creatinine after 60 minutes (Figure 6C) tended to be lower in AC6−/− versus WT mice.
Whole-Kidney Phosphate Handling in Anesthetized Mice
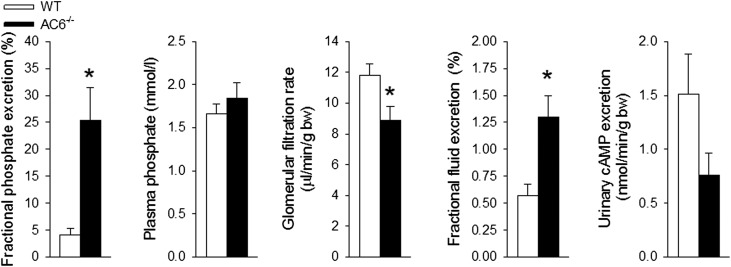
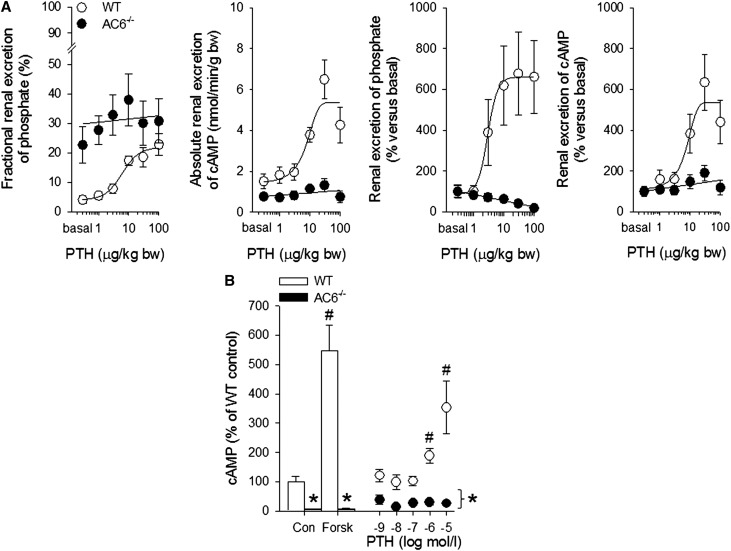
Renal clearance experiments showed a significantly higher absolute (WT: 24±8 versus AC6−/−: 133±35 nmol/min, P<0.05) and fractional urinary Pi excretion after vehicle application (Figure 7). Plasma Pi and the amount of filtered Pi (WT: 580±45 versus AC6−/−: 502±50 nmol/min, P was NS) were comparable between genotypes, whereas GFR was modestly lower in AC6−/− versus WT mice (Figure 7). Urinary cAMP excretion tended to be lower in AC6−/− mice associated with greater fractional fluid excretion, consistent with their previously reported NDI.27 Bolus application of PTH to WT mice (Figure 8) dose-dependently increased absolute and fractional urinary Pi excretion and urinary cAMP excretion. In contrast, AC6−/− mice (Figure 8) responded to PTH with a slight decrease in absolute urinary Pi excretion, and PTH did not change the higher fractional urinary Pi excretion and absolute urinary cAMP excretion (all doses not significantly different from vehicle).
Figure 7.
Clearance experiments show higher fractional phosphate excretion and confirm the phosphate wasting phenotype of AC6−/− mice. Higher fractional phosphate excretion in AC6−/− mice. Acute bolus application of vehicle (0.85% NaCl) in clearance experiments under anesthesia resulted in comparable plasma phosphate levels between the genotypes, whereas AC6−/− mice have a modestly lower GFR associated with a significantly higher fractional phosphate excretion. AC6−/− mice have a significantly higher fractional fluid excretion. Although urinary cAMP excretion tended to be lower, it did not reach statistical significance (n=6–8/genotype). bw, body weight. *P<0.05 versus WT.
Figure 8.
PTH-induced urinary cAMP, phosphate excretion, and cAMP formation in renal cortical tubule suspensions are impaired in AC6−/− mice. (A) Bolus application of PTH in WT mice dose-dependently increased fractional urinary Pi excretion and urinary cAMP excretion. In contrast to WT mice, the phosphaturic effect and cAMP formation in response to PTH are absent in AC6−/− mice. (B) Stimulation of cAMP formation by forskolin (Forsk; 10 µmol/L) or PTH is absent in freshly isolated renal cortical tubule suspensions of AC6−/− compared with WT mice. Protein concentration was adjusted to 60 µg/vial. Experiments were performed with phosphodiesterase inhibition (0.5 mmol/L 3-isobutyl-1-methylxanthine; n=6–8/genotype for clearance experiments and n=5/genotype for isolated cortical tubules). bw, body weight. *P<0.05 versus WT. #P<0.05 versus control same genotype.
Effect of PTH on Renal Cortical cAMP Formation
Freshly isolated renal cortical tubule suspensions from WT mice significantly increased cAMP formation after forskolin stimulation; however, this response was absent in AC6−/− mice. In similar suspensions, PTH increased cAMP formation in WT but not AC6−/− mice (Figure 8B).
Effect of PTH on Npt2a and Npt2c Trafficking in AC6−/− Mice
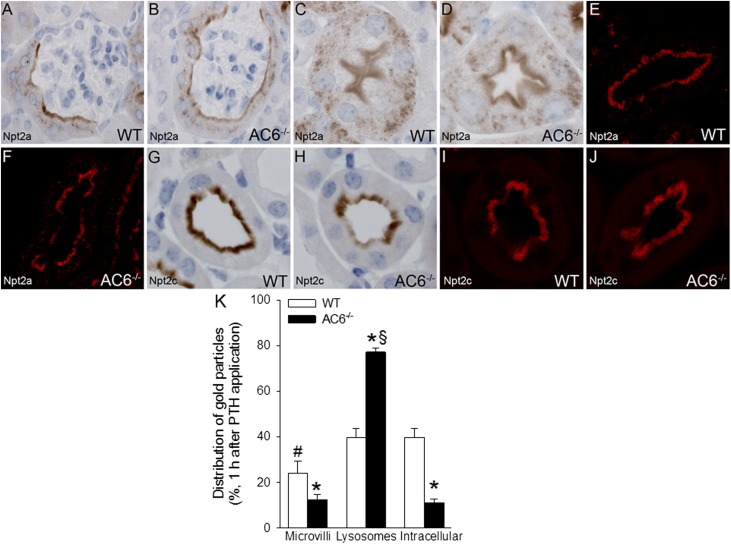
To study the in vivo response to PTH receptor activation, we administered PTH to WT and AC6−/− mice and studied kidneys by immunohistochemistry. Npt2a labeling and distribution were (Figure 9), in contrast to control conditions (Figures 2 and 3), similar between WT and AC6−/− mice in the early S1 segment, with staining observed in the microvilli and intracellularly in a punctate pattern. In the late S3 segment, no observable difference was detected in Npt2a labeling or the distribution between genotypes. Immunogold labeling showed that, in WT mice, compared with control conditions, the distribution of Npt2a gold particles after PTH treatment was different, with a reduction in the number of gold particles residing in the microvilli and an increase of gold particles in lysosomes and intracellular compartments (compare Figure 4 with Figure 9). In contrast, PTH treatment did not significantly change the distribution of Npt2a in AC6−/− mice. After PTH treatment, the distribution of Npt2c was similar in WT and AC6−/− mice with predominant labeling of the microvilli and showing a similar distribution compared with control conditions (Figure 5).
Figure 9.
PTH treatment results in comparable immunolocalization of Npt2a and Npt2c in WT and AC6−/− mice. (A and B) After PTH treatment (200 µg/kg), immunohistochemistry shows that Npt2a labeling and distribution were similar between WT and AC6−/− mice in the early (S1) segment of the proximal tubule, with staining observed in the apical microvilli and intracellularly. (C and D) No observable difference in Npt2a labeling or distribution between WT and AC6−/− mice in the late (S3) segment of the proximal tubule. (E and F) Quantitative laser-scanning confocal microscopy confirmed the similar distribution of Npt2a in WT and AC6−/− mice after PTH treatment. (G–J) After PTH treatment, the distribution of Npt2c was similar in WT and AC6−/− mice, with predominant labeling of the apical brush border. No differences were observed in Npt2c distribution compared with control conditions (Figure 4). (K) In WT mice, compared with control conditions (Figure 4), the distribution of Npt2a gold particles after PTH treatment was shifted, with fewer particles residing in the apical microvilli compared with lysosomes and intracellular compartments. In contrast, after PTH treatment, the distribution of Npt2a in AC6−/− mice is similar to control conditions (Figure 4) (n=4/genotype). *P<0.05 versus corresponding WT group. #P<0.05 versus lysosomes and intracellular in WT. §P<0.05 versus microvilli and intracellular in AC6−/−.
Discussion
Much information has been learned about the molecular determinants of PTH-regulated phosphate transport, but little is known about the role and molecular identity of the AC isoform(s) involved. The present studies show, for the first time, that almost all of the PTH-stimulated cAMP formation and urinary Pi excretion are mediated by AC6. We show that AC6 plays a vital role in Pi homeostasis and that mice lacking AC6 achieve Pi homeostasis only by having compensatory elevated PTH and FGF-23 levels. This defect in urinary Pi excretion is associated with and potentially, the consequence of reduced levels of cAMP, leading to effects on the abundance and distribution of Npt2a and Npt2c.
AC6−/− mice had 7-fold higher PTH compared with WT mice. We excluded primary hyperparathyroidism, because acute activation of the calcium-sensing receptor completely suppressed PTH. In patients with pseudohypoparathyroidism, the calcium-sensing receptor agonist cinacalcet reduced PTH.33 Vice versa, in a mouse model of primary hyperparathyroidism, cinacalcet was ineffective in suppressing PTH.34 The exclusion of primary hyperparathyroidism in AC6−/− mice was supported by very low PTH when fed a zero Pi diet.
Why do AC6−/− mice show renal phosphate wasting and not reduced Pi excretion? Hypothetically, AC6−/− mice should show renal PTH resistance, impaired Pi excretion, hyperphosphatemia, and hyperparathyroidism. Although AC6−/− mice show an absent cAMP and phosphaturic response to PTH, the elevated PTH possibly activates phosphatidylinositol-specific PLC (PI-PLC),35 leading to Npt2a/Npt2c redistribution. Consistent with this hypothesis, continuous infusion of PTH to mice with a PTH receptor inactive for PI-PLC signaling but an intact AC/cAMP/PKA signaling pathway can only transiently increase urinary Pi excretion, indicating that PI-PLC is important for the sustained phosphaturic effect of PTH.7 It is possible that AC6 is part of a negative feedback loop, which when absent, fails to suppress PTH. Alternatively, AC6 may play a secondary role to limit PI-PLC signaling events.35 Additionally, because FGF-23 reduces Npt2a and Npt2c expression and increases urinary Pi excretion,12 the approximately 2-fold increase in FGF-23 levels in AC6−/− mice might aggravate renal phosphate wasting in AC6−/− mice. Although the increased FGF-23 levels are not as severe compared with the drastically increased levels in Hyp,36 FGFR1−/−/FGFR4−/−,37 or Klotho−/−38 mice, even a minor elevation in the FGF-23 signaling pathway may affect urinary Pi excretion.
Consistent with elevated PTH and FGF-23, AC6−/− mice showed a significantly reduced abundance of Npt2a and Npt2c. Using immunohistochemistry and confocal microscopy, Npt2a was localized along the entire length of the proximal tubule in both genotypes, although AC6−/− mice had less intense staining compared with WT, especially in the S1 segment. Our basal immunogold electron microscopy clearly highlights that, in the absence of AC6, the majority (approximately 80%) of Npt2a is localized in secondary lysosomes. It might reflect that the majority of Npt2a has entered the degradative pathway.39 The reductions of Npt2a protein abundances observed in Western blotting of membrane preparations are comparable with the reduced localization of Npt2a gold particles in microvilli of AC6−/− mice. Our previous studies in AC6−/− mice indicated a mild Bartter syndrome caused by reduced NKCC2 expression and NDI caused by impaired aquaporin-2 phosphorylation and trafficking. At this point, it is unclear if these diseases can contribute to the high PTH/FGF-23 and renal phosphate wasting in AC6−/− mice. To our knowledge, Npt2a expression has only been studied in type II Bartter syndrome (renal outer medullary potassium channel-type)40 and lithium-induced NDI.41 Both studies found reduced Npt2a expression; however, the underlying mechanism was never determined. In addition, AC6 might regulate Npt2a/Npt2c function by modulating the phosphorylation of other proteins involved in apical membrane insertion/retention of Npt2a/Npt2c.
In contrast to studies localizing Npt2c mainly to the S1 and weaker in the S2 segment,6,17 our antibody16,42,43 identified Npt2c along the entire length of proximal tubules, with clear transitions from a labeled S3 segment to an unlabeled thin descending limb of Henle’s loop. Our antibody previously gave a strong signal in the outer stripe of the outer medulla in WT mice, consistent with Npt2c expression in S3 segments.16 The reason for this discrepancy remains unknown; however, it might relate to species differences.
Why is FGF-23 increased and 1,25 (OH2)D suppressed in AC6−/− mice? PTH and FGF-23 are part of a negative feedback loop (suppressing each other). However, our data in AC6−/− mice indicate that elevated PTH can offset this negative feedback. A recent study indicates that PTH has a stimulatory effect on FGF-23 release,7 and mice with a constitutively active PTH receptor, overexpressed in osteocytes, have significantly elevated FGF-23 compared with WT mice, a response partially mediated by a cAMP-dependent mechanism.44 Patients with constitutively active PTH receptors (e.g., Jansen metaphyseal chondrodysplasia) also show increased FGF-23, despite hypophosphatemia, possibly indicating that FGF-23 could be governed by other factor(s), including activation of PTH receptors.45 Based on our data, AC6 in bone does not seem to be the enzyme mediating this stimulatory effect, because AC6−/− mice show increased and not reduced FGF-23. The well known regulators of FGF-23 do not seem to be the culprits, and additional work is needed to identify these regulatory factors, possibly involving a kidney bone crosstalk. FGF-23 and PTH have opposite effects on 1,25 (OH2)D: FGF-23 inhibits and PTH stimulates 1,25 (OH2)D. In AC6−/− mice, the suppression of 1,25 (OH2)D dominates (along with reduced Npt2a expression), possibly as a consequence of the unopposed activation of FGF-23. In contrast, a study in humans showed that 18-hour PTH treatment increases 1,25 (OH2)D and FGF-23, indicating that 1,25 (OH2)D is a potent stimulator of FGF-23 secretion.46 Additional studies are needed to better understand the interactions of these hormones or if additional factors, like circulating cleaved forms of Klotho,47 are involved in PTH/FGF-23 regulation.
To assess the role of AC6 for renal Pi transport, we used an acute oral hyperphosphatemic model. After 7 days on zero Pi intake, a situation maximizing the potential contribution for each of the Pi transport mechanisms,32 AC6−/− mice show evidence for impaired renal Pi excretion, with consecutively increased plasma Pi after 30 and 60 minutes, in addition to a tendency for lower urinary Pi/creatinine. Vice versa, when mice lacking intestinal Npt2b were exposed to a comparable acute hyperphosphatemic model, the increase in plasma Pi was impaired after 30 and 60 minutes.48
Is AC6 mediating the acute phosphaturic response to PTH and cAMP formation? Our data indicate that AC6 is the major isoform required for the acute phosphaturic effect and cAMP formation in response to PTH. The timeframe of the phosphaturic response in our experiments (15 minutes) is consistent with other studies, showing a rapid inhibition of renal Pi transport and Npt2a abundance as early as 5 minutes.4,49 A recent study in mice provides evidence that the cAMP/PKA signaling pathway is important for acute PTH-induced phosphaturia and reduction of Npt2a staining.6,50 The maximum effect of PTH-induced fractional Pi excretion observed in WT mice was comparable with the fractional Pi excretion observed after vehicle application and under all tested PTH concentrations in AC6−/−, possibly indicating that AC6−/− mice are at their maximum Pi excretion capacity. Confirming the in vivo results under clearance conditions, freshly isolated renal cortical tubule suspensions from AC6−/− lacked a cAMP response to forskolin or PTH. The impaired renal forskolin response in this study is consistent with the renal forskolin response of an AC6−/− mouse made by another group.30 AC5 and AC6 are the only calcium-inhibitable AC isoforms,21,24 and PTH-simulated AC activity can be inhibited by increasing calcium concentrations, showing that AC5 and/or AC6 are mediating the PTH response.51 The impaired forskolin-stimulated cAMP formation in renal cortex (current study), renal inner medulla,27 and whole kidney30 of AC6−/− mice indicates that AC6 plays a prominent role for renal cAMP formation.
Acute application of PTH leads to a rapid internalization of Npt2a from the brush border membrane and its subsequent routing to lysosomes.4,6,52 Our immunogold electron microscopy shows that, 1 hour after PTH treatment (a timeframe shown to result in almost complete retrieval of Npt2a from the brush border membrane6,42), there is a shift of Npt2a from the microvilli to intracellular compartments and lysosomes; however, this acute PTH-induced Npt2a trafficking is absent in AC6−/− mice. Moreover, 1 hour after PTH application, both genotypes had a comparable amount of Npt2a gold particles in the microvilli, which may explain why fractional urinary Pi excretion was not significantly different at the highest PTH doses used in our clearance studies. An explanation for the number of Npt2a gold particles in microvilli of WT mice after PTH application still being higher than the number of gold particles found in AC6−/− mice under basal conditions might relate to the continuously elevated PTH and/or FGF-23. Our data support other studies indicating a different time course in the regulation of Npt2c versus Npt2a. Npt2c retrieval in response to PTH is not complete until 4–8 hours after treatment.17,42 Consistent with these studies, we did not see a difference in Npt2c abundance or distribution in either genotype after PTH administration.
In summary, we are just beginning to understand the roles of different AC isoforms for hormone-mediated regulation of renal transport. We recognize that our study has limitations because of the complex regulation of Pi homeostasis by the elevated PTH and FGF-23 in AC6−/− mice, thereby hindering our ability to decipher the extent of each hormone on, for example, the reduction in renal Npt2a and Npt2c expression/abundance. However, our results show that, of nine different AC isoforms, AC6 determines PTH-induced cAMP formation and Npt2a and Npt2c expression and trafficking. Furthermore, we show that these effects are functionally relevant for urinary Pi excretion and FGF-23 levels. As a result, mice lacking AC6 are a new model of renal phosphate wasting, despite having absent PTH-induced cAMP formation.
Concise Methods
All animal experimentation was conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and approved by the local Institutional Animal Care and Use Committee. Age-matched adult male and female AC6−/− and WT littermates were generated as described53 using heterozygous breeding; their genetic background is a mix of 129Sv/J and C57BL/6. Mice were fed either a control diet (0.6% Pi, 0.8% Ca2+; Harlan Teklad, Madison, WI) or for studying the effect of Pi intake, a zero Pi diet (0% Pi, 0.8% Ca2+; Harlan Teklad). Experimental male mice were between 3 and 6 months old.
Analysis of AC6−/− Mice with Free Access to Fluid
Mice were kept on control diet or zero Pi diet for 7 days with free access to food and water. Spontaneously voided urine was collected for determination of Pi, Ca2+, and creatinine (all determined photometrically; Thermo Fisher Scientific, Middleton, VA). Blood was drawn from the retro-orbital plexus for determination of Pi, Ca2+, PTH (1–84; ALPCO Immunoassays, Salem, NH), FGF-23 (Immutopics International, San Clemente, CA), and 1,25 (OH)2D (Immunodiagnostic Systems, Scottsdale, AZ). In another set of mice, kidneys were harvested on control diet to determine renal cortical abundance of Npt2a and Npt2c as described below.
PTH Response to Ca2+-Sensing Receptor Activation and FGF Receptor Blockade
The calcimimetic NPS R-568 (Tocris Biosciences, Minneapolis, MN) was applied (30 mg/kg by oral gavage, 10 µl/g body wt of 0.5% carboxymethylcellulose) to activate Ca2+-sensing receptors. At baseline (0 minutes) and after 30 minutes, blood was taken from the retro-orbital plexus under brief isoflurane anesthesia for determination of PTH levels. For FGF receptor blockade, mice were treated every 12 hours for 6 days with PD173074 (50 mg/kg in Kolliphor EL:ethanol:water [7.5:2.5:90] by oral gavage, 1% of body weight; LC Laboratories, Woburn, MA).54,55 Blood was taken before and at the end of the treatment period, and urine was collected daily.
Acute Hyperphosphatemic Mouse Model
Animals were placed on a zero Pi diet for 1 week. Baseline blood and urine samples were taken at 0 minutes. Animals were then gavaged with vehicle (sterile water, 1% of body weight) or a 0.5 mol/L NaH2PO4 solution. Blood was collected after 30 and 60 minutes, and urine was collected at 60 minutes postgavage. Plasma Pi as well as urinary Pi and creatinine were measured as described above.
Effect of PTH on Urinary Pi and cAMP Excretion in Renal Clearance Experiments
Mice were anesthetized with thiobutabarbital/ketamine and prepared for clearance experiments.56,57 A catheter was placed in the femoral artery for continuous BP recording. For assessment of two-kidney GFR, the jugular vein was cannulated for continuous maintenance infusion of 2.25 g/dl BSA in 0.85% NaCl at a rate of 0.5 ml/h per 30 g body wt. [3H]inulin was added to the infusion to deliver 5 µCi/h. After 1 hour of equilibration, intravenous bolus application of vehicle (0.5 µl/g body wt of 0.85% NaCl over 1 minute) was followed by application of increasing doses of PTH (1, 3, 10, 30, and 100 µg/kg; 1–34 Human; GenScript, Piscataway, NJ). After each bolus, allowing 5 minutes for drug distribution, urine was quantitatively collected through a bladder catheter for 15 minutes to determine urinary Pi. Plasma and urinary Pi concentrations were measured as described above. Urinary cAMP concentration was assessed by RIA.58,59
Freshly Isolated Renal Cortical Tubule Suspensions
Renal cortex was isolated using a modification of the method described by Guder,60 and cAMP accumulation was measured as described.27,59 Tubule suspensions were stimulated with PTH (1 nmol/L to 10 µmol/L; GenScript), forskolin (10 µmol/L; Ascent Scientific, Princeton, NJ), or vehicle for 15 minutes at 37°C in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (pretreated for 10 minutes, 0.5 mmol/L; Sigma-Aldrich, St. Louis, MO). Reactions were terminated by the addition of ice-cold 10% TCA (vol/vol), and the cAMP content of TCA extracts was determined by RIA.58,59
Cortical Abundance and mRNA Expression of Npt2a and Npt2c
Kidneys were removed, and renal cortex was dissected and prepared for Western blotting.26,27 Proteins were transferred to nitrocellulose membranes and immunoblotted with Npt2a (1:1000) and Npt2c (1:2500).16,42 Chemiluminescent detection was performed with ECL Plus (Amersham, Piscataway, NJ). Densitometric analysis was performed by ImageJ Software (National Institutes of Health). β-Actin was used as loading control (dilution 1:20,000; Sigma-Aldrich). Mice were euthanized, and renal inner medullae and brain were rapidly removed. In a different set of mice (n=3/genotype), whole-kidney RNA was isolated and reverse-transcribed into cDNA. Quantitative PCR was performed as described previously27,59 using 8 ng RNA/reaction, and it was used in conjunction with primer pairs (forward/reverse) specific for the following murine genes: Npt2a, 5′-GGCTCCAACATTGGCACTAC-3′/5′-ACAGTAGGATGCCCGAGATG-3′; Npt2c, 5′-TACCCCCTCTTCTTGGGTTC-3′/5′-CAGTCTCAAGACAGGCACCA-3′. Data analysis used the ΔΔ cycle threshold method, and they were normalized to 18S ribosomal RNA expression and compared with WT expression.
Immunohistochemistry and Preparation of Tissue for Microscopy
All procedures have been described in detail previously.61 Labeling was visualized by use of a peroxidase-conjugated secondary antibody for light microscopy (P448; Dako, Glostrup, Denmark).
Immunofluorescent Labeling of Kidney Sections, Confocal Laser-Scanning Microscopy, and Image Quantification
Tissue preparation, sectioning, and labeling were performed as previously described.26 Semiquantitative imaging was performed using 8–10 individual images from a labeled section per experimental mouse per genotype as described previously.26
Quantitative Immunogold Electron Microscopy
All tissue processing, staining, and counting procedures have been described in detail previously.62 Briefly, tissue from mice either under basal conditions or after 60 minutes of PTH treatment (200 µg/kg, 2 µl/g body wt in 0.85% NaCl intravenously)4,50 were assessed after labeling with Npt2a. A minimum of six complete proximal tubule cells from different tubules per animal was analyzed from sections oriented approximately at right angles to the apical cell membrane, and they showed negligible background over mitochondria and nuclei. Gold particles within 40 nm of the apical brush border were classified as microvilli, gold particles labeling defined structures morphologically resembling lysosomes were classified as lysosomes, and all other gold particles were classified as intracellular.
Statistical Analyses
The data are expressed as mean±SEM. Unpaired and paired t tests were performed, as appropriate, to analyze for statistical differences between groups. All quantitative data from confocal laser-scanning microscopy were analyzed using one-way ANOVA followed by Newman–Keuls multiple comparisons tests between the groups. For all statistical analyses, P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank N. Aroonsakool, E.-M. Locke, I.-M. Paulsen, and M. Sharik for technical assistance.
Development of the AC6−/− mice was supported by National Institutes of Health Grants P01HL6694 and HL088426 and Veterans Affairs Grant 1101BX001515. This work was supported, in part, by National Institutes of Health Grant R01DK066029 (to M.L.), O'Brien Center for Acute Kidney Injury Research Grant P30DK079337 (to T.R.), American Heart Association Grant 10SDG2610034 (to T.R.), American Society of Nephrology Carl W. Gottschalk Research Scholar Grant (to T.R.), Satellite Healthcare, a not-for-profit renal care provider (to T.R.), the Lundbeck Foundation, the Danish Medical Research Council, the Novo Nordisk Foundation (R.A.F.), and the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101102/-/DCSupplemental.
References
- 1.Levi M, Blaine J, Breusegem S, Takahashi H, Sorribas V, Barry N: Renal phosphate-wasting disorders. Adv Chronic Kidney Dis 13: 155–165, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bergwitz C, Jüppner H: Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 61: 91–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forster IC, Hernando N, Biber J, Murer H: Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med 34: 386–395, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA: The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 69: 495–503, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K: Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol 292: F395–F403, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Picard N, Capuano P, Stange G, Mihailova M, Kaissling B, Murer H, Biber J, Wagner CA: Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflugers Arch 460: 677–687, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Song L, Liu M, Segawa H, Miyamoto K, Bringhurst FR, Kronenberg HM, Jüppner H: Activation of a non-cAMP/PKA signaling pathway downstream of the PTH/PTHrP receptor is essential for a sustained hypophosphatemic response to PTH infusion in male mice. Endocrinology 154: 1680–1689, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iida-Klein A, Guo J, Drake MT, Kronenberg HM, Abou-Samra AB, Bringhurst FR, Segre GV: Structural requirements of parathyroid hormone/parathyroid hormone-related peptide receptors for phospholipase C activation and regulation of phosphate uptake. Miner Electrolyte Metab 21: 177–179, 1995 [PubMed] [Google Scholar]
- 9.Iida-Klein A, Guo J, Takemura M, Drake MT, Potts JT, Jr., Abou-Samra A, Bringhurst FR, Segre GV: Mutations in the second cytoplasmic loop of the rat parathyroid hormone (PTH)/PTH-related protein receptor result in selective loss of PTH-stimulated phospholipase C activity. J Biol Chem 272: 6882–6889, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Bacic D, Schulz N, Biber J, Kaissling B, Murer H, Wagner CA: Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflugers Arch 446: 52–60, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Lederer ED, Sohi SS, McLeish KR: Parathyroid hormone stimulates extracellular signal-regulated kinase (ERK) activity through two independent signal transduction pathways: Role of ERK in sodium-phosphate cotransport. J Am Soc Nephrol 11: 222–231, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M: FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 297: F282–F291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biber J, Hernando N, Forster I, Murer H: Regulation of phosphate transport in proximal tubules. Pflugers Arch 458: 39–52, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Madjdpour C, Bacic D, Kaissling B, Murer H, Biber J: Segment-specific expression of sodium-phosphate cotransporters NaPi-IIa and -IIc and interacting proteins in mouse renal proximal tubules. Pflugers Arch 448: 402–410, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Nie X, Arrighi I, Kaissling B, Pfaff I, Mann J, Barhanin J, Vallon V: Expression and insights on function of potassium channel TWIK-1 in mouse kidney. Pflugers Arch 451: 479–488, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Giral H, Lanzano L, Caldas Y, Blaine J, Verlander JW, Lei T, Gratton E, Levi M: Role of PDZK1 protein in apical membrane expression of renal sodium-coupled phosphate transporters. J Biol Chem 286: 15032–15042, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K: Internalization of renal type IIc Na-Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol 288: F587–F596, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Yu S, Yu D, Lee E, Eckhaus M, Lee R, Corria Z, Accili D, Westphal H, Weinstein LS: Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsalpha) knockout mice is due to tissue-specific imprinting of the gsalpha gene. Proc Natl Acad Sci U S A 95: 8715–8720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germain-Lee EL, Schwindinger W, Crane JL, Zewdu R, Zweifel LS, Wand G, Huso DL, Saji M, Ringel MD, Levine MA: A mouse model of albright hereditary osteodystrophy generated by targeted disruption of exon 1 of the Gnas gene. Endocrinology 146: 4697–4709, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Litman D, Rosenberg MJ, Yu S, Biesecker LG, Weinstein LS: A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin Invest 106: 1167–1174, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieg T, Kohan DE: Regulation of nephron water and electrolyte transport by adenylyl cyclases. Am J Physiol Renal Physiol 306: F701–F709, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen T, Suzuki Y, Poyard M, Miyamoto N, Defer N, Hanoune J: Expression of adenylyl cyclase mRNAs in the adult, in developing, and in the Brattleboro rat kidney. Am J Physiol 273: C323–C330, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Bek MJ, Zheng S, Xu J, Yamaguchi I, Asico LD, Sun XG, Jose PA: Differential expression of adenylyl cyclases in the rat nephron. Kidney Int 60: 890–899, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Defer N, Best-Belpomme M, Hanoune J: Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400–F416, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Wu CS, Lin JT, Chien CL, Chang WC, Lai HL, Chang CP, Chern Y: Type VI adenylyl cyclase regulates neurite extension by binding to Snapin and Snap25. Mol Cell Biol 31: 4874–4886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, Vallon V: Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC. Am J Pathol 182: 96–106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieg T, Tang T, Murray F, Schroth J, Insel PA, Fenton RA, Hammond HK, Vallon V: Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol 21: 2059–2068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE: Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol 302: F78–F84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos KP, Bugaj V, Mironova E, Stockand JD, Ramkumar N, Rees S, Kohan DE: Adenylyl cyclase VI mediates vasopressin-stimulated ENaC activity. J Am Soc Nephrol 24: 218–227, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chien CL, Wu YS, Lai HL, Chen YH, Jiang ST, Shih CM, Lin SS, Chang C, Chern Y: Impaired water reabsorption in mice deficient in the type VI adenylyl cyclase (AC6). FEBS Lett 584: 2883–2890, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Chabardès D, Firsov D, Aarab L, Clabecq A, Bellanger AC, Siaume-Perez S, Elalouf JM: Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J Biol Chem 271: 19264–19271, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Radanovic T, Wagner CA, Murer H, Biber J: Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P(i) diet of the type IIb Na(+)-P(i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 288: G496–G500, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Neary NM, El-Maouche D, Hopkins R, Libutti SK, Moses AM, Weinstein LS: Development and treatment of tertiary hyperparathyroidism in patients with pseudohypoparathyroidism type 1B. J Clin Endocrinol Metab 97: 3025–3030, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawata T, Imanishi Y, Kobayashi K, Kenko T, Wada M, Ishimura E, Miki T, Nagano N, Inaba M, Arnold A, Nishizawa Y: Relationship between parathyroid calcium-sensing receptor expression and potency of the calcimimetic, cinacalcet, in suppressing parathyroid hormone secretion in an in vivo murine model of primary hyperparathyroidism. Eur J Endocrinol 153: 587–594, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Hruska KA, Moskowitz D, Esbrit P, Civitelli R, Westbrook S, Huskey M: Stimulation of inositol trisphosphate and diacylglycerol production in renal tubular cells by parathyroid hormone. J Clin Invest 79: 230–239, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrow EG, Summers LJ, Schiavi SC, McCormick JA, Ellison DH, White KE: Altered renal FGF23-mediated activity involving MAPK and Wnt: Effects of the Hyp mutation. J Endocrinol 207: 67–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattineni J, Alphonse P, Zhang Q, Mathews N, Bates CM, Baum M: Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Renal Physiol 306: F351–F358, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Keusch I, Traebert M, Lötscher M, Kaissling B, Murer H, Biber J: Parathyroid hormone and dietary phosphate provoke a lysosomal routing of the proximal tubular Na/Pi-cotransporter type II. Kidney Int 54: 1224–1232, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, Wang T, Verrey F, Geibel JP, Giebisch G, Hebert SC, Loffing J: Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Renal Physiol 294: F1373–F1380, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Kwon TH, Laursen UH, Marples D, Maunsbach AB, Knepper MA, Frokiaer J, Nielsen S: Altered expression of renal AQPs and Na(+) transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 279: F552–F564, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Lanzano L, Lei T, Okamura K, Giral H, Caldas Y, Masihzadeh O, Gratton E, Levi M, Blaine J: Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone. Am J Physiol Cell Physiol 301: C850–C861, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breusegem SY, Takahashi H, Giral-Arnal H, Wang X, Jiang T, Verlander JW, Wilson P, Miyazaki-Anzai S, Sutherland E, Caldas Y, Blaine JT, Segawa H, Miyamoto K, Barry NP, Levi M: Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol 297: F350–F361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T: Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49: 636–643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown WW, Jüppner H, Langman CB, Price H, Farrow EG, White KE, McCormick KL: Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab 94: 17–20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ: Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res 24: 1681–1685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE: Circulating αKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest 122: 4710–4715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC: Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20: 2348–2358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lötscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek HK, Biber J, Murer H, Kaissling B: Rapid downregulation of rat renal Na/P(i) cotransporter in response to parathyroid hormone involves microtubule rearrangement. J Clin Invest 104: 483–494, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagai S, Okazaki M, Segawa H, Bergwitz C, Dean T, Potts JT, Jr., Mahon MJ, Gardella TJ, Jüppner H: Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem 286: 1618–1626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellorin-Font E, Martin KJ: Regulation of the PTH-receptor-cyclase system of canine kidney: Effects of calcium, magnesium, and guanine nucleotides. Am J Physiol 241: F364–F373, 1981 [DOI] [PubMed] [Google Scholar]
- 52.Traebert M, Roth J, Biber J, Murer H, Kaissling B: Internalization of proximal tubular type II Na-P(i) cotransporter by PTH: Immunogold electron microscopy. Am J Physiol Renal Physiol 278: F148–F154, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JXJ, Roth DM, Hammond HK: Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117: 61–69, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, Ullrich RT, Menon R, Maier S, Soltermann A, Moch H, Wagener P, Fischer F, Heynck S, Koker M, Schöttle J, Leenders F, Gabler F, Dabow I, Querings S, Heukamp LC, Balke-Want H, Ansén S, Rauh D, Baessmann I, Altmüller J, Wainer Z, Conron M, Wright G, Russell P, Solomon B, Brambilla E, Brambilla C, Lorimier P, Sollberg S, Brustugun OT, Engel-Riedel W, Ludwig C, Petersen I, Sänger J, Clement J, Groen H, Timens W, Sietsma H, Thunnissen E, Smit E, Heideman D, Cappuzzo F, Ligorio C, Damiani S, Hallek M, Beroukhim R, Pao W, Klebl B, Baumann M, Buettner R, Ernestus K, Stoelben E, Wolf J, Nürnberg P, Perner S, Thomas RK: Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2: 62ra93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wöhrle S, Bonny O, Beluch N, Gaulis S, Stamm C, Scheibler M, Müller M, Kinzel B, Thuery A, Brueggen J, Hynes NE, Sellers WR, Hofmann F, Graus-Porta D: FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J Bone Miner Res 26: 2486–2497, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V: P2Y₂ receptor activation decreases blood pressure and increases renal Na⁺ excretion. Am J Physiol Regul Integr Comp Physiol 301: R510–R518, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rieg T, Richter K, Osswald H, Vallon V: Kidney function in mice: Thiobutabarbital versus alpha-chloralose anesthesia. Naunyn Schmiedebergs Arch Pharmacol 370: 320–323, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V: Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Rieg T, Pothula K, Schroth J, Satriano J, Osswald H, Schnermann J, Insel PA, Bundey RA, Vallon V: Vasopressin regulation of inner medullary collecting ducts and compensatory changes in mice lacking adenosine A1 receptors. Am J Physiol Renal Physiol 294: F638–F644, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Guder WG: Stimulation of renal gluconeogenesis by angiotensin II. Biochim Biophys Acta 584: 507–519, 1979 [DOI] [PubMed] [Google Scholar]
- 61.Moeller HB, Knepper MA, Fenton RA: Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandberg MB, Maunsbach AB, McDonough AA: Redistribution of distal tubule Na+-Cl- cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291: F503–F508, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.